Quantity versus quality: A balance between egg and clutch size among Australian amphibians in relation to other life-history variables
Abstract
Due to resource limitations and physical constraints of the reproducing female, a trade-off must be made between the number of eggs she produces and their size per clutch. This generally results in an inverse relationship between egg and clutch size, which has been found repeatedly across animal groups. Few studies have investigated this relationship with respect to selection pressures, environmental variables and other life-history traits. We aimed to test current hypotheses regarding the trade-off between egg and clutch size among the three Australian Anuran families (Hylidae, Myobatrachidae and Microhylidae). Specifically, we used a comparative phylogenetic approach to look at the influence of environmental selection pressures (egg-laying location, environment persistence and bioregion) and life-history traits (female body size, egg development type, parental care level, breeding period and temporal breeding pattern) on this trade-off. As expected, a strong inverse relationship was found between egg and clutch size. Smaller clutches of larger eggs tended to be produced by species with smaller female sizes that (i) oviposit terrestrially and arboreally compared with aquatically, (ii) have prolonged compared with explosive breeding periods, (iii) directly develop compared with having a feeding tadpole stage and (iv) exhibit high compared with low levels of parental care. These findings show that the shift towards producing larger eggs in smaller clutches is associated with the transition away from the ancestral amphibian reproductive pattern. We highlight how the balance made when provisioning finite resources to egg within a clutch differs between species that have evolved diverse life histories, providing a framework for future examinations of the trade-off between egg and clutch sizes among anurans.
INTRODUCTION
The partitioning of a female's finite resources towards reproduction defines how a species has adapted to the selection pressures of the environment in which it resides and has considerable influence on both parent and offspring fitness (Stearns 1976). A trade-off must be made between the total resources invested towards each clutch and the number of clutches produced over reproductive life, given that resources expended on current reproduction reduces a female's potential survival and, thus, additional chances to reproduce (Williams 1966). A trade-off must also be made between the number of eggs produced per clutch and their size, given a female's finite egg-carrying capacity and reproductive reserve (Lack 1967; Smith and Fretwell 1974). Understanding what drives the balance between egg size and number when provisioning resources to a clutch is a central issue in evolutionary biology (Smith and Fretwell 1974; Marshall et al. 2010; Lasne et al. 2018). It is now understood that there are many life-history variables that influence this trade-off between egg and clutch size (Murphy 1968; Stearns 1976; Haywood and Perrins 1992; Adolph and Porter 1993, Mickaill et al. 2020, Beranek et al. 2021, Hope et al. 2021). Yet for many taxa, the manner in which this trade-off shifts with respect to other variables has not been examined using robust natural history datasets.
The trade-off between egg and clutch size is reflected in their general inverse relationship (Parker and Begon 1986; Dziminski et al. 2009), with selection favouring the production of large eggs (provided there are few) or many eggs (provided they are small). This selection process is influenced by the effect of egg size on offspring survival under a given set of environmental conditions and egg number on fecundity, which is assumed to result in the evolution of an optimal egg size (Smith and Fretwell 1974; Lloyd 1987; Roff 1992; Einum and Fleming 2004). However, it is possible for multiple egg size optima to exist, particularly in stochastic environments, and may result in variable egg sizes within a clutch, temporal egg size differences between a female's clutches, differences between females within a population and between breeding years, as well as spatial differences between populations that are exposed to different environmental conditions (Kaplan and Cooper 1984; McGinley et al. 1987; Crump 1984; Dziminski et al. 2009). Information regarding the extent of variability in life-history parameters remains absent in the literature for many species, particularly on whether this variability is adaptive or not. Examining the provision of maternal investment in terms of mean values for each species may thus be an effective means of obtaining general yet critical information on the relationship between egg and clutch sizes within large taxonomic groups.
An inverse relationship between egg and clutch size has been shown repeatedly among amphibians (Crump 1974; Crump and Kaplan 1979; Duellman 1989; Hödl 1990; Perotti 1997). As most amphibians lay externally (i.e. oviparous), variables that have influenced this relationship are strongly related to the environmental conditions offspring are exposed to throughout embryogenesis and later developmental stages. The likelihood of offspring survival is influenced by competition, predation, sub-optimal temperature and moisture levels (Wilbur 1987; Werner and McPeek 1994; Skelly 1996; Wellborn et al. 1996; Skelly et al. 1999; Hendry et al. 2001; Babbitt et al. 2003; Semlitsch et al. 2015), which are likely to vary considerably both spatially and temporally. In temporary aquatic systems, the high risk of desiccation would be expected to select for the production of smaller eggs that develop more quickly (McLaren and Cooley 1972; Salthe and Duellman 1973; Kuramoto 1975; Kaplan 1985), as this may result in shorter developmental periods and an improved chance of offspring metamorphosing before water levels recede. In variable environments where offspring survival is low or unpredictable, producing smaller eggs may also provide a fecundity advantage. Producing many smaller eggs can increase the chance of at least some offspring surviving to reproductive age by allowing for an increase in clutch size (Salthe 1969; Crump 1984). It may also allow for much greater genetic diversity within the offspring pool by allowing for more maternal–paternal gene combinations to be produced (Smith 1978). Larger eggs should be selected at the expense of fecundity when offspring survival is more likely, such as in more permanent, stable systems where offspring development is not constrained by system ephemerality and as larger offspring may have a competitive advantage (Pianka 1970; Einum and Fleming 1999; Moran and Emlet 2001). The production of larger eggs is also apparent with the withdrawal of anuran egg deposition from aquatic environments, likely improving the egg's resistance to evaporative water loss in terrestrial environments, and/or facilitating direct development (Bogart, 1981, Roberts, 1981, Bradford and Seymour, 1988).
Alongside the conditions that define the egg deposition environment, numerous life-history traits have likely influenced the evolution of egg and clutch size among amphibians. In particular, species in this group exhibit a wide range of parental care levels, ranging from egg attendance through to more energetically expensive activities, such as offspring feeding and movement (Crump 1996; Zamudio et al. 2016; Furness and Capellini 2019). Egg size is expected to be directly related to the level of parental care that is provided as this will determine the level of exposure of offspring to the external environment (Summers et al. 2005; Furness and Capellini 2019) and may buffer them from sub-optimal conditions. Other behavioural traits, such as the length of the breeding period and whether breeding is explosive or prolonged, also influence exposure to risk factors (Morin et al. 1990; Petranka and Thomas 1995) and may be related to clutch traits.
The Australian anurans provide an ideal model to investigate factors associated with the trade-off between egg and clutch size. They possess a rich diversity of reproductive patterns, as well as settings of egg deposition and conditions of subsequent offspring development (Anstis 2017). Furthermore, a large proportion of Australian amphibians are derived from two large and likely monophyletic lineages (Hylidae and Myobatrachidae) with a long evolutionary history of isolation on the continent (Tyler 1971; Heyer and Liem 1976; Farris et al. 1982; Hutchinson and Maxson 1987). This is favourable for analysing the influence of the environment on reproduction, as reproductive patterns found in these families likely represent the radiation of a few common ancestral genotypes into various ecological niches. In light of this, we used a comparative phylogenetic approach to investigate the relationship between egg and clutch size among the Australian anurans, including whether (i) mean egg size is inversely related to mean clutch size and (ii) whether the relationship between egg and clutch sizes are related to female body size, environmental selection pressures (egg-laying location, environment persistence and bioregion) and life-history traits (development type, parental care level, breeding period and temporal breeding pattern). We obtained natural history data of 128 Australian anurans from three major groupings (Hylidae, Myobatrachidae and Microhylidae), constituting 53% of currently described Australian species. Data were sourced from Anstis (2017), the most complete and up to date dataset on the reproductive biology of Australian anurans, providing a unique opportunity to compare the trade-off between egg and clutch size in this group.
METHODS
Data collection
The partitioning of clutch resources between egg size and number, along with factors pertaining to reproductive pattern, were identified for each species using data collated by Anstis (2017). The basis of our data collection was focused on the mean size of eggs, clutches and females of each species. We also collected data on the location in which egg deposition occurred (bioregion, habitat type and ephemerality), the development type of offspring following hatching, the breeding period of adults, and the extent of parental care provided to offspring. We must highlight that this does not encompass all possible life-history variables, which are likely to have an effect on the trade-off between egg and clutch size. For example, precise measurements of conditions of offspring development (e.g. temperature; Duellman and Trueb 1986; Bradford 1990; Beattie et al. 1992) have not been collected for a large number of species but should be analysed when they become available in future studies. For our life-history variables of interest, we were able to obtain a near complete dataset for 128 Anuran species from Anstis (2017). Partially complete data for a small number of species were completed by sourcing online databases (Oliveira et al. 2017; AmphibiaWeb 2018). To enable future comparisons to be conducted using the same methodology, we have provided precise definitions for each of our variables of interest (Table 1).
| Variable | Variable type | Description |
|---|---|---|
| Egg size | Continuous |
Mean diameter (mm) of the ovum or fertilized egg cell during the initial stages of embryogenesis. For consistency, we included measurements made prior to Gosner stage 9 of development (Gosner 1960) |
| Clutch size | Continuous |
Mean total number of eggs oviposited by females during a single cycle of oogenesis. As some species are known to temporally or spatially partition their clutch (e.g. species in the Pseudoprhyne genera), we only included total clutch size values |
|
Female size Development type |
Continuous Categorical |
Mean snout-vent length (SVL; mm) of adult females Development is lacking a feeding tadpole stage, including direct developers (feeding tadpoles absent) or includes a feeding tadpole stage (feeding tadpoles present) |
| Parental care | Categorical |
Parental attendance by one or both parents is only provided during egg development or not at all (low), or provided during and beyond larval development (high) |
| Breeding period | Categorical |
Breeding restricted to a particular period of the year (seasonal) or occurring throughout the year (continuous) |
| Temporal breeding pattern | Categorical |
Calling period occurring <1 month (explosive) or > 1 month (prolonged), as described by Wells (1977) |
| Egg-laying location | Categorical |
Initial environment in which ovipositioning occurs (aquatic, arboreal and terrestrial). Species with ambiguous egg deposition environments, such as some Mixophyes' which oviposit in water before splashing them onto overhanging rocky surfaces, were defined by initial deposition environment |
| Habitat persistence | Categorical |
Egg deposition environment present throughout the breeding period (permanent), including permanent pools or rivers, or restricted (temporary), including terrestrial or aquatic environments that periodically dry out, or both (mixed) |
| Bioregion | Categorical | Location of the breeding environment (tropical, temperate or desert). Bioregions were initially identified using the Interim Biogeographical Regionalisation for Australia (Department of Agriculture, Water and the Environment 2012) and subsequently reclassified as either tropical, temperate or desert. The bioregion encompassing the largest portion of the species distribution was included |
It must be recognized that samples sizes over which measurements of egg, clutch and female size were calculated differed between species, reflecting limitations in natural history data. Even though various factors are known to cause interspecific variations in egg and clutch size, such as female size and condition (Gibbons and McCarthy 1986; Long 1987), we assumed that mean estimates obtained for each species were representative. However, a caveat which we note for our study is that data were obtained from populations in discrete locations and years that may not reflect spatial and temporal differences in life-history variables seen within and between populations; in particular, the likely differences in egg, clutch and female size across a specie's climatic and geographic ranges. It is also possible that some species may show within-female variations that are a result of adaptive bet-hedging or plasticity (Bernardo 1996; Lips 2001). However, it is unlikely that other key life-history traits, such as whether eggs are deposited aquatically or terrestrially differ widely between a species' populations.
Phylogeny
Phylogenetic data, including topologies and branch lengths, were obtained for the major three Australian anuran groups (Microhylidae, Hylidae and Myobatrachidae) based on a NNI-optimized maximum likelihood phylogeny (amph_shl_new) from Jetz and Pyron (2018). The relationships between 29 species not represented in Jetz and Pyron (2018) were obtained from alternatives sources, particularly Pyron and Wiens (2011), and manually added to our phylogeny. Among microhylid frogs, relationships among and between Cophixalus and Austrochaperina species were also obtained from Hoskin (2004). Among myobatrachid frogs, relationships were obtained from additional sources for some Heleioporus (Morgan et al. 2007), Crinia, Geocrinia and Bryobatrachus (Read et al. 2001), Uperoleia (Catullo and Keogh 2014), Pseudophryne (Donnellan et al. 2012), Taudactylus and Philoria (Byrne et al. 2002), and Neobatrachus (Novikova et al. 2020). The phylogenetic relationship of one hylid, Litoria myola, not defined in any study was estimated based on its recent split from Litoria genimaculata (Hoskin, 2007). Branch length data not available in Jetz and Pyron (2018) were imputed for species based on the average branch lengths obtained for all other species within their respective family groupings.
Statistical analyses
Closely related species that share a more recent common ancestor are likely to have similar traits irrespective of the current environmental conditions they are exposed to (Felsenstein 1985). We accounted for phylogeny when examining the relationship between egg and clutch sizes among Australian anurans by running phylogenetic generalized least squares (PGLS) models (Grafen 1989; Martins and Hansen 1997). Using PGLS modelling, it is possible to examine the relationship between variables of interest while accounting for shared evolutionary history, thereby controlling for potential non-independence that arises in species comparisons.
The degree to which trait evolution is phylogenetically correlated can be set for each PGLS model using the parameter lambda λ, with values between 0 (trait evolution is phylogenetically independent) and 1 (trait evolution follows Brownian motion) (Pagel 1999; Freckleton et al. 2002; Revell 2010). The maximum likelihood estimate of lambda was incorporated into our models, with likelihood ratio tests performed to compare the lambda value obtained for each model with lambda values of 0 and 1. We tested for the presence of multicollinearity in our models by calculating the variance inflation factor (VIF) for fixed effects using the car package (Fox & Weisberg 2019) and found no values larger than 1.5. Model residuals were also checked for normality and heteroscedasticity. All modelling was performed using R version 3.5.1 (R Core team 2018). Ancestral states were reconstructed using maximum likelihood with the fastAnc function in phytools (Revell 2012).
Model 1
We investigated whether there was an inverse relationship between egg and clutch size among species by running a PGLS model (Model 1) in which egg size was the response variable and clutch size was a fixed effect. Both variables were log transformed prior to analysis to improve normality of residuals.
Model 2
Given the strong inverse relationship between egg size and clutch size that reflects the trade-off between these two life-history traits, we ran an additional PGLS model (Model 2) in which we examined this trade-off based on the partitioning of total clutch volume per egg. To do so, we calculated the volume of each egg based on the volume of a sphere (V = 4/3πr3), which we divided by total clutch volume (egg volume × total egg number per clutch) to obtain the proportion of clutch volume that made up each egg; a value we refer to as egg investment. Large values are indicative of the partitioning of a larger portion of total clutch volume to fewer eggs (i.e. a larger investment per egg per clutch). We analysed how the trade-off between egg size and clutch size in terms of egg investment differed with respect to our life-history variables of interest, including female size, development type, parental care level, breeding period, breeding type, egg-laying location, breeding habitat and region. For each categorical variable, we were primarily interested in comparing differences in egg investment values between levels. As proportional data do not often satisfy linearity assumptions required for regression analysis, we both log and logit transformed egg investment values (see Warton and Hiu 2011). The model including log transformed data was found to be the most parsimonious based on the Akaike's information criterion (AIC) value and has subsequently been presented. Female size was also log transformed prior to analysis to improve normality of residuals.
RESULTS
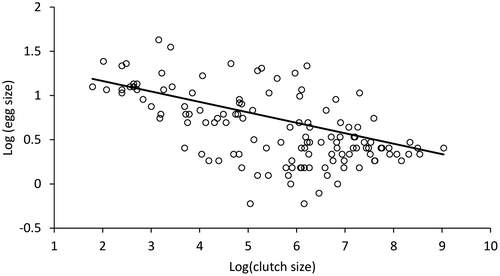
Between species, there were considerable differences in mean egg size (0.8–5.1 mm), mean clutch size (6–8000 eggs) and mean female size (16.6–135 mm). The smallest eggs (diameter = 0.8 mm) were produced by Litoria microbelos, while the largest eggs (diameter = 5.1 mm) were produced by Myobatrachus gouldii. The largest female species examined, Litoria splendida (SVL = 118 mm) and Litoria infrafenata (SVL = 135 mm), possessed mean clutch sizes of over 8000 and 4000 eggs, respectively, compared with the smallest species, Cophixalus hosmeri (SVL = 17 mm) and Geocrinia vitelina (SVL = 18 mm), with mean clutch sizes of only 6 and 11 eggs respectively (Fig. 1). Litoria aurea had the second-largest clutch size and had the fifth-largest female length (SVL = 108 mm). Phylogenies with ancestral state reconstructions for egg, clutch and female size can be found at Gould et al. (2020).
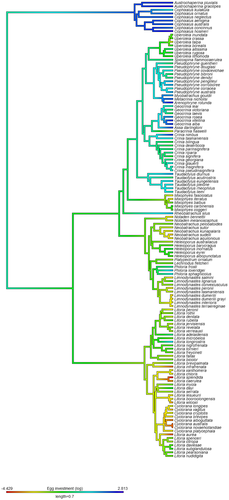
Species were mostly from the families Myobatrachidae (60%), Hylidae (33%), with only a small number from Microhylidae (7%). When considered together, a majority of species across all three families were prolonged breeders (83%), possessed a feeding tadpole stage of development (84%) and exploited environments that were aquatic (71%) and temporary (53%). Most species had seasonal breeding (85%) and low levels of parental care (98%), and were found in temperate or tropical bioregions (54% and 41%, respectively) compared with deserts (5%). However, this general pattern differed across families. A majority of hylids presented with an ancestral reproductive mode with few species laying away from aquatic sites (2%), providing high levels of parental care (0%) or without a tadpole feeding stage (0%). This is compared with the myobatrachids where a greater proportion of species laid away for aquatic sites (35%), provided high levels of parental care (12%) and lacked feeding tadpoles (14%), and microhylids where a majority laid away from aquatic sites (100%), had high levels of parental care (100%) and lacked feeding tadpoles (100%).
Model 1—the relationship between egg and clutch size
The PGLS model comparing clutch size to egg size had an adjusted R2 of 0.1927. There was a significant inverse relationship between egg and clutch size (Fig. 1), with a 1% increase in clutch size resulting in a 12% decrease in egg size (T = −5.5740, P < 0.0001). The maximum likelihood estimated model value of lambda was 0.981 (CI: 0.918, 0.997), which was significantly different to 0 (P < 0.0001) and 1 (P = 0.0003). We defined this relationship between egg and clutch size in terms of egg investment per clutch, which differed within and between the major Australia anuran groupings (Fig. 2).
Model 2—effects on egg investment per clutch
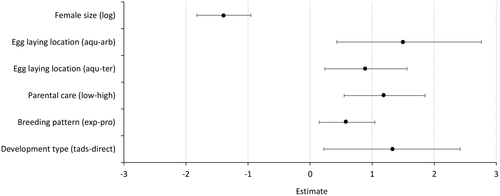
The PGLS model comparing the partitioning of total clutch volume per egg against all life-history variables had an adjusted R2 of 0.5088 (Fig. 3). There was a significant inverse relationship between egg investment and female size, with a 1% increase in female size resulting in a 0.38% decrease in egg investment (T = −6.2314, P < 0.0001). Larger eggs in smaller clutches were found for species exploiting terrestrial (increase in mean egg investment of 146%, T = 2.6116, P = 0.0087), and arboreal (increase in mean egg investment of 393%, T = 2.6857, P = 0.0083) compared with aquatic egg-laying locations, those with high compared with low levels of parental care (increase in mean egg investment of 233%, T = 3.6223, P = 0.0004), prolonged compared with explosive breeding periods (increase in mean egg investment of 81%, T = 2.5997, P = 0.0105) and those with direct development compared with those with a tadpole feeding stage (increase in mean egg investment of 272%, T = 2.3446, P = 0.0208). The maximum likelihood estimated model value of lambda was 0.88 (CI: 0.692, 0.960), which was significantly different to 0 (P < 0.0001) and 1 (P < 0.0001).
DISCUSSION
We demonstrate a clear inverse relationship between egg and clutch among the Australian anurans. This mirrors results obtained by Byrne et al. (2003) for Australian myobatrachids, as well as other amphibian groups (Crump 1974; Crump and Kaplan 1979; Duellman 1989; Hödl 1990; Perotti 1997), and was expected based on the widely known trade-off between these two life-history variables (Parker and Begon 1986). Furthermore, we show that egg investment per clutch differs in relation to various life-history variables. Smaller clutches of larger eggs tended to be produced by smaller female species that (i) oviposit terrestrially or arboreally, (ii) possess high levels of parental care, with (iii) direct development and (iv) prolonged breeding patterns; many of which are features of species that do not possess the ancestral amphibian reproductive mode (Duellman and Trueb 1986; Gomez-Mestre et al. 2012). By accounting for phylogenetic context, we have been able to test key evolutionary hypotheses concerning how the trade-off between egg and clutch size differs with other life-history variables among the Australian amphibians.
The relationship between egg, clutch and female size
The link between fecundity and size has been shown repeatedly in amphibian assemblages (Lemckert and Shine 1993; Prado and Haddad 2005; Gomez-Mestre et al. 2012; Silva et al. 2020) and occurs as the maximum volume of a clutch that an individual can produce will be constrained by the internal body space available for ovarian development. We found that larger female species produced larger clutches of smaller eggs, though the reasons for this positive correlation between female size and egg investment remain undetermined. Egg size should be selected to optimize offspring survival irrespective of female size, which is apparent among the Australian anurans as some of the smallest species possess some of the largest eggs. For example, female Austrochaperina pluvialis have a mean length of 29 mm but the same egg size as that of Mixophyes coggeri, which have a mean length of 104 mm. Although not tested as part of this study, it is possible that the evolution of a larger female size at sexual maturity may be an adaptive means of increasing the number of offspring produced per clutch (Wells 2007; but see Shine 1988) and may increase female fitness in environments where offspring survival is low. A stronger pressure for increased clutch size compared with increased egg size may explain our finding.
Temporal breeding pattern and habitat persistence
Larger clutches of smaller eggs were recorded in species that possess explosive compared with prolonged breeding patterns. An explosive breeding strategy is characteristic of amphibians that exploit temporary environments (Wells 1977; Laufer et al. 2015; Gould et al. 2021), such as ephemeral pools, where there are limited optimal periods for reproduction and/or restrictions on the availability of breeding sites due to their hydrological regimes. The selection for small eggs allows for the production of a larger clutch that increases fecundity and improves the odds of at least some offspring making it to reproductive age via a sheer increase in offspring number (Salthe 1969; Crump 1984). This is advantageous in temporary environments that are unpredictably variable, where adults are provided unreliable cues to determine when suitable conditions arise for reproducing (Moran 1992; Rungeand and Sherman 2002), leading to an increased risk of offspring mortality. This is in contrast to temporary environments that are more reliable, such as arid pools where reproduction only occurs after large rainfall events (Roberts 1981), where the selection for large clutches over large eggs is not as advantageous or apparent.
The smaller size of eggs produced by explosive breeding species may also be attributed to the relationship between egg size and development duration in temporary sites. When breeding sites are only available for short periods of time, it is highly advantageous for adults to produce eggs which can complete development as quickly as possible before the system becomes inhospitable for their continued survival, which may partly occur by reducing egg size (Salthe & Duellman 1973; Kaplan 1980). It has been repeatedly emphasized that larger anuran eggs have slower development than smaller eggs (McLaren and Cooley 1972; Salthe and Duellman 1973; Kuramoto 1975; Kaplan 1985), which may occur as a result of oxygen limitations in larger eggs that slow the rate of embryonic tissue synthesis (Seymour and Bradford 1995). While smaller eggs may allow for more rapid hatching, they are also likely to give rise to smaller offspring upon hatching (Salthe and Duellman 1973; Kaplan 1980); a correlation generally seen among animals (e.g., Guinnee et al. 2007). Under some environmental conditions, there could be a cost of having smaller eggs if offspring have less competitive ability and are more vulnerable to predation, particularly if they take longer to reach size refuges (Shine 1978; Travis 1980; Hayes et al. 2009). Of course, external factors besides egg size play a critical role in developmental rates during incubation, such as environmental temperature (Duellman and Trueb 1986; Bradford 1990; Beattie et al. 1992), which must also be considered. Nevertheless, it is possible that the selection for small eggs by explosive breeders has two complimentary benefits, allowing rapid egg development while also increasing female fecundity via the possibility for a larger clutch.
Even after hatching has occurred, egg size can continue to directly impact the developmental period of amphibian offspring. Results from studies relating egg size to tadpole development among amphibians have been contradictory and primarily single species focused (e.g. Crump 1984; Kaplan 1985; Berven and Chadra 1988). Such differences between studies may be related to the conditions tadpoles are exposed to post-hatching. In particular, studies on the California newt, Taricha torosa and wood frog, Rana sylvatica have shown contrasting developmental rates between large and small conspecific eggs that is influenced by the availability of food and/or conspecific density (Kaplan 1985). However, further investigation into the relationship between egg size and post-hatching development between species is required. Offspring post-hatching are able to exploit environmental resources, which influences developmental rate (Doughty & Roberts 2003). Amphibian tadpoles of some species are also known to have plastic developmental times, delaying or quickening the onset of metamorphosis in response to whether conditions are favourable or not for continued tadpole growth (Wilbur and Collins 1973; Newman 1992; Loman & Claesson 2003). This complexity of tadpole development must be considered in future studies to determine whether smaller egg sizes are selected to increase developmental rates both before and after hatching.
Parental care level
Our results suggest that it is advantageous to produce fewer, larger eggs with increasing levels of parental care. We found that the total clutch volume partitioned to each egg in species with high levels of care was three times the amount partitioned to species with low levels of care. This conforms to predictions from r-k type selection theory (Pianka 1970) and is in accordance with a majority of previous studies that show large egg and small clutch sizes are strongly associated with extended parental care (Summers et al. 2005; Summers et al. 2007; Vági et al. 2019). Since increased investment reduces offspring exposure to environmental risk factors (Shine 1978), it is more advantageous to invest more clutch resources per egg as the chances of survival are likely to be comparatively much greater than for eggs that are left unattended. In order to facilitate increasing levels of care, however, there may be an upper limit to the number of offspring that such care can be adequately provided to Lack (1954); Cody (1966); Dugas et al. (2016); Furness and Isabella (2019), which may also select for a smaller clutches. This would explain the small clutches in Australian species that carry or brood their young, such as Assa darlingtoni (Anstis 2017).
Egg-laying environment and development type
Smaller clutches of larger eggs were also found among species that have withdrawn egg deposition from aquatic systems, supporting work conducted by Byrne et al. (2003) on the Australian myobatrachids. In particular, more than double the amount of total clutch volume was partitioned to the eggs of terrestrial compared with aquatic species, and more than three times the amount for arboreal compared with aquatic species. This life history of laying eggs arboreally or terrestrially removes many of the threats associated with having offspring stages reliant on free-standing water to complete metamorphosis (Denver et al. 1998), including the risk of water drying up before development can be completed. Larger eggs buffer offspring from desiccation better than smaller eggs, given that a smaller surface area relative to volume reduce rates of evaporative water loss (Salthe 1965; Bogart 1981; Roberts 1981; Bradford and Seymour 1988; Mitchell 2002). However, those laid out of water are still vulnerable to changes in moisture levels, which may explain their climatic distribution of terrestrial and arboreal egg-laying across primarily tropical rainforests (Bradford and Seymour 1988; Anstis et al. 2007); though both do occur in other bioregions where reliable moisture is present (Roberts 1981). Some species have intermediate reproductive modes and require free-standing water at some point for offspring to complete development, such as Pseudophryne australis whose embryos develop to an advanced stage on land before nests are flooded and tadpoles hatch into water (Thumm and Mahony 2002). For such species, large eggs may confer an adaptive advantage by providing a large yolk supply that provides prolonged nourishment if hatching is delayed to coincide with a flooding event (Byrne et al. 2003).
The production of large eggs is also apparent in species that have evolved direct development, with the total clutch volume partitioned to each egg per clutch with direct development being four times the amount partitioned to eggs which hatch into a feeding tadpole stage. Among the Australian anurans, relatively few species have evolved this life history (M. gouldii, both Arenophryne species, all microhylids and Metacrinia nichollsi), all of which are terrestrial egg depositors (Anstis 2017). In order for an egg to complete direct development, it must possess a comparatively larger supply of energy for the embryo to bypass the tadpole stage (Summers et al. 2007). Presumably, this can only occur with an increase in the yolk content, resulting in a relatively larger egg. Although direct development is found in species with different egg sizes, suggesting that an absolutely large egg is not necessary for direct development to be achieved, a relative increase in egg size has been found among Urodela when species are grouped based on adult body size (Salthe 1969), suggesting the selection for a relative increase in egg size in direct-developing amphibians when compared to their closest non-direct-developing counterparts. Given these findings, the transition to terrestrial egg laying and direct development from the ancestral mode have likely both driven the selection for an enlarged egg size in tandem, albeit at the expense of a large clutch.
Bioregion
Although egg sizes did not differ across bioregions, we propose that physical microhabitat has an indirect effect by selecting for traits that alter the manner in which eggs are exposed to external risk factors. For example, while terrestrial breeding is considered to be confined to species inhabiting tropical environments, the Australian terrestrial breeder M. gouldii deposits its eggs subterraneously in moist sandy soil in semi-arid environments during periods of reliable rainfall (Poynton 1964; Roberts 1981; Roberts 1984). Thus, the conditions eggs are exposed to are not truly reflective of those that generally define the bioregion at all other times outside of the breeding period. Other terrestrial and direct developing species in this group, including Arenophryne rotunda and M. nichollsi, also exploit temperate or arid environments, highlighting the possibility for such reproductive patterns to be selected for in areas far removed from the tropics so long as there is some sort of reliable moisture source available. Future studies must therefore define the precise microhabitat of egg deposition, as larger geographical scales may not accurately reflect the true conditions offspring are exposed to.
CONCLUSION
For our study, we have used mean life-history values of egg, clutch and female sizes for each species. These may not be truly representative of the life-history complexity found among some species in this group where fitness benefits have been obtained by selecting for the deliberate production of variable egg sizes within and between clutches (Fox et al. 1997; Einum and Fleming 2004; Olofsson et al. 2009). Indeed, intra-clutch variability has been recorded for some Australian anurans (Dziminski and Alford 2005). There may also be differences between females within populations, as well as spatial differences between females from different populations. While this was not analysed in the current study given the lack of such fine-scale detail for Australian anurans, future studies will find merit in considering life-history data collected over the entirety of each species' range to account for even subtle variability.
An inverse relationship between egg and clutch size shown among the Australian anurans highlights the balance that must be made when provisioning finite resources for reproduction. We provide evidence that several life history and environmental variables are related to and have likely influenced the trade-off between egg and clutch sizes in this group. While the variables analysed in this study are not exhaustive, they show the potential benefit of conducting analyses on large taxonomic groups in order to better understand the complex interdependencies that exist between species' reproductive traits and egg-laying environments.
ACKNOWLEDGEMENT
We dedicate this study to the incredible and comprehensive natural history work of Marion Anstis, which has allowed the formulation of this study. Open access publishing facilitated by The University of Newcastle, as part of the Wiley - The University of Newcastle agreement via the Council of Australian University Librarians. WOA Institution: The University of Newcastle Blended DEAL: CAUL 2022.
AUTHOR CONTRIBUTIONS
John. Gould: Conceptualization (equal); data curation (lead); formal analysis (lead); investigation (equal); methodology (equal); project administration (lead); visualization (lead); writing – original draft (lead); writing – review and editing (lead). Chad Beranek: Conceptualization (equal); data curation (equal); investigation (equal); methodology (equal); writing – original draft (equal); writing – review and editing (equal). Jose Valdez: Formal analysis (equal); methodology (equal); writing – review and editing (supporting). Michael Mahony: Conceptualization (lead); investigation (supporting); writing – original draft (supporting); writing – review and editing (equal).
CONFLICT OF INTEREST
There is no conflict of interest.




