Evidence for the effect of serotonin receptor 1A gene (HTR1A) polymorphism on tractability in Thoroughbred horses
Summary
Tractability, or how easily animals can be trained and controlled, is an important behavioural trait for the management and training of domestic animals, but its genetic basis remains unclear. Polymorphisms in the serotonin receptor 1A gene (HTR1A) have been associated with individual variability in anxiety-related traits in several species. In this study, we examined the association between HTR1A polymorphisms and tractability in Thoroughbred horses. We assessed the tractability of 167 one-year-old horses reared at a training centre for racehorses using a questionnaire consisting of 17 items. A principal components analysis of answers contracted the data to five principal component (PC) scores. We genotyped two non-synonymous single nucleotide polymorphisms (SNPs) in the horse HTR1A coding region. We found that one of the two SNPs, c.709G>A, which causes an amino acid change at the intracellular region of the receptor, was significantly associated with scores of four of five PCs in fillies (all Ps < 0.05) and one PC in colts (P < 0.01). Horses carrying an A allele at c.709G>A showed lower tractability. This result provides the first evidence that a polymorphism in a serotonin-related gene may affect tractability in horses with the effect partially different depending on sex.
Introduction
Tractability, or how easily animals can be trained and controlled, is an important behavioural trait for the management and training of domestic animals. Individuals with low tractability may be troublesome in many practical situations. Among many potential factors that could affect tractability, anxiety may be particularly important. Individuals showing high anxiety should be less tractable. Serotonin – a neurotransmitter – and its receptors play a crucial role in regulating anxiety in many animal species including humans (Lucki 1998; Fernandez & Gaspar 2012). In particular, one of the subtypes of the serotonin receptor, 1A [HTR1A; 5-hydroxytryptamine (serotonin) receptor 1A, G protein-coupled], is important for modulation of anxiety (Ramboz et al. 1998; Gross et al. 2002; Richardson-Jones et al. 2011; Piszczek et al. 2015).
Polymorphisms in a gene coding for HTR1A have also been found to be associated with many psychiatric disorders including anxiety-related disorders (Drago et al. 2008). For example, a single nucleotide polymorphism (SNP) located in the HTR1A promoter region, named g.–1019C>G, increases the risk of depression and suicide (Lemonde et al. 2003) and panic disorder (Straube et al. 2014).
In domestic animals, the association between serotonin receptor genes and behaviour remains unclear. A few studies have examined the effect of some serotonin receptor genes on aggression in dogs. Whereas one study on Golden Retrievers reported no effect of SNPs in serotonin receptor genes (HTR1A, HTR1B and HTR2A) on aggression (Van Den Berg et al. 2008), a study on English Cocker Spaniels showed a significant effect of other serotonin receptor genes (HTR1D and HTR2C) on aggression (Vage et al. 2010). In the horse HTR1A gene, two SNPs have been found in the coding region (Momozawa et al. 2007). These SNPs are non-synonymous substitutions, which may change the function of the protein due to change in coded amino acids. However, the effect of these SNPs on equine behaviour has not been tested.
In this study, we investigated the association between tractability in Thoroughbred horses and HTR1A gene polymorphisms. We assessed tractability of Thoroughbred horses by giving a questionnaire to caretakers working on a farm and tested the association between tractability scores and HTR1A genotype. We focused on two non-synonymous SNPs in the exon region reported by Momozawa et al. (2007) because resulting amino acid changes may affect the function of the protein, which may, in turn, lead to differences in behaviour.
Materials and methods
Subjects
We assessed the tractability of 167 one-year-old Thoroughbred horses (81 colts and 86 fillies) reared at the Hidaka Training and Research Center, Japan Racing Association, in Hokkaido, North Japan, between 2011 and 2013. Horses were purchased at yearling sales and were introduced to the centre in the first summer after they were one year old. They received breaking and training to be racehorses for 10 months, from autumn to the following spring. Full-siblings were not included in the analysis.
Behavioural evaluation
The same three caretakers evaluated the tractability of horses at stabling, breaking and training using a three-point scale questionnaire (1, easy; 2, somewhat difficult; 3, difficult) consisting of 17 items. At stabling, three items were evaluated: reactions to body inspection, the first shower and the first grooming. During breaking, eight items were evaluated as follows: patting with towels, familiarization with straps, attaching a roller, attaching long reins, driving, riding in the box, riding in the lunging ring and riding on an 800-metre track. During training, three items were evaluated: number of spills, familiarization with getting on trailers and familiarization with passing through starting gates. Caretakers also recorded their impressions about the horses' delicacy, independency and rebelliousness. A detailed definition of each item is shown in Table 1. All horses were given a three-point score (1, 2 or 3) for each item reflecting all three caretakers' consensus decision. Details of the criteria for each item are shown in Table S1.
| Period | Item | Definition |
|---|---|---|
| Stabling | Body inspection | Reaction to body inspection upon arrival at the farm |
| First showering | Reaction to the first showering | |
| First grooming | Reaction to the first grooming | |
| Breaking | Patting by towels | Patting the whole body at the box until the horse shows no sign of fear |
| Familiarization with strap | Attaching straps on the belly at the box as preparation for attaching a roller | |
| Attaching roller | Attaching a roller to the horse at the lunging ring | |
| Attaching long reins | Attaching two long reins and lunging at the ring | |
| Driving | A human stands behind the horse and drives it to walk using long reins | |
| Riding in box | A rider leans over on the horse and then rides on back in the box | |
| Riding in lunging ring | A rider leans over on the horse and then rides on back in the lunging ring | |
| Riding on 800-m track | Walking with a rider on the back at 800-m track in a group | |
| Training | Spill | Number of spills at training (1, zero or one time; 2, two or three times; 3, four or more times) |
| Familiarization with trailer | Familiarization with getting on the trailer | |
| Familiarization with gate | Familiarization with passing through a starting gate | |
| Caretaker's impression | Delicacy | Difficulty in getting accustomed to stimuli |
| Independency | Difficulty in getting accustomed to staying alone | |
| Rebelliousness | Rebelliousness with caretakers or riders |
A principal components analysis with varimax rotation was conducted to contract the behavioural data. The number of components to be extracted was determined by a parallel analysis. For the parallel analysis and the principal components analysis, we used a polychoric correlation coefficient matrix to estimate the correlation of the three-point scale data more adequately (Olsson 1979). Items that had a loading score higher than 0.50 were considered to belong to the principal component. Principal scores of each horse were calculated as mean scores of items that belonged to each principal component.
Genotyping
Genomic DNA was extracted from blood samples using a MagExtractor™ genome kit on a MagExtractor System MFX®-2100 (TOYOBO Life Science). We designed a primer pair (forward: 5′-CTTTCTACATCCCGCTGCTG-3′ and reverse: 5′-CGTTGCGCTCATTTTTCTTC-3′) to amplify 338 base pairs of the equine HTR1A exon region (GenBank: AB264325), which contained two SNPs reported previously (Momozawa et al. 2007). Polymerase chain reaction (PCR) was conducted using a TaKaRa LA Taq with GC buffer in a 10-μl reaction volume containing about 20 ng of template DNA. The PCR thermocycling consisted of denaturation at 95 °C for 2 min, followed by 35 cycles of 95 °C for 30 s, 60 °C for 30 s, 74 °C for 1 min and then the final extension at 74 °C for 10 min. PCR products were purified using a High Pure PCR Product Purification Kit (Roche Diagnostics). Nucleotide sequences of the PCR products were determined by a Big Dye Terminator v3. 1 Cycle Sequencing Kit and an ABI 3130xl Genetic Analyzer (Applied Biosystems). SNPs were detected visually by mega software ver. 6.0 (Tamura et al. 2013) and finchtv software ver. 1. 4. 0 (Geospiza). All samples were sequenced at least twice.
Association analysis
The association between tractability scores and genotype was tested by two-way factorial analysis of variance (ANOVA). Sex and genotype were used as factors. All statistical analyses were conducted using r software ver. 3. 1. 2 (R Foundation for Statistical Computing).
Ethics
This study adhered to the ethical guidelines of Kyoto University and was approved by the Animal Experiments Committee of the Graduate School of Letters, Kyoto University.
Results
Behavioural traits
According to the parallel analysis, we constructed five principal components (PCs). These five PCs explained 74% of the total variance of the data. The loading matrix of 17 items on each PC is shown in Table 2. According to the loading value, all items were classified into one of the five PCs. Internal consistencies (Cronbach's α) ranged from 0.46 to 0.79.
| Period | Item | PC1 | PC2 | PC3 | PC4 | PC5 |
|---|---|---|---|---|---|---|
| Stabling | Body inspection | 0.22 | 0.85 | 0.18 | −0.20 | 0.12 |
| First showering | 0.28 | 0.80 | 0.07 | 0.08 | 0.14 | |
| First grooming | 0.09 | 0.86 | −0.12 | 0.24 | 0.07 | |
| Breaking | Patting by towels | 0.12 | 0.14 | 0.08 | 0.30 | 0.82 |
| Familiarization with strap | 0.15 | 0.17 | 0.25 | 0.10 | 0.86 | |
| Attaching roller | 0.57 | 0.03 | −0.05 | −0.24 | 0.38 | |
| Attaching long reins | 0.76 | 0.17 | 0.20 | 0.16 | 0.35 | |
| Driving | 0.71 | 0.22 | 0.15 | 0.32 | 0.16 | |
| Riding in box | 0.87 | 0.26 | 0.12 | −0.07 | −0.10 | |
| Riding in lunging ring | 0.83 | 0.16 | 0.16 | 0.12 | 0.03 | |
| Riding on 800 m track | 0.67 | 0.06 | 0.38 | 0.30 | 0.03 | |
| Training | Spill | 0.12 | 0.02 | −0.04 | 0.55 | 0.19 |
| Familiarization with trailer | 0.04 | −0.14 | 0.83 | −0.20 | 0.20 | |
| Familiarization with gate | 0.37 | 0.18 | 0.71 | 0.11 | 0.11 | |
| Caretakers' impression | Delicacy | 0.31 | 0.14 | 0.71 | 0.46 | 0.08 |
| Independency | 0.20 | 0.25 | 0.43 | 0.60 | −0.28 | |
| Rebelliousness | 0.01 | −0.01 | 0.04 | 0.90 | 0.17 | |
| Contribution ratio | 0.22 | 0.14 | 0.13 | 0.13 | 0.11 | |
| Cumulative contribution ratio | 0.22 | 0.37 | 0.50 | 0.63 | 0.74 | |
| Cronbach's α | 0.79 | 0.70 | 0.63 | 0.46 | 0.73 |
- Loadings higher than 0.50 are in bold. Contribution ratio, cumulative contribution ratios and internal consistency (Cronbach's α) of each PC are also shown.
The meanings of the PCs were interpreted based on their constituent items. PC1 reflected the tractability involved mainly with attaching equipment, driving and backing because it contained six items related to the middle or late period of breaking. PC2 explained the tractability at the introduction to a horse's new environment because it contained all three items at stabling (body inspection, showering and grooming). PC3 reflected the tractability of familiarization with large novel objects because it contained items related to familiarization to trailers and starting gates. PC4 reflected mainly caretakers' impression about independence and rebelliousness. Finally, PC5 reflected tractability at the very beginning of breaking because it contained the two items: ‘patting by towels’ and ‘familiarization with straps’.
Genotyping
We confirmed two non-synonymous SNPs (c.709G>A and c.771G>C) reported previously (Momozawa et al. 2007); no novel polymorphisms were found. Genotype frequencies of these SNPs are shown in Table 3. The frequencies of minor alleles were 0.05 for c.709G>A and 0.10 for c.771G>C. Observed heterozygosity (Ho) was 0.10 for c.709G>A and 0.21 for c.771G>C. The genotype frequencies of these SNPs did not deviate from Hardy–Weinberg equilibrium (Fisher's exact test, P = 0.38 for c.709G>A and P = 0.22 for c.771G>C). Linkage disequilibrium between c.709G>A and c.771G>C was weak (r2 = 0.0065). We divided subjects into separate groups based on the existence of minor alleles (for c.709G>A, A+: A/A or A/G and A–: G/G; for c.771G>C, C+: C/C or C/G and C–: G/G) for the following association analysis because of low frequencies of minor alleles for both SNPs.
| G/G | A/G | A/A | Total | |
|---|---|---|---|---|
| c.709G>A | ||||
| Colt | 73 | 8 | 0 | 81 |
| Filly | 77 | 8 | 1 | 86 |
| Total | 150 | 16 | 1 | 167 |
| G/G | C/G | C/C | Total | |
|---|---|---|---|---|
| c.771G>C | ||||
| Colt | 63 | 18 | 0 | 81 |
| Filly | 69 | 17 | 0 | 86 |
| Total | 132 | 35 | 0 | 167 |
Association analysis
We tested the association between the five PC scores and genotype by five independent two-way factorial ANOVAs. Behavioural scores and genotypes for all 167 horses are shown in Table S2. For PC1, tractability during the middle or late period of breaking, the interaction between sex and the c.709G>A genotype was significant (F(1, 163) = 7.90, P = 0.0055). The simple effect of sex was significant in the A+ group (F(1, 163) = 11.64, P = 0.0008) but not in the A– group (F(1, 163) = 1.77, P = 0.19). In the A+ group, fillies showed higher scores than did colts. The simple effect of c.709G>A was significant in fillies (F(1, 163) = 15.03, P = 0.0002) but not in colts (F(1, 163) = 0.04, P = 0.84). A+ fillies showed higher scores than did A– fillies (Fig. 1a).
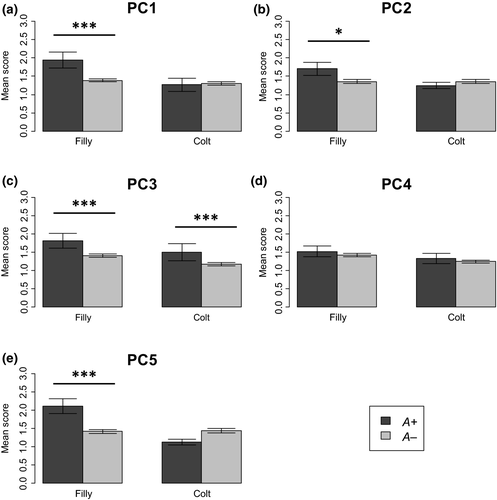
A similar result to PC1 was seen in PC2, tractability at stabling. The interaction between sex and c.709G>A was marginally significant (F(1, 163) = 3.21, P = 0.075). The simple effect of sex was marginally significant in the A+ group (F(1, 163) = 3.63, P = 0.059) but not in the A– group (F(1, 163) = 0.002, P = 0.97). In the A+ group, fillies tended to have higher scores than did colts. The simple effect of c.709G>A was significant in fillies (F(1, 163) = 3.98, P = 0.048) but not in colts (F(1, 163) = 0.34, P = 0.56). A+ fillies showed higher scores than did A– fillies (Fig. 1b).
For PC3, tractability reflecting familiarization with gates and trailers, both the main effects of sex and c.709G>A were significant (sex: F(1, 163) = 6.70, P = 0.011; c.709G>A: F(1, 163) = 11.70, P = 0.008). The interaction was not significant (F(1,163) = 0.13, P = 0.72). Fillies showed higher scores than did colts, and the A+ group showed higher scores than did the A– group (Fig. 1c).
For PC4, independence and rebelliousness, the main effect of sex was marginally significant (F(1,163) = 3.05, P = 0.083). Fillies tended to show higher scores than did colts (Fig. 1d). However, the main effect of c.709G>A and the interaction were not significant (c.709G>A: F(1, 163) = 0.74, P = 0.39; interaction: F(1,163) = 0.004, P = 0.95).
Finally for PC5, tractability at the beginning of breaking, a result similar to those for PC1 and PC2 was seen (Fig. 1e). The interaction between sex and c.709G>A was significant (F(1, 163) = 14.96, P = 0.0002). The simple effect of sex was significant in the A+ group (F(1, 163) = 16.13, P = 0.0001) but not in the A– group (F (1, 163) = 0.04, P = 0.85). The simple effect of c.709G>A was significant in fillies (F(1, 163) = 15.27, P = 0.0001) but not in colts (F(1, 163) = 2.65, P = 0.11). c.771G>C showed no significant associations with any PCs (all P-values were greater than 0.05).
Discussion
We found a significant association between tractability, as assessed by a questionnaire survey of caretakers, and the HTR1A:c.709G>A genotype in Thoroughbred horses. However, the effect of c.709G>A interacted with sex. In fillies, c.709G>A had a significant effect on four of five PCs, the exception being PC4. Fillies carrying an A allele consistently showed higher scores than did G/G genotype fillies. In colts, the effect of c.709G>A was found only in PC3. The tendency was the same as in fillies; colts carrying an A allele showed higher scores. These results suggest that the A allele of c.709G>A may be linked with lower tractability in horses with its effect more salient in fillies.
A sex difference in the effect of c.709G>A may be caused by sex differences in systems that regulate anxiety. A recent study showed a sex difference in the regulation of stress by HTR1A receptors in rats (Goel et al. 2014). A sex-dependent effect of genetic polymorphisms was reported in the serotonin transporter gene (5HTT) polymorphism in humans; individual differences in resting-state electroencephalographies (EEGs) were affected by 5HTT genotype in females but not in males (Volf et al. 2015). Such a sex difference in the stress mediation system with serotonin may exist in horses.
Principal components in this study may represent different kinds of anxiety. PC3, which was affected by c.709G>A in both sexes, seems to represent anxiety for novel visual stimuli because it contains items related to familiarization with starting gates and trailers. This could be investigated by performing a novel object test and comparing scores with PC3. In contrast, PC1, PC2 and PC5, which were affected by c.709G>A only in fillies, seem to represent anxiety for direct body contact at stabling (PC2) or breaking (PC1 and PC5) because they contain items involving mainly direct touching by humans or attaching equipment. The interaction among sex, types of anxiety and genotype should be tested more precisely in the future.
In this study, A/G heterozygote individuals showed less tractability than did G/G homozygote individuals. However, whether the effect of the allele is additive remains unclear because we found only one A/A homozygote individual in our subjects. The frequency of the A allele is likely to be very low, and replication using a larger sample is necessary to investigate whether A/A individuals show a ‘very difficult’ trait. As for c.771G>C, although we found no significant association, it would be worth testing whether C/C individuals show less tractability.
The molecular function of c.709G>A remains unknown. c.709G>A causes a change in amino acids from glycine to arginine at position 237 of the HTR1A protein (Momozawa et al. 2007). According to the UniProt database (accession no: Q0EAB6), amino acid position 237 is located in the third intracellular loop (ICL3) of the HTR1A protein, which is a region interacting with G protein (Nichols & Nichols 2008). In human HTR1A, several SNPs were found in the coding region (Drago et al. 2008). One of them, causing a p.Gly272Asp substitution, was also found in ICL3 and was associated with a depressive disorder (Suzuki et al. 2004). However, other studies have found no significant effects of p.Gly272Asp (Kawanishi et al. 1998; Nishiguchi et al. 2002; Yu et al. 2006). Thus, the effect of p.Gly272Asp remains unclear. Another SNP located in ICL3, causing a p.Arg219Leu substitution, was reported to be associated with impaired signal transduction by expression analysis using human cells (Bruss et al. 2005), but its effect on behaviour is still unknown. In the non-coding region, a SNP in the promoter region, g.–1019C>G, reduces the expression of HTR1A receptors, leading to decreased serotonergic neurotransmission, and increased risk of depression and suicide (Lemonde et al. 2003). Horse c.709G>A may also affect the function of the receptor, possibly causing loss of the function of the receptor. However, the mechanism needs to be confirmed by molecular experiments.
In conclusion, our results suggest that a genetic polymorphism in HTR1A gene affects tractability during the rearing period of racing horses. This is the first evidence showing that a serotonin-related gene polymorphism may affect individual differences in a behavioural trait in horses. An interaction between sex and genotype was also found. These results may have implications for horse management or training practices once replication with a larger sample and confirmation of molecular function are completed. It would be interesting to study the effect of this polymorphism or tractability scores on the racing performance of horses in the future. Anxiety-related traits assessed by this study should be important because race horses have to cope with various types of stress involved with racing competition. It may be worthwhile to pursue the race performances of horses used in this study.
Acknowledgements
We thank caretakers at the Hidaka Training and Research Center who cooperated on behavioural evaluation. We also thank Noboru Manabe (The University of Tokyo) for his useful suggestions at the beginning of this project and James Anderson and Robert Ogden (Kyoto University) for their careful editing of the text. This study was supported by the Cooperation Research Program of the Wildlife Research Center, Kyoto University. Financial support was provided by the Japan Society for the Promotion of Science (JSPS) Grants-in-Aids for Scientific Research No. 255327 to YH, Nos. 25118005 and 25290082 to MI-M, Nos. 25118002 and 25240020 to KF.




