Nest-associated vocal behaviours of the south-eastern red-tailed black cockatoo, Calyptorhynchus banksii graptogyne, and the Kangaroo Island glossy black cockatoo, C. lathami halmaturinus
Abstract
Animal vocalisations can signify diverse behavioural contexts, knowledge of which can be applied in bioacoustic monitoring programs. Australia’s endemic black cockatoos (Calyptorhynchus sp., family Cacatuidae) are highly vocal species that are threatened in many locations. In this study, we describe the nest-associated vocal behaviours of two endangered subspecies of black cockatoo, the south-eastern red-tailed black cockatoo, C. banksii graptogyne and the Kangaroo Island glossy black cockatoo, C. lathami halmaturinus. Breeding success is limiting their recoveries and nest monitoring is challenging, but vocal recordings might provide valuable long-term information hard to obtain otherwise. We recorded daily vocal activity at wild nests of both cockatoos using autonomous sound recorders. Combined with behavioural observations and video footage, we identified vocalisations characteristic of six behavioural contexts at nests: birds in flight, while perched, during begging (adult females), during courtship displays (adult males), when entering or sitting near to the nest hollow entrance (adult females), and from nestlings. Linear discriminant analysis on 12 acoustic measurements correctly classified 58.4% of calls of the red-tailed black cockatoo (n = 907 calls from eight nests) and 62.9% of calls of the glossy black cockatoo (n = 1632 calls from 11 nests). In both subspecies, the female nest call and nestling calls are the most conspicuous vocal indicators of active nesting, and therefore should be considered for their bioacoustic potential. Other adult vocalisations indicate a range of behavioural contexts that could be informative for monitoring nesting behaviour, and its association to habitat features, in these endangered subspecies.
Introduction
Animal vocalisations can signify diverse behavioural states. For vocal species, sound data collected in bioacoustic studies can therefore indicate particular behavioural contexts, which can benefit conservation if they provide new insights into a population’s state, trajectory or response to management (Teixeira et al. 2019). For example, critical life history events like mating and recruitment may be detected via context-specific vocalisations (e.g. African elephant copulation; Poole 2011) or changes in group-level vocalisations (e.g. a shift in acoustic energy of Iberian Wolf packs when juveniles are present; Palacios et al. 2016). Therefore, knowledge on the behavioural contexts associated with particular vocalisations can improve the resolution and application of bioacoustic data beyond population metrics such as species presence-absence, population density or abundance. As technology advances, the efficiency and cost-effectiveness of acquiring data from species’ vocalisations are likely to improve, warranting improved incorporation of vocal behaviour into bioacoustic monitoring programs. To achieve this, the vocal behaviours that could provide conservation-relevant data must be described for the species of interest.
Parrots are among the most social, intelligent and vocally complex avian species (Bradbury & Balsby 2016; Cussen 2017). They are one of few taxa able to learn new vocalisations throughout life (open-ended vocal learning), an ability hypothesised to be an adaptation to their highly social foraging behaviours (Bradbury & Balsby 2016). Australia’s endemic black cockatoos, comprising five species of genus Calyptorhynchus (family Cacatuidae), are highly social parrots, often found in large, noisy flocks (Higgins 1999). Nesting is semi-communal, with nests often aggregated in the landscape (Johnstone et al. 2013; D. Teixeira, pers. obs., 2018). Clutches comprise one or two eggs, and overall reproductive output is low. Eggs hatch after about four weeks’ incubation and fledging occurs ten to twelve weeks later (Higgins 1999; Johnstone & Kirkby 2008). Fledgling cockatoos have a long parental dependency period (Higgins 1999), possibly up to 24 months in some populations (forest red-tailed black cockatoo, C. banksii naso; Johnstone et al. 2013). As in other parrots, young cockatoos likely learn their early vocalisations from their parents, with whom they have contact at the nest, and develop their adult repertoire from social interactions in larger flocks during the fledgling period.
Given their sociality, it is likely that black cockatoos’ different vocalisations reflect different behavioural contexts. This is shown in other cockatoos, including the closely-related Carnaby’s black cockatoo, C. latirostris (Saunders 1983), and the palm cockatoo, Probosciger aterrimus (Zdenek et al. 2015). As such, black cockatoos are good candidates for bioacoustic monitoring of behaviour, with potential benefits for conservation. Indeed, every black cockatoo species is listed as threatened under state or national legislation in at least part of its range, and bioacoustics could aid monitoring. Currently, monitoring and management vary among species and populations, but often rely on citizen science activities coordinated by non-profit organisations (e.g. Birdlife Australia’s Great Cocky Count). These activities usually involve counting birds in flocks, such as at roosts or drinking sites. Such data can be useful for understanding trends in flock size, demographic structure and occupancy in the landscape. However, methods for collecting data from other contexts, particularly during the breeding season, are limited. If behaviour-specific vocalisations can be reliably identified, then obtaining data from other contexts, such as nesting, should be achievable using bioacoustic methods.
In this study, we investigated the nest-associated vocalisations of two endangered subspecies of black cockatoo, the Kangaroo Island glossy black cockatoo, C. lathami halmaturinus, and the south-eastern red-tailed black cockatoo, C. banksii graptogyne. We focussed specifically on vocal behaviours associated with nesting because efforts to monitor breeding in these subspecies have been restricted, despite their recoveries being limited by breeding success (Russell et al. 2018; Berris et al. 2018). The Kangaroo Island glossy black cockatoo’s population size is the smallest of any black cockatoo (373 individuals counted in the 2016 census; Berris et al. 2018). Until 2016, when the recovery program’s funding was reduced dramatically, up to 50 nests were monitored each breeding season. However, human resource requirements were substantial, and the funding reductions have seen monitoring efforts greatly reduced. For the south-eastern red-tailed black cockatoo, whose declining population numbers about 1400 individuals, monitoring of breeding has always been limited (Russell et al. 2018). Nests are difficult to monitor because they are remote, rare in the landscape and are often on private land, making it challenging to observe nests through to fledging or failure. Breeding success is inferred from the demographic structure of flocks and increases in the proportion of male birds over recent years suggests a decrease in breeding output (Russell et al. 2018). For this reason, a high priority for conservation is to develop efficient methods for nest monitoring.
This study aimed to describe the nest-associated vocalisations of the south-eastern red-tailed black cockatoo and the Kangaroo Island glossy black cockatoo. For these subspecies, the only adult vocalisation reported from nests is the nest call of the Kangaroo Island glossy black cockatoo, given by adult females when entering or prospecting a nest hollow (Pepper 1996). Vocalisations of nestlings are not formally described in either red-tailed or glossy black cockatoos, although Cameron (2009) in studying the eastern subspecies of glossy black cockatoo, C. lathami lathami, defined late-stage nests as those where nestlings gave ‘harsh growling’ calls upon the return of the nesting female. Here, for both subspecies, we aimed to qualitatively describe the behavioural contexts associated with each call type given at nests and to provide quantitative acoustic measurements for each. We hypothesised that adult and nestling birds give unique vocalisations in various behavioural contexts, and that these are distinct in acoustic structure. This knowledge may reveal important information about critical life history events and, more specifically, the potential for bioacoustics to provide a novel method with which to monitor nesting behaviour.
Methods
Study sites
Data for the south-eastern red-tailed black cockatoo were collected from areas near Casterton and Edenhope in south-west Victoria, Australia. The cockatoos commonly nest in dead and isolated river red gums, Eucalyptus camaldulensis, that occur in livestock paddocks. Given their relative ease of observation and accessibility, paddock trees were the focus of data collection for this study. Nests were located through active searching in spring and summer. Typically, cockatoos in flight were detected by their calls and then followed to identify if they approached a nest hollow. This method allowed active nests to be confirmed without the need for tree-climbing, which is normally unsafe as the trees are dead. Some trees were inspected with a pole-mounted camera at later stages of nesting to confirm their continued activity.
On Kangaroo Island, data for the glossy black cockatoo were collected from several nesting areas that are routinely monitored by the state government. To increase the cockatoos’ breeding opportunities, many artificial nest hollows have been installed on the island, and these have been successfully used by the cockatoos for many years (Berris et al. 2018). Nesting occurs in several habitat types, including conservation estates, roadsides, regenerated woodlands and residential and agricultural areas (M. Barth, pers. obs., 2018), all of which are represented in this study. No preference was given to either natural or artificial hollows for this study. Nest activity was confirmed via afternoon observations, as in the red-tailed black cockatoo, via nest inspection with a pole-mounted wireless camera, or via a female’s presence at the nest hollow entrance. Laying is thought to peak in March and April (M. Barth, pers. obs., 2018). For most nests, monitoring began during these months.
Acoustic data collection
For each active nest, an autonomous sound recorder (Frontier Labs Bioacoustic Audio Recorder, https://frontierlabs.com.au/) was installed on the nest tree or a nearby tree. If not on the nest tree, recorders were within 5 m of the nest tree to ensure that it was closer to the nest of interest than to other nests; therefore, the loudest vocalisations could be confidently assigned to the nest of interest. Recorders were approximately 8–30 m from the nest hollow, depending on whether they were fixed to the nest tree or a neighbouring tree. We chose to install recorders at this height, rather than at the hollow, because this is what would be more feasible in a bioacoustic monitoring program.
Installed sound recorders were programmed to record for three hours per day, beginning at 2.5 h before sunset (sunset-based schedule). This time period was chosen because this is when the cockatoos are most active at the nest (R. Hill, M. Barth, & D. Teixeira, pers. obs., 2018). In addition, once a week, recording began half an hour before sunrise and ended half an hour after sunset (full-day schedule). This was done to capture any unexpected activity at the nest during the day. If a nestling was observed at a nest (i.e. a late-stage nest), we attempted to update schedules to record at the full-day schedule every day, to maximise the likelihood of recording the fledging event. This was not achieved for every nest. Recorders remained in place until after the observed or expected date of fledging, unless nest failure was confirmed sooner. All recordings were made using an omnidirectional microphone, with a fixed gain of 20 dB, at a sample rate of 44.1 kHz. Microphones had an 80 Hz high-pass filter to reduce the effects of low-frequency noise (e.g. wind). Recorders were fitted with four rechargeable lithium ion batteries and one 128 GB SanDisk memory card, which were replenished at approximately 6-week intervals. All recordings were made in uncompressed wave (.wav) format.
Behavioural classification
To describe the behavioural contexts of vocalisations recorded, observations of cockatoos were carried out at and near nest trees. This included nests that were monitored with autonomous sound recorders, as well as other nests that were opportunistically observed. Cockatoos were filmed using a Canon 5D mark III DSLR camera and Canon 100–400 II IS USM telephoto lens with a Rode VideoMic Pro microphone attached. Observation distance was usually a minimum of 10 m from the cockatoos’ location but varied between sessions. Observations were usually made in the late afternoon and early evening when the cockatoos are most active at the nest. Recording usually commenced when the cockatoos were in plain sight and actively vocalising. Individuals were not marked but could be identified by their association with a nest. Observation length varied and depended primarily on the length of time that the birds were within visual and auditory distance. Observations were usually terminated by the cockatoos flying out of sight or by the female entering the nest hollow. Observations were also terminated in poor weather (rain or high wind). We did not standardise observation time, frequency or recording length among nests, since most observations were opportunistic and dependent on recording conditions and nest status. Where possible, preference was given to nests where a nestling was observable at the hollow entrance. Behavioural observations were conducted in both early and late stages of nest development, although nestlings were only observed at late stages (D. Teixeira, pers. obs., 2017, 2018).
Call types and their associated behavioural contexts (Table 1) were putatively described from field observations and video footage. Sound files were extracted from videos using Adobe Media Encoder CC 2017. Spectrograms (Hann window; window size = 1024 samples; hop size = 512 samples; 50% overlap) and waveforms (oscillograms) were inspected using RavenPro 1.5 (Cornell Lab of Ornithology, Ithaca, NY) and viewed in tandem with videos to determine the behaviours associated with each call type. Behaviours for both subspecies were classified into broad categories in line with the ethogram provided by Pepper (1996) for the Kangaroo Island glossy black cockatoo. Since the camera records sound in compressed MPEG-4 AVC/H.264 format, spectrograms created from video data were used only for visual purposes when classifying putative call types and behavioural contexts. Quantitative measurements of each call type (Appendix S1) were only taken from recordings made with the autonomous sound recorders.
| Call type (behaviour) | Caller identity | Behavioural context | Description | Notes |
|---|---|---|---|---|
| Flight | Adult male and female | Adults give flight calls when flying to and from the nest tree |
Typically loud with harmonics. May contain pulsatile elements. Common at nests. Reliably recorded every day Take-off call subtype: Given upon take-off flight. Shorter in duration. May show a downward inflection RTBC: Clear harmonics, with or without pulsatile elements. Males and female calls are usually not overlapping. Take-off call is usually the final call of the day, given by the male as he leaves the nest tree to roost. Take-off call is common at RTBC nests because the male tends not to roost in the nest tree (isolated paddock tree) GBC: Often contain pulsatile elements. Male and female flight calls are often overlapping. Take-off call is less common at GBC nests because the male tends to roost in the nest tree or a nearby tree |
Did not differentiate between the sexes |
| Begging | Adult female | Female begs in the presence of her mate to elicit allofeeding |
Often given when the pair is perched on the nest tree or a nearby tree. During incubation and brooding, a female may beg from within the nest hollow in response to her mate’s flight call. The main purpose of begging appears to be to elicit allofeeding from the male; however, females also appear to beg to maintain contact with their mate. Calls are given repetitively. Begging bouts can be long in duration. Calls are highly variable in structure within and among individuals. Calls vary from loud to soft. Common at nests RTBC: Commonly contain harmonics and deterministic chaos. Some calls appear largely chaotic with little harmonic structure. Subharmonics are sometimes present GBC: Usually show harmonics, with or without deterministic chaos and frequency jumps. Subharmonics are sometimes present |
Highly variable in structure |
| Display (courtship) | Adult male | Male displays to his mate in courtship, to maintain pair bonds and to instigate copulation |
Display calls are given by adult males when perched close to their mate. Calls appear to function in courtship and in maintaining the pair bond. Given prior to copulation. Display involves non-vocal elements, namely head-bobbing and tail-panning wherein the male’s red tail feathers are displayed to the female. Call is given repetitively. Display bouts can be long in duration. Call contains two elements. Second element is usually louder. Usually soft RTBC: First element is harmonic with a dominant frequency band at around 1.8 kHz. Other harmonic elements are clear. Harmonic at 3.6 kHz can also be high in energy. First element usually contains some noise. Second element is short and broadband without clear harmonics. Second element is usually louder but elements can be similar in energy. GBC: First element is soft with most energy around 3.4 kHz. Other harmonic elements are usually low in energy and less clear. Second element is short and broadband with harmonics. Second element typically much louder than the first element |
Termed the kwee-chuck call for the glossy black cockatoo by Pepper (1996). Not heard every day |
|
Nest (nest entry) |
Adult female | Given by females when near the nest or entering the nest hollow |
Very pulsatile. Varies from loud to soft. Appears to function in communication with her mate and the nestling. Female will vocalise once sitting at the nest hollow entrance. Sometimes given when perched near the hollow. Can be drawn out and relatively long in duration RTBC: Guttural, ‘purring’ sound. Pulsatile GBC: Guttural, ‘growling’ sound. Can be very similar to nestling call, but pulses are more distinct (less noisy) |
Not given every time nest is visited or entered |
| Nestling | Nestling | Given by large nestlings in the nest hollow or when sitting at the nest hollow entrance |
Call given in the presence of parents. Calling begins upon parents’ return to the nest. Can resemble females’ nest call. Subtypes 1 and 2 are common once the nestling is close the fledging; reliably heard every day Subtype 1: Very loud, broadband, chaotic. May contain harmonics towards the end of the call. Nestling stimulated upon parents’ first return to the nest. Can be associated with wing-flapping in older nestlings Subtype 2: Softer call but otherwise similar in structure to subtype 1. Nestling is less stimulated Subtype 3 (begging): Soft, highly variable. Can resemble female begging calls. Appears to function as a close contact call with the adult female. Elicits allofeeding by female. Sounds high-pitched and ‘squeaky’ RTBC: Very noisy and chaotic. Sounds ‘throaty’ or ‘wheezy’. Not pulsatile. Harmonics sometimes present. GBC: Noisy, chaotic call, often with some harmonic structure. Highly pulsatile. Can be very similar to females’ nest call, but more chaotic and less harmonic |
Type 3 is soft; can be difficult to detect on a recorder. Identified from a small sample of nests with video footage, but expected at every nest |
| Perch |
Adult male (RTBC) Adult male and female (GBC) |
Given when perched on or near the nest tree |
Perch calls comprise several, variable contact calls given by adult birds when perched on or near the nest tree. Appear to maintain contact between male, female and nestling in hollow. In the GBC, both sexes commonly call when perched. In the RTBC, only male calls are reliably heard in recordings. Perch calls range from loud to soft. Male perch call (subtype 2) is most common. Recorded often. After female has entered the nest hollow for the night, male will call intermittently, often for a long duration. Perch calls are often the final vocalisations of the day. Subtypes 2 and 3 (soft perch calls) often given in response to female begging RTBC: Given by male. Always begins with pulsatile or chaotic elements. Has a ‘crackling’ quality to the sound Male loud perch call (perch subtype 1): Loud. Sounds ‘trumpet-like’. Clear harmonic structure in second part of call. Often with subharmonics. First part of call is chaotic and pulsatile. First part of call may be lacking, showing only harmonics; typically given soon after landing on the nest tree Male soft perch call (perch subtype 2): Beginning of call is highly pulsatile, with a ‘crackling’ sound quality, ending in harmonics. Can be loud or soft. Typically given when male is perched on the nest tree, including after the female has entered the hollow Male soft perch call (perch subtype 3): Soft, guttural. Lacks harmonics but otherwise is similar to subtype 2. Entire call is chaotic or pulsatile GBC: Highly variable, graded calls. Subtypes 1, 2 and 3 are given by adult males. Subtypes 4, 5 and 6 are given by adult females. Perch calls by both adult birds are commonly given at nests, often in interactions Male loud perch call (perch subtype 1): Contains harmonics. May contain some pulses and chaos. Less common than soft perch calls. Usually loud but can be relatively soft. May precede take-off Male soft perch call (perch subtype 2): Most common adult male soft perch call. Usually very soft, ‘fuzzy’ sound. Often appears as a single, wide frequency band between 2.5 and 4.5 kHz, ending with a downward inflection. Louder variations have additional high and low-frequency bands but maintain the ‘fuzzy’ quality to the sound. Common at nests. Often given in response to female perch calls or begging. Given when the female is in the nest hollow Male soft perch call (perch subtype 3): Very similar to subtype 2. Begins as subtype 2 but abruptly changes to end with a louder harmonic element. Some individuals appear to use subtype 3 more often than subtype 2. Female loud perch call (perch subtype 4): Clear harmonics. Sometimes shows frequency modulation. Often given when the female is perched high on the nest tree or on a nearby tree Female loud, alarm perch call (perch subtype 5): Loud, pulsatile call. Can resemble nest entry call. Given when female is alarmed near the nest, usually when defending the nest tree from other birds (e.g. other glossy black cockatoos or galahs) Female soft perch call (perch subtype 6): Soft. Resembles male perch call subtype 2 but contains more than one frequency band. Often appears as two dominant frequency bands around 3–3.5 and 6–6.5 kHz. Uncommon and easily masked by other sounds |
Graded contact calls. Soft perch calls are easily masked by other sounds and therefore appear to be uncommon in sound recordings |
Quantitative structure of vocalisations
To efficiently select calls for quantitative analysis, we used recordings made in the final two weeks before fledging (or failure), as this time period represents the complete repertoire of conspicuous vocalisations at nests. This includes nestlings, which can be difficult to detect in earlier weeks (D. Teixeira, pers. obs., 2019). Most selections were made from nests where nestlings were recorded. Additional nests were included to increase sample sizes of adult calls, if required (see below). Selections were not made on days or during time periods where recording conditions were poor (e.g. high wind or rain).
For the south-eastern red-tailed black cockatoo, selections were made from recordings obtained at eight nests. For the Kangaroo Island glossy black cockatoo, selections were made from 11 nests. Calls were manually selected and annotated from spectrograms (Hann window; window size = 1024 samples; hop size = 512 samples; 50% overlap) and waveforms using RavenPro 1.5. For each selection, upper and lower bounds (i.e. low frequency and high frequency of the selection box) were set via inspection of the spectrogram, while start time and end time were set via inspection of the waveform. For each call type, we aimed to select a minimum of 20 calls per nest, to sufficiently represent within-individual variation in call structure (Fischer et al. 2013). For flight calls, we initially aimed to annotate at least 40 calls per nest, because both male and female adult birds give this call, however this was difficult to achieve for the glossy black cockatoo because male and female flight calls are often overlapping and therefore unsuitable for acoustic analysis.
Calls that were selected were chosen ad hoc from those that showed relatively high signal to noise ratio on the spectrogram and were not overlapping with other calls or background noise. Each call selected was categorised by call type (behavioural category), age (adult or nestling) and, except for flight calls, sex (adult birds only). Quantitative measurements recorded for each selected call were as follows: (i) low frequency (Hz), (ii) peak frequency (kHz), (iii) centre frequency (kHz), (iv) aggregate entropy (bits), (v) average entropy (bits), (vi) minimum entropy (bits), (vii) maximum entropy (bits), (viii) delta time (seconds), (ix) interquartile range duration (seconds), (x) peak amplitude (U), (xi) peak frequency contour average slope (Hz ms−1) and (xii) peak frequency contour maximum slope (Hz ms−1; Appendix S1). We excluded high frequency measurements (e.g. high frequency, delta frequency and interquartile range bandwidth) because high frequency components were often attenuated by distance. This differed among nests because the distance of the sound recorder to the nest hollow varied, as did the distance between the sound recorder and the vocalising birds when not in the nest hollow (i.e. the birds’ position in the nest tree or nearby trees varied).
Statistical analysis
To examine for differences in putative call types (response variable), we conducted linear discriminant analyses on the acoustic measurements (predictors; Appendix S1) using the MASS package in R (Venables & Ripley 2002; R Core Team 2019). Acoustic measurements were inspected for normality and transformed where necessary. For each subspecies, the data (n = 907 selections of calls for the red-tailed black cockatoo; n = 1632 selections of calls for the glossy black cockatoo) were randomly divided into two separate datasets, one as training data (70% of the original dataset), from which the discriminant models were built, and the other as test data (30% of the original dataset). To account for different units of acoustic predictors, test data were centred and scaled using the caret package (Kuhn 2008). Test data were used to classify call types. To further confirm model performance, each discriminant model was tested using leave-one-out cross validation on the complete dataset. Results were very similar between the two approaches (Appendix S3). Finally, each discriminant model was tested using a Multivariate Analysis of Variance (manova). Plots were made using ggplot2 in R (Wickham 2016).
Results
For the south-eastern red-tailed black cockatoo, 23 nests from eight locations were located over two breeding seasons. All nests were located on livestock farms, except for one nest that was in an artificial hollow in a plantation of Australian blue gum, E. globulus. In total, four nests were in artificial nest hollows. The remainder were most often in large, dead river red gums, E. camaldulensis. One nest was found in a live river red gum. Nesting occurred from September through March. Nestlings were recorded at nine nests. For the Kangaroo Island glossy black cockatoo, data were recorded from 28 nests in eight locations over two breeding seasons. Eighteen nests were in artificial nest hollows. Natural nest hollows were all in live sugar gums, E. cladocalyx. Nesting occurred from March through November. Nestlings were recorded at 15 nests. Observed behaviours at nests were similar for the Kangaroo Island glossy black cockatoo and the south-eastern red-tailed black cockatoo.
South-eastern red-tailed black cockatoo
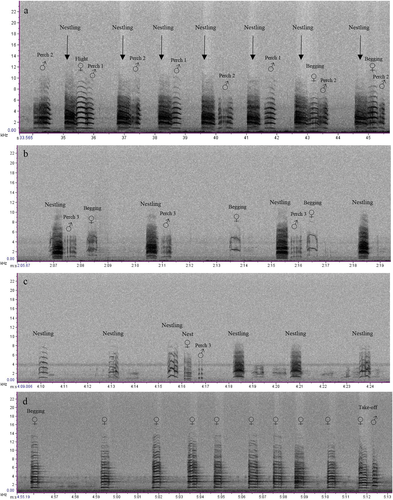
For the red-tailed black cockatoo, we putatively described 11 call types (including subtypes) from six behavioural categories (Table 1 and Fig. 1). Flight calls were typically loud and harmonic in structure, given by adult birds (male and female) when flying to and from the nest tree (Fig. 1a). We considered the take-off call (adult male and female) to be a subtype of the flight call, differing by having more arched frequency components (downward inflection; Fig. 1b). Begging calls given by adult females were highly variable in structure and amplitude. These calls clearly exhibited non-linear phenomena, including deterministic chaos, frequency jumps and subharmonics, often within a single bout of begging (Fig. 1c,d). Begging calls typically elicited head-bobbing and allofeeding from the adult male. Early in the nesting period, females could often be heard begging from inside the nest hollow in response to the approaching males’ flight calls. Display calls were sometimes given by males in response to females’ begging. Display calls were highly stereotypical and repetitive (Fig. 1f) and involved head-bobbing and fanning of the tail feathers. At two nests, display calls were given soon after alarm calling. Perch calls and nestling calls each comprised three subtypes, differentiated by their apparent loudness and harmonic, chaotic or pulsatile structure. Perch calls (three subtypes) were given by adult males when the adult female was near or inside the nest hollow, presumably to maintain contact with the female (Fig. 1j–l). Perch calls were usually the final calls given in the day, except for take-off and flight calls as the male went to roost. Females sometimes called when perched soon after flight, but we considered these calls to be flight calls because they were not obviously different in sound or spectrographic structure. Adult females’ nest call (or nest entry call) was highly pulsatile, sometimes resembling nestlings’ calls (Fig. 1e). These calls were not detected every day. The most commonly recorded nestling calls were loud and broadband (Fig. 1g). These calls usually began as the parents were flying to the nest. These calls were easily heard up to 30 m from the nest and were distinct on spectrograms. Two subtypes of nestling calls (nestling subtype 2 and subtype 3; Fig. 1h,j) were quieter and given some time after the parents’ arrival at the nest. These softer varieties were more difficult to detect on spectrograms. Vocal behaviours and interactions between individuals at the nest were evident in spectrograms (Fig. 2).
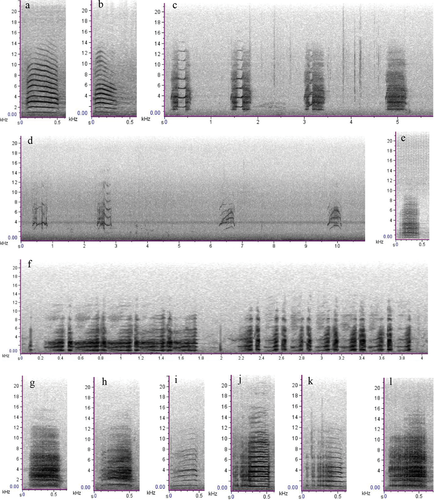
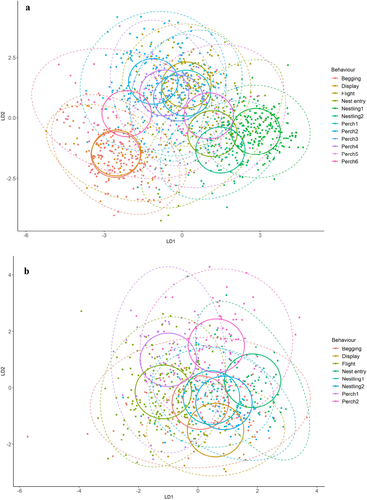
Acoustic measurements were obtained from a total of 939 selections of annotated vocalisations, representing 11 call types (including subtypes) from eight nests, for the south-eastern red-tailed black cockatoo. Descriptive statistics of each call type’s acoustic measurements are provided in Appendix S4. The sample size (i.e. number of annotated vocalisations) of each call type varied between n = 5, for nestling subtype 3 and n = 251 for the flight call. Three call subtypes (perch subtype 3, nestling subtype 3 and take-off) had fewer than 20 annotations and were therefore excluded from subsequent analyses. The final dataset used for analyses, therefore, comprised 907 selections of vocalisations. Linear discriminant analysis correctly classified 58.4% of calls (manova: Wilk’s λ = 0.16, F = 22.518, P < 0.001; Fig. 3). LD1 and LD2 explained 68.0% of the overall variance in the model. Accuracy was highest for the display call (73.7%), followed by the flight call (72.0%), and lowest for nestling subtype 2 (28.6%; Appendix S2). Nestling subtype 2 was most often misclassified as nestling subtype 1 or begging (Appendix S2). Perch subtype 1, with an accuracy of 34.8%, was commonly misclassified as a flight call (Appendix S2).
Kangaroo Island glossy black cockatoo
We putatively described 14 call types (including subtypes) from six behavioural categories for the Kangaroo Island glossy black cockatoo (Table 1 and Fig. 4). Flight calls were typically loud and overlapping, given by the adults when flying to and from the nest (Fig. 4a). Begging calls were given by females and usually elicited allofeeding from the male. Begging calls usually contained harmonics (Fig. 4b) but were highly variable in structure and showed clear non-linear phenomena (Fig. 4c). Display calls were given by males in the presence of the female. Display calls were highly stereotypical and repetitive (Fig. 4d) and involved head-bobbing and fanning of the tail feathers. Adult females gave nest calls when perched at, or near, the nest hollow entrance, but not on every occasion. Nest calls were highly pulsatile in structure, giving the call a ‘growling’ characteristic (Fig. 4e). Perch calls were given by adult males and females when perched on or near the nest tree. They comprised six graded call types (Fig. 4i–o). Perch subtypes 2 and 3, given by males, were the most common perch calls observed in the field and in sound recordings (Fig. 4j–l). They are usually the final calls recorded on any given day. Female perch calls (subtypes 4, 5 and 6) were less commonly observed (Fig. 4m–o). Nestling calls were typically loud, given in response to the parents’ arrival at the nest (subtype 1; Fig. 4f). Nestling calls were highly resemblant of the female nest call. Nestling subtype 2 was softer, given when the nestling appeared less stimulated following the arrival of its parents (Fig. 4g). Nestling subtype 3 was the softest subtype, given only when the female was close to the nestling (Fig. 4h). Vocal behaviours and interactions between individuals at the nest were evident in spectrograms (Fig. 5).
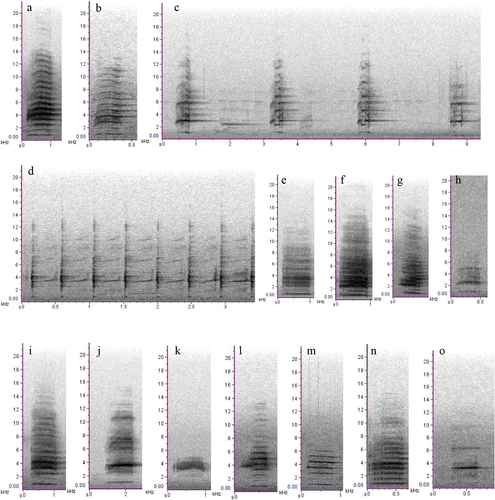
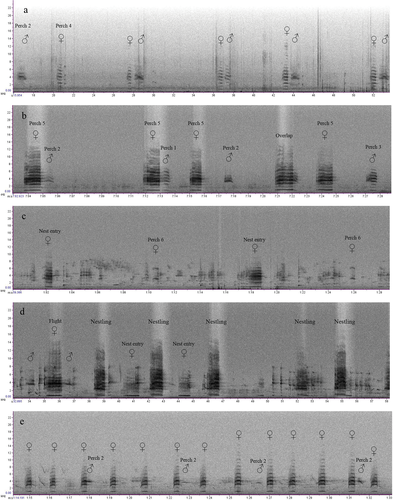
Acoustic measurements were obtained from a total of 1641 selections of annotated vocalisations, representing 14 call types (including subtypes) from 11 nests, for the Kangaroo Island glossy black cockatoo. Descriptive statistics of each call type’s acoustic measurements are provided in Appendix S4. The sample size of call types varied from n = 9, for the take-off call, to n = 303 for nestling subtype 1. Due to low sample size, the take-off call was excluded from further analysis. Therefore, the final dataset used for analyses comprised 1632 selections. Linear discriminant analysis correctly classified 62.9% of calls (manova: Wilk’s λ = 0.04, F = 47.362, p < 0.001; Fig. 3). LD1 and LD2 explained 81.2% of the overall variance in the model. Nestling subtype 1 had the greatest classification accuracy (87.8%) followed by begging (84.7%; Appendix S2). Nestling subtype 2 had an accuracy of 73.2% and misclassifications were mostly nestling subtype 1 (Appendix S2). There was misclassification among flight calls and perch calls (Appendix S2). Perch subtypes 1 and 3 had no correct classifications (Appendix S2). Perch subtype 1 was mostly classified as flight and perch subtype 5 was mostly classified as the female nest call (Appendix S2).
Discussion
Bioacoustic sound recordings can provide a rich source of behavioural data for species whose vocal diversity is known (see review by Teixeira et al. 2019). Particularly for social species, which usually exhibit more complex repertoires (Freeberg et al. 2012; Leighton 2017), vocalisations can indicate specific behaviours, demographics (e.g. age and sex of the caller) and interactions among individuals. Bioacoustic studies often focus on what vocalisations a species makes; this helps determine presence-absence, a common objective of bioacoustic studies. However, the ability to understand from vocalisations who the signaller is and why they are vocalising (behavioural context) can greatly improve the resolution of data acquired from bioacoustic programs (Teixeira et al. 2019). Through context-specific vocalisations, bioacoustics could help to monitor species’ behaviours and the relationship to habitat features, and thereby inform conservation decision-making. A necessary first step, then, is to understand a species’ vocal repertoire to the extent required for monitoring or conservation.
In this study, we provide the first descriptions of the diversity of nest-associated vocal behaviours of two endangered subspecies of black cockatoo, the Kangaroo Island glossy black cockatoo, C. lathami halmaturinus and the south-eastern red-tailed black cockatoo, C. banksii graptogyne. Through behavioural observations, we found that these subspecies gave distinct vocalisations in each of six behavioural contexts at nests. Specifically, vocalisations were identified from birds in flight, while perched, during begging (females), during courtship displays (males), when entering or sitting near to the nest hollow entrance (females), and from nestlings when in the presence of their parents. This knowledge can be used to develop novel nest monitoring methods using bioacoustic technology. This is important because bioacoustics using remote sound recorders allows for data to be collected at spatial and temporal scales much greater than that feasible by human observers. This offers an advantage for these subspecies as traditional monitoring is limited by human survey effort and available funding. Even where active nests are known, monitoring their subsequent development and outcome (fledging or failure) is difficult or, in many cases, is not achieved. Moreover, bioacoustics could be used to monitor not only known active nests, but also potential nests, such as tree hollows of unknown status, hollows used in previous years, and newly deployed artificial nest hollows.
The female nest call and the nestling calls are the most conspicuous vocal indicators of active nesting in these subspecies. These calls are loud, distinct and are, to the best of our knowledge, the only calls that are unique to active nests (D. Teixeira, R. Hill, & M. Barth, pers. obs., 2018). These calls, therefore, are most relevant to bioacoustic monitoring programs. The female nest call appears to function in close-range communication with the nestling and with the adult male when he is perched on the nest tree. In both subspecies, but especially the Kangaroo Island glossy black cockatoo, the nest call resembled the nestling call, which reduced linear discrimination (Appendix S2). This may be a product of nestlings learning their calls from the adult female, who is the only parent to enter the nest hollow in these species. Late-stage nestling calls are characteristically loud upon the parents’ arrival to the nest tree (subtype 1) in both subspecies. These loud calls were clear and easily identified in spectrograms of sound recordings. Calls become softer and less stimulated after the parents’ arrival (subtype 2), but the acoustic structure is otherwise similar. Although discrimination accuracy varied (glossy black cockatoo: 87.8% and 73.2% for subtypes 1 and 2, respectively; red-tailed black cockatoo: 54.6% and 28.6% for subtypes 1 and 2, respectively; Appendix S2), nestling calls of both subspecies were distinct to the human ear and unlike other call types, except for some cases of the female’s nest call (D. Teixeira, pers. obs., 2019).
Female begging calls were highly variable within and between individuals of both subspecies. Calls showed a range of nonlinear phenomena including deterministic chaos, subharmonics and frequency jumps. The acoustic structure of calls observed on spectrograms often varied substantially within a single begging bout (Figs 1c,4c). Though largely untested in birds, one hypothesis regarding nonlinear sound states that the more variable and random (nonlinear) a call is, the less likely a receiver is able to ignore it (Blumstein & Récapet 2009). That is, nonlinearity in animal communication has possibly evolved to attract and maintain attention to increase fitness. For example, in African elephants, Loxodonta africana, infant roars increase in chaos with the urgency of the situation (Stoeger et al. 2011). In red deer, Cervus elaphus, the harsher the males’ roars, the more attention they receive from potential mates (Reby & Charlton 2012). It is possible, therefore, that the highly nonlinear structure of female begging calls functions to limit habituation by the male and consequently increase his provisioning of the female. Since females are solely responsible for provisioning the nestling, it is plausible that female begging has been subject to strong selection pressures to increase provisioning rates. Likewise, the soft, begging-like nestling call (subtype 3) also appeared to be nonlinear and has possibly evolved to stimulate allofeeding by the female. Moreover, since both subspecies are highly specialised in diet, begging call structure may relate to the availability or quality of food in the habitat near nests and, subsequently, nestling body condition or the likelihood of breeding success. Alternatively, the soft, high-pitched characteristics of the female and nestling begging calls may function to limit detection by nest predators during allofeeding (c.f. loud calls given in other contexts).
The male display call is highly stereotypical and repetitive in both subspecies and involves head-bobbing and tail-fanning. The call contains two elements; the first element is longer in duration and may show harmonics, while the second element is a short, broadband ‘chuck’. Characterised by its rhythm, with each two-element call being repeated over time, the call may function as a signal of male fitness. Recent literature on rhythm in birds, though in its infancy, suggests the possibility of sexual selection for highly rhythmic calls or other sounds. For instance, palm cockatoos, Probosciger aterrimus, are renowned for their drumming behaviour, wherein males use a tool (a stick) to drum on a tree branch or a nest hollow. Drumming is most often directed towards females. Individualised drumming styles, including variations in rhythm, suggest that information about the male may be conveyed to females (Heinsohn et al. 2017). In budgerigars, Melopsittacus undulatus, experiments show that females prefer rhythmic sounds, which may relate to a preference for rhythm (as yet untested) in the head-bobbing sexual display given by males of the species (Hoeschele & Bowling 2016). In the current study, observed display calls were always given by adult males and directed towards their bonded females. Often, females seemingly ignored the display, or lunged to the male, or moved to a different position in the tree (D. Teixeira, pers. obs., 2017, 2018). Copulation sometimes followed the display call and was, in all observations, preceded by it (D. Teixeira, pers. obs., 2018; copulation not observed in the red-tailed black cockatoo). Thus, the call appears to be a sexual display by males to elicit copulation, or to reinforce the pair bond, to which females respond variably. Selection for rhythm may be acting on the call, in which case individualised rhythmic features may provide a bioacoustic index of male fitness.
Notwithstanding, the display call appears to have at least two secondary functions. Pepper (1996) noted that, in the Kangaroo Island glossy black cockatoo, the display call (referred to as the kwee-chuck call) was sometimes given by unpaired juvenile males when perched prominently, suggesting a secondary function in dominance. Pepper (1996) also noted that the call was given after disturbance by human observers. This concords with two opportunistic findings in sound recordings from nests of the south-eastern red-tailed black cockatoo. In both cases, display calls were given following a period of alarm calling or loud banging sounds in the hollow. Cockatoos were not detected in recordings thereafter, which suggests that display calls may accompany nest failure in some cases (e.g. a predation event). The function of the display call in such a context is not yet clear.
We classified perch calls as any vocalisation that did not resemble another call type and was given by adult birds when perched on the nest tree. These represented a range of graded contact calls, which were sometimes difficult to differentiate on spectrograms, and classification accuracy was mixed (Appendix S2). These results support the hypothesis that animal calls are often graded, variations of each other, rather than distinct categories; thus categorisation is somewhat subjective (Fischer et al. 2016). In parrots, loud contact calls tend to elicit a vocal response from conspecifics and, therefore, are generally thought to function in establishing connections between individuals (Bradbury 2003). This appeared to be the case in this study, where males and females, and sometimes nestlings, would often engage in vocal exchanges while perched on the nest tree (Figs 2,5). In both subspecies, the males’ soft perch call (referred to as subtype 2) was relatively common and detected most days. This call provided a clear acoustic signal of the birds’ presence at the nest tree. These soft contact calls were given by males after landing on the nest tree, after the female had entered the hollow, as well as in response to female begging. Therefore, its function appeared mostly one-way, directed from male to female, usually without response. Parrots’ soft contact calls often do not elicit responses and are thought to function in coordinating flock movements through vegetation. Indeed, in the glossy black cockatoo, this soft perch call appears synonymous with the feeding call shown in Pepper (1996) wherein it was noted ‘mated pairs gave soft, short calls at intervals while foraging’. Like the display call, perch calls appear to function in several behavioural contexts.
Vocal behaviours in this study were described from bioacoustic methods that align with those likely to be feasible in a larger monitoring program for these subspecies. Specifically, sound data were collected from nest trees, usually in the late afternoon as this is when birds are most active at nests (D. Teixeira, pers. obs., 2017, 2018), from approximately 8–30 m distance from the nest hollow. Tree-climbing was avoided as it is unsafe for nests in dead trees, which are important for the south-eastern red-tailed black cockatoos, and has high human resource costs. Vocalisations were clearer from recorders that were closer to the nest hollows (e.g. where hollows were lower to the ground), although loud calls, including nestling calls, were easily identified in recordings from all distances included in this study. Since loud nestling calls are one of the most useful indicators of active nesting, we believe that the approach used here is appropriate for these subspecies. A limitation is that this requires a sound recorder at every nest tree monitored. However, since the cockatoos often nest in loose aggregations, it is possible that a smaller number of recorders could monitor several nests simultaneously. Nest location could be measured from the time difference in the arrival of calls at each recorder (Stevenson et al. 2015). Designing an appropriate recorder array requires an understanding of the distances at which key vocalisations can be detected by the sound recorders in each habitat type (e.g., forest vs paddock). This was not explicitly examined in this study but warrants attention as it could reduce the number of sound recorders required. Another important consideration is post-processing sound data using automated or semi-automated recognition methods (Blumstein et al. 2011; Crump & Houlahan 2017; Priyadarshani et al. 2018). Although not examined here, we believe that nestling calls are a good candidate for automated detection, since they are loud, distinct and are a good indicator of active nesting.
This study aimed to describe the nest-associated vocal behaviours of the Kangaroo Island glossy black cockatoo and the south-eastern red-tailed black cockatoo, to provide the knowledge necessary for the development of a bioacoustic nest monitoring program. Nest monitoring is important for understanding how breeding activity varies across the landscape, which can help inform management decisions. For instance, two important conservation actions for these subspecies are managing fire impacts to feeding habitat (especially close to nests) and supplementing natural nest hollows with artificial nest hollows. However, spatial prioritisation of these actions could be better informed by a greater understanding of the habitat features that influence the choice of nest location and the likelihood of fledging success. Acquiring sufficient data to test relevant hypotheses is resource intensive if using traditional human-observer methods. Moreover, using traditional methods, it is not feasible to collect behavioural data such as nest visitation rates by the adult birds or the date of fledging or nest failure. The vocal behaviours described in this study suggest that a wide range of behavioural data could be extracted from sound data. Bioacoustics can, therefore, aid monitoring by reducing human survey effort while also providing a range of behavioural data. With continued advances in recording technology and automated sound processing, it is foreseeable that bioacoustics could provide daily data from potential and active nests, with human field effort limited to the deployment and retrieval of sound recorders.
Conclusion
Both subspecies examined in this study are nationally endangered and breeding success is a limiting factor in their recoveries (Berris et al. 2018; Russell et al. 2018). However, monitoring of breeding is difficult in both subspecies, largely because of accessibility and resource restrictions. This potentially limits conservation decision-making as it pertains to nesting. Bioacoustics may help address this issue. For both subspecies, the most important objective of nest monitoring is to confirm the birds’ daily presence or absence at nests. To this end, we conclude that most useful calls for bioacoustic nest monitoring are female nest call and loud nestling calls. These call types are the most conspicuous signs of active nesting, since they are loud, distinct and unique to active nests. They are usually easily identifiable to the human ear and on spectrograms. Additionally, because they are relatively stereotypical and loud, these calls could be the focus of automated or semi-automated detection of nesting activity. Failure to detect these calls on any given day could indicate nest failure or successful fledging.
Further, with knowledge on vocal behaviour, bioacoustics can be used to monitor not only nesting activity, but also specific behaviours of the cockatoos at nests. It is also possible that calls are individually distinct, as shown in the palm cockatoo (Zdenek et al. 2018) and Carnaby’s black cockatoo (Saunders 1983), in which case bioacoustics may help monitor nest site fidelity. Indeed, bioacoustics offers a range of monitoring options for black cockatoos and this study provides a preliminary description of conservation-relevant vocalisations for two highly threatened subspecies. As bioacoustic technology and analytical methods continue to advance and become more accessible, large-scale bioacoustic nest monitoring programs could be implemented for the conservation benefit of the Kangaroo Island glossy black cockatoo and the south-eastern red-tailed black cockatoo.
Species nomenclature
Calyptorhynchus lathami subsp. halmaturinus
Calyptorhynchus banksii subsp. graptogyne
Acknowledgements
Many people contributed to this study. In-kind field support was provided by the south-eastern red-tailed recovery team and the Kangaroo Island glossy black cockatoo recovery program. In particular, we thank Tim Burnard, Evan Roberts, Karleah Berris and Torren Welz. We thank the landowners who permitted us to work on their properties and the citizens who reported their sightings of the black cockatoos. We thank the members of the Ecosounds Lab at the Queensland University of Technology, especially Prof. Paul Roe, Dr. Anthony Truskinger, Dr. Michael Towsey and Dr. Phil Eichinski, for help with sound data handling and storage. We thank Dr. Simone Blomberg for statistical guidance. This work was supported by an Australian Postgraduate Award, the National Environmental Science Programme’s Threatened Species Recovery Hub and the Glossy Black Conservancy.
Author contributions
Daniella Teixeira: Conceptualization (lead); data curation (lead); formal analysis (lead); funding acquisition (lead); investigation (lead); methodology (lead); project administration (lead); resources (lead); software (lead); visualization (lead); writing-original draft (lead); writing-review & editing (lead). Richard Hill: Conceptualization (supporting); investigation (supporting); project administration (supporting); supervision (supporting); writing-review & editing (supporting). Michael Barth: Investigation (supporting); project administration (supporting); writing-review & editing (supporting). Martine Maron: Conceptualization (supporting); funding acquisition (supporting); methodology (supporting); supervision (supporting); writing-review & editing (supporting). Berndt J. van Rensburg: Conceptualization (supporting); funding acquisition (supporting); methodology (supporting); project administration (supporting); resources (lead); software (lead); supervision (lead); writing-review & editing (supporting).
Conflict of interest
There are no known conflicts of interest.
Ethics statement
This work was conducted under animal ethics approval number SBS/076/17/VIC and SBS/DEWNR/219/17 issued by The University of Queensland Animal Ethics Committee.




