Digging up the dirt: Quantifying the effects on soil of a translocated ecosystem engineer
Abstract
Digging mammals are often considered ecosystem engineers, as they affect important properties of soils and in turn nutrient exchange, vegetation dynamics and habitat quality. Returning such species, and their functions, to areas from where they have been extirpated could help restore degraded landscapes and is increasingly being trialled as a conservation tool. Studies examining the effects of digging mammals have largely been from arid and semi-arid environments, with little known about their impacts and importance in mesic systems. To address this knowledge gap, we investigated the ecological role of a recently introduced population of eastern barred bandicoots (Perameles gunnii) on Churchill Island, Victoria, south-eastern Australia, from which all digging mammals have been lost. We quantified the annual rate of soil turnover by estimating the number of foraging pits bandicoots created in 100-m2 plots over a 24-h period. Foraging pit counts could not be completed in each season, and the overall turnover estimate assumes that autumn/winter months represent turnover rates for the entire year; however, this is likely to fluctuate between seasons. Ten fresh and ten old pits were compared to paired undug control sites to quantify the effect soil disturbance had on soil hydrophobicity, moisture content and soil strength. Plots contained between zero and 64 new foraging pits each day. We estimated that an individual eastern barred bandicoot digs ~487 (95% CI = 416–526) small foraging pits per night, displacing ~13.15 kg (95% CI = 11.2–14.2 kg) of soil, equating to ~400 kg (95% CI = 341–431 kg) of soil in a winter month. Foraging pits were associated with decreased soil compaction and increased soil moisture along the foraging pit profile. Eastern barred bandicoots likely play an important role in ecosystems through their effects on soil, which adds to an increasing body of knowledge suggesting restoration of ecosystems, via the return of ecosystem engineers and their functions, holds much promise for conserving biodiversity and ecological function.
Introduction
Ecosystem engineers have distinct and often substantial effects on ecosystem structure, composition and function, influencing resource availability for sympatric species (Jones et al. 1994). Globally, the presence of ecosystem engineers has been associated with important changes such as increased species richness and altered fire regimes (Arribas et al. 2014; Law et al. 2017; Waldram et al. 2008; Wright et al. 2002). The loss of ecosystem engineers as part of the current global extinction crisis is of great concern, as extinction of a species may also mean the loss of important ecological functions (Fleming et al. 2013). Such a situation is prominent in Australia, where during the last 200 years over one third of global mammal extinctions (Garkaklis et al. 2003; Woinarski et al. 2015) have occurred, including six digging mammal species that fall within the ‘critical weight range’ (CWR) of 35–5500 g (Johnson & Isaac 2009). Losses of these species, considered to be ecological engineers, and their functions, have been linked to historical and ongoing landscape degradation (Martin 2003; Eldridge & James 2009).
Bioturbation, the ‘stirring and churning of sediments by organisms’ (Gabet et al. 2003), can have positive effects on ecosystem health. A soil-foraging species’ activities can modify, maintain or create habitat by directly or indirectly influencing resource availability for other species (Jones et al. 1994; Fleming et al. 2013). For example, the soil disturbances created by Australia’s greater bilby (Macrotis lagotis) increase nutrient availability throughout the landscape (James & Eldridge 2007), similarly Pocket Gophers (Thomomys bottae) in North America alter plant demography through changing soil development rates (Moloney et al. 1992). The effect digging species exert on their ecosystem depends largely on the mass of soil they turn over (Martin 2003). In semi-arid regions of Western Australia that experience a Mediterranean-type climate, brush-tailed bettongs (Bettongia penicillata) and southern brown bandicoots (Isoodon obesulus) can turnover 4.8 and 3.9 tonnes of soil annually, respectively (Garkaklis et al. 2004; Valentine et al. 2013). Animals such as these disrupt the soil surface crust, exposing more porous soil beneath, in turn improving soil respiration and reducing soil hydrophobicity (Whitford & Kay 1999; Jones et al. 2006). This increases water infiltration and moisture retention (Laundre 1993; Valentine et al. 2017) and reduces surface compaction (Bancroft et al. 2005).
Digging mammals may have a greater influence on soil properties and ecosystem health in resource-limited environments such as arid and semi-arid regions than in more mesic and productive environments where conditions, such as soil moisture, are not as limited (Crain & Bertness 2006; Eldridge & James 2009). Historical records indicate that prior to European settlement, Australia had areas of land that contained soft textured, friable soil that was rich and fertile, which was likely to have been sustained by abundant and widespread native digging mammals (Bride 1983; Martin 2003). However, European-driven, anthropogenic disturbance initiated extensive range declines and extinctions of Australia’s soil cultivating marsupial species, and consequently, soil and ecosystem health are thought to have been substantially degraded (Watson 2009). Considerable research has examined the influence soil-foraging species have on arid and semi-arid environments (Garkaklis et al. 2000, 2004; Bragg et al. 2005; Eldridge & Mensinga 2007; James et al. 2010; Eldridge et al. 2012; Verdon et al. 2016); however, much less is known about what role digging species play in mesic environments. We address this knowledge gap by examining the effects on soil properties of an insular population of eastern barred bandicoots (Perameles gunnii) (unnamed Victorian subspecies), translocated onto Churchill Island, south-eastern Australia.
- To quantify the rate and amount of soil turned over by eastern barred bandicoots.
- To examine what factors, including habitat type and weather, contribute to spatial and temporal variation in bandicoot foraging activity.
- To examine the effects of bandicoot soil disturbance on physical soil properties (soil moisture content, soil penetration resistance and infiltration rates).
We predicted that eastern barred bandicoots would turn over similar amounts of soil to closely related species of a similar body size, such as southern brown bandicoots (3.9 tonnes) and brush-tailed bettongs (4.8 tonnes) (Garkaklis et al. 2004; Valentine et al. 2013) and that a majority of foraging activity would occur in open habitat and vary seasonally (Dufty 1994; Winnard 2010; Winnard et al. 2013). We also predicted that bandicoots would decrease soil compaction, consistent with similar disturbances made by wedge-tailed shearwaters and mole rats in other systems (Bancroft et al. 2005; Hagenah & Bennett 2013), and increase soil moisture, similar to the effects of bilby (M. lagotis), echidna (Tachyglossus aculeatus) and brush-tailed bettong diggings (B. penicillata) (Garkaklis et al. 1998; Eldridge & Mensinga 2007; Chapman 2013).
Methods
Study site
Churchill Island (57 ha) is adjacent to Phillip Island at the entrance of Westernport Bay in south-east Victoria, Australia (38.4992° S, 145.3379° E). Temperature on the island ranges from a mean maximum annual temperature of 18.7°C to an annual mean minimum of 11.7°C, and average annual rainfall is 620 mm (BoM weather station #086373). Throughout the months of April, May and June 2017, during field data collection, Churchill Island received ~30% of the average annual rainfall (approx. 190.2 mm), with the average rainfall for this time of year being 196.1 mm (Australian Bureau of Meteorology 2017).
Churchill Island is dominated by compact loam and clay soil, alternating to sandier substrate along the intertidal zone. The classified texture of the soil falls into a range of categories including sand/loam, loam and loamy/clay (CSBP Soil & Plant Analysis Laboratory 2016). There are two major habitat types on the island: closed woodland and open grassy pasture. Overall, the open pastures have a high relative abundance of introduced grass species, with several non-native genera occupying the area. Microlaena stipoides is the only native grass species, which is dominant in a small section of pasture. The closed woodland habitat has a more complex vegetation structure, with Melaleuca, Banksia, Allocasuarina and Eucalyptus species comprising most of the overstorey. The mid-storey is dominated by Acacia longifolia sophorae and Rhagodia candolleana. The understorey is predominately made up of sprawling herbs such as Tetragonia implexicoma and grasses including M. stipoides and Rytidosperma racemosum.
Study species
The mainland subspecies of the eastern barred bandicoot (Perameles gunnii) is a 750 g (Seebeck 2001) ground-dwelling marsupial that falls within the critical weight range (CWR) (Burbidge & McKenzie 1989) and previously inhabited mesic ecosystems across Victoria. This species is listed as extinct in the wild under the Advisory List of Threatened Vertebrate Fauna in Victoria 2013, as a result of predation by introduced predators and the extensive loss of its natural grassland/grassy woodland habitat in the Victorian Basalt Plains (Dufty 1994; The State of Victoria Department of Sustainability and Environment 2013). Currently, the eastern barred bandicoot only survives in captivity, in predator-barrier fenced sanctuaries or on predator-free islands (Coetsee 2016; Parrott et al. 2017). Eastern barred bandicoots dig small conical pits (average 2–4 cm wide and 3–5 cm deep) in the soil when foraging for subterranean invertebrates (Dufty 1991; Mallick et al. 1997) and prefer to nest in structurally complex habitat but forage in open grassy areas (Dufty 1994; Winnard et al. 2013).
To assist this species’ recovery, 16 individuals (eight males and eight females) were released onto predator-free Churchill Island (57 ha), Victoria, Australia, in August 2015, and an additional 4 in October 2015 (Rendall et al. 2018). Population estimates for the island now exceed 120 individuals (D. Sutherland, unpublished data, 2018). Historical accounts indicate eastern barred bandicoots have never been found on Churchill Island; however, another bandicoot species (species unknown) was observed on Churchill Island, therefore reintroducing a digging mammal onto the island could help restore ecosystem processes with an analogue species (Grant 1803).
Investigating foraging activity
Foraging pit density
Bandicoot foraging pits were counted in 100-m2 plots (dimensions: 25 m × 4 m) over Churchill Island to investigate the number of excavations created per night. Comparisons between the two major habitat types were made in order to detect the effect spatial variation and different vegetation had on foraging activity. Through power analysis, it was deemed appropriate to sample 45 plots in order to successfully detect differences in foraging activity over the island. Sample sizes were proportional to the available area in each habitat type; 30 plots were allocated to the open pastures, and the closed woodland habitat had 15 plots (Fig. 1). Using Manifold GIS, plots were systematically stratified over the island from a random origin (Fig. 1) and had pre-determined random directional bearings to decide the way each plot ran.

The number of foraging pits created in 24 h was recorded by sampling within the 100-m2 plots. Two transects (2 m × 25 m) covering each half of the plot were walked, and pre-existing foraging pits encountered were marked with flagging tape on the top of a small piece of wire which was inserted into the ground adjacent to the digging. After 24 h, each new, unmarked foraging pit with a distinct spoil heap (pile of ejected soil) was recorded. Small investigative nose pokes were excluded. Churchill Island is rabbit-free and has no other digging species that bandicoot foraging pits could be mistaken for. This was repeated once a month over the months of April, May and June, 135 separate counts in total, to allow for temporal variation in foraging activity. This timeframe, however, is too short to determine seasonal variation in foraging activity. The overall average number of foraging pits within a plot was extrapolated across the landscape to estimate how many digs would be created in one hectare over 24 h.
The study primarily focused on the two major habitats on the island: closed woodland and open grazed pasture. However, to examine the effect human influences might have on bandicoot foraging behaviour, a third habitat type, open ungrazed/undisturbed habitat (Fig. 1), was added to the field data collection in May and June for comparison with the closed woodland and open grazed pasture. Using a haphazard approach, an extra four sites were randomly selected from a mapped extent of this area to examine the number of foraging pits created per night in the ungrazed/undisturbed habitat (Fig. 1).
Foraging pit morphology
Plaster of Paris was used to make moulds of a subsample of 40 fresh foraging pits from the closed woodland and open grazed pastures (n = 80; 40 moulds from each habitat). These foraging pits were haphazardly selected but spread evenly across the island to avoid bias in pit size. Pits were selected based on having a conical shape with average length and width measurements at the soil surface. The dimensions of an average foraging pit were determined by measuring the width (at the soil surface) and the depth of these moulds. The average volume of a pit was obtained by placing each mould into a known volume of water (600 mL) and measuring the amount of water (mL) displaced upon mould submersion. To examine differences in foraging pit morphology between the two main habitat types (closed woodland versus open grazed pastures), the width (mm) and depth (mm) of foraging pit moulds were compared using a one-way analysis of variance (anova) within the R statistical environment (version 3.4.1) (R Core Team 2018). Volume (mL) of digs was dependent on the variable shapes and depth of digs, resulting in positively skewed data. Therefore, a generalised linear model with a Poisson distribution was used; however, this was found to be over dispersed (dispersion = 6.85) and a negative binomial distribution was considered more appropriate (dispersion = 1.13; Venables & Ripley 2002).
Spatial and temporal variation in foraging activity
The number of foraging pits created each night was compared between all habitat types (closed woodland, open grazed pastures and open ungrazed) and among month of survey (April, May and June). These data had a Poisson distribution however when the distribution was applied to a generalised linear model, it was over dispersed (dispersion = 9.89). A negative binomial distribution was found to be more appropriate (dispersion = 1.05).
Plot-level soil displacement
To investigate whether the amount of soil displaced from the 100-m2 plots in each habitat type (closed woodland and open grazed pasture) differed, the mass of soil displaced per plot was estimated. The average foraging pit volume from each habitat type was multiplied against each plot-level foraging pit count to quantify the plot-level volumes of soil removed (e.g. to obtain this data set for the open grazed habitat – the average number of digs in open habitat plots was multiplied by the average volume of pits in the open habitat. The same was done for the closed habitat). Volume of soil removed per 100 m2 (log10 transformed data) was compared between habitat types (closed woodland and open grazed pasture) with a one-way anova.
Soil properties
We examined soil strength, water infiltration rates and soil moisture levels to determine the effects of soil disturbance by bandicoots. Ten fresh foraging pits (<72 h; Fig. 2a) and ten old foraging pits (>2 weeks; Fig. 2b) were haphazardly selected from each habitat type. Each foraging pit was paired with an adjacent undug control site, each pit was at least 0.5 m from the undug soil testing site to avoid confounding factors. Each foraging site had no other bandicoot disturbances within a one-metre vicinity to prevent confounding effects (Valentine et al. 2017). Within each foraging pit, the soil properties were measured at three microsites (located on top of the spoil heap, midway down the slope of the pit and at the base of the pit). In the undug paired control sites, tests were conducted at a single random location. There was no rain in the three days prior to testing these soil properties to ensure precipitation was not influencing results. Each soil property was tested on a single occasion within the same day to obtain the most accurate results.

Soil moisture content
A Lincoln 24-inch soil moisture metre was used at a depth of 50 mm within the undug soil and within fresh and old foraging pits to determine whether bandicoot foraging altered soil water-holding capacity (Valentine et al. 2017). As these data had a Gaussian distribution, soil moisture between dug and undug soil was compared using a linear mixed effects model.
Soil hydrophobicity (water repellence) was measured between dug and undug soil; however, due to a large amount of rainfall prior to data collection, all soils were hydrophilic, resulting in little variation in the data so no comparisons were possible (see Appendix S1, S2, S3 & S4).
Soil strength
To determine whether bandicoots altered soil strength, a Humboldt H4200 soil penetrometer was used at each microsite in fresh and old pits and the nearby undug soil to measure the amount of force (measured in kg cm−2) required to break through the soil surface (Bancroft et al. 2005). These data were zero-inflated; therefore, a zero-inflated generalised linear mixed effects model was run in the ‘GLMMadaptive’ package (Rizopoulos 2019). Soil strength (converted to g cm−2) was compared under each treatment, with microsite included as a zero-inflated fixed effect.
Model selection process
Numerous variables were considered to play a role in the soil condition. To determine how bandicoot activity influenced the soil, a model selection process was used to highlight what factors were the most influential (Table 1). All soil property models only contained data collected from the closed woodland and the open grazed pastures, and excluded the open ungrazed habitat data, as data from this habitat type were collected at a later stage. Habitat type (open/closed), pit age (fresh/old) and microsite (spoil heap, midway down slope, pit base and undug) were fixed factors indicated by a priori knowledge. Interaction terms between the parameters, habitat and microsite, age and microsite, were included in the global models to begin the model selection process. A random blocking factor of soil test site ID was used to account for paired sites that may have spatially similar soil properties.
| Model | d.f. | AICc | Δi | wi |
|---|---|---|---|---|
| Soil moisture | ||||
| Habitat + Age + Microsite + Habitat:Microsite + Age:Microsite | 14 | 609.1 | 0 | 0.659 |
| Habitat + Age + Microsite + Age:Microsite | 11 | 610.5 | 1.36 | 0.334 |
| Habitat + Microsite + Habitat:Microsite | 10 | 618.0 | 8.87 | 0.008 |
| Soil strength | ||||
| Habitat | 8 | 1513.7 | 0 | 0.916 |
| Habitat + Microsite | 11 | 1518.8 | 5.05 | 0.073 |
| Habitat + Microsite + Age | 12 | 1522.6 | 8.85 | 0.011 |
The global model was reduced in a backwards stepwise process, decreasing the number of interactions or fixed factors included, by dropping predictors with the smallest (non-significant) F statistics (Quinn & Keough 2002). Models were selected and ranked according to Akaike’s information criterion corrected for small sample sizes (AICc), delta AICc (difference between AICc values = Δi) values and Akaike weights (wi) (Wagenmakers & Farrell 2004). The assumptions and goodness of fit of all statistical models used in this study were assessed by testing for over or under dispersion and constructing Q-Q plots, boxplots and inspecting the spread of fitted values vs. residuals to assess homogeneity of variance.
Population estimate
The abundance of bandicoots was estimated using the capture histories of marked individuals from live trapping over four consecutive nights (13–16 June 2017), using robust design mark–recapture models (Pollock et al. 1990). Wire cage traps (length × width × height: 50 × 18 × 20 cm) were systematically set across the island and baited with a rolled oats, peanut butter and golden syrup mixture. The health and condition of captured bandicoots was assessed, and each individual was marked with a passive integrated transponder (PIT) tag. Population abundance was estimated in the statistical package RMark (Laake 2013) that calls on the statistical program MARK (White & Burnham 1999).
Soil bulk density
To calculate the average bulk density of the soil and determine the mass of soil ejected by bandicoots when digging, cores of soil samples (n = 20) of a known volume (40 mm × 100 mm) were taken from haphazardly selected sites across the island (10 cores from the closed habitat and 10 from the open). These were oven-dried for 72 h at 105°C, and the dry mass was weighed and divided by the volume of the soil core (Brown & Wherrett 2017).
Quantifying soil turnover rate
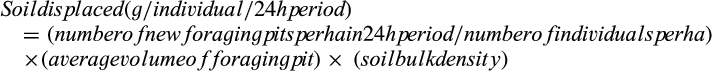
Results
Investigating foraging activity
Foraging pit density
Between zero and 64 new foraging pits were created per night within a plot, and the number of new foraging pits varied throughout survey months. After 24 h in April, there was an average of eight (95% CI = 6–10) new foraging pits per plot, in May there were 16 (95% CI = 11–21) and June averaged 10 (95% CI = 7–12). The mean number of foraging pits created in 24 h within 100 m2 throughout the entire study period, regardless of habitat type, was 11.3 (95% CI = 9–13), which extrapolated to 1113 new foraging pits per hectare. With a population density of two bandicoots per hectare, this meant that each individual made ~487 new foraging pits over the hectare. As eastern barred bandicoots are strictly nocturnal, it was assumed that there are approximately 12 suitable foraging hours (Dufty 1991, 1994); therefore, the estimated digging rate for winter is 40.6 (95% CI = 34.6–43.8) small foraging pits per hour.
Foraging pit morphology
Pit width differed between habitat types (F1, 78 = 60.16, P < 0.001). In open pasture, foraging pits were 32 mm (95% CI = 29–35 mm) wide compared with the closed woodland where pits were 48 mm (95% CI = 45–51 mm) wide. Habitat type also affected foraging pit depth (F1, 78 = 30.64, P < 0.001), where foraging pits in the grazed pastures were approximately 34 mm deep (95% CI = 30–37 mm) and in the closed woodland habitat 47 mm deep (95% CI = 43–50 mm). Foraging pits in the woodland habitat (mean vol: 42 mL; 95% CI = 37–48 mL) were more than double the average volume (Z = −8.95, P < 0.001) than those in the open grazed pasture (mean vol: 17 mL; 95% CI 15–20 mL). Regardless of habitat type, the average foraging pit depth was 40.01 mm (95% = 37.38–42.82 mm) and width at soil surface was 39.75 mm (95% = 37.06–42.44 mm), having an average volume of 29.63 mL (95% = 25.21–34.04 mL).
Spatial and temporal variation in foraging activity
Spatial variation in digging activity was found between the three habitat types. The open grazed pastures contained 50% more foraging pits than the closed woodland habitat (Z = 2.26, P = 0.02; Fig. 3). The undisturbed habitat had 96% more foraging pits created compared with the closed woodland habitat (Z = 1.69, P = 0.09) and 31% more than the grazed pastures (Fig. 3). Pairwise comparisons indicated that human/stock disturbance had minimal effects on habitat use, as no statistical difference between foraging pit numbers in the two open habitats were detected (Z = 0.59, P = 0.55). However, power was low due to the small number of ungrazed, open sites sampled. Temporal variation in digging rates between survey months was not significant. Statistical significance was only apparent when comparing the months of May and April (Z = 3.38, P < 0.001) with 97% more foraging pits excavated in May (Fig. 3).
Plot-level soil displacement
The total volume of soil displaced from each plot was 38% lower for plots in closed woodland habitat than plots in the open pasture. High variability in foraging activity within each of these habitat types meant there was no statistical difference in the quantity of soil displaced between open grazed pastures and closed woodland plots (F1, 133 = 3.21, P = 0.08).
Soil properties
Soil moisture content
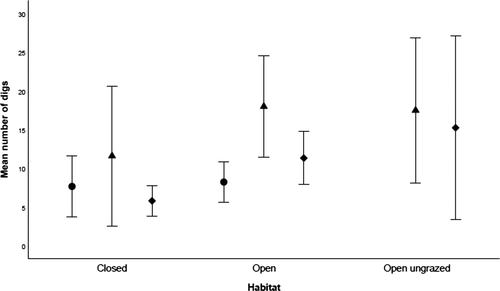
There was model selection uncertainty with the top two soil moisture models receiving support and the only variance between these two models was the removal of the habitat and microsite interaction (Table 1). The global model was selected as the most appropriate. Habitat type affected soil moisture levels on Churchill Island, as the model without the habitat parameter had less support (Δi = 25.93—where Δi is the next ranked model without the respective parameter present). Undisturbed soil in the open habitat was up to 14% drier than the soil in the foraging pit. In the closed woodland habitat, pits contained 30% more moisture than the surrounding undug soil. Soil moisture varied between microsite locations along the foraging pit profile, and this was an important parameter in explaining moisture levels (Δi = 37.85; Fig. 4). The most significant variations in soil moisture were found when comparing the spoil heap to all other microsite locations. The spoil heap consistently recorded the lowest moisture levels, with soil moisture increasing down the pit profile (Fig. 4). When comparing the spoil heap to microsites within the foraging pit, midway down the slope of the pit had 67% more moisture and the pit base contained 91% more moisture. Foraging pit age was also an important predictor of soil moisture (Δi = 8.87; Table 1). The interaction between microsite and age was apparent (Δi = 13.01), particularly when pairwise comparisons contained fresh spoil heaps, as they had less moisture than all other combinations. The model selection uncertainty stemmed from the interaction between habitat and microsite location. This interaction affected soil moisture variation, although support for its inclusion in the top model was not strong (Δi = 1.36; Table 1).
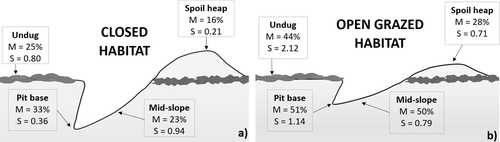
Soil strength
The soil strength top model, which contained the parameter habitat, was strongly supported (Δi = 5.05; Table 1). The undug soil in grazed pasture (where stock frequented) demonstrated greater penetration resistance (2.1 kg cm−2, 95% CI = 1.5–3.0 kg cm−2) than the soil in the closed woodland (0.8 kg cm−2, 95% CI = 0.6–1.1 kg cm−2; Fig. 4). Age of foraging pits did not influence soil compaction, as when this factor was included into the model, it obtained less support (Δi = 8.85; Table 1). The zero-inflated fixed effect component of the model was supported in the model. Undug microsites were found to have less zeros (P < 0.05) than either the base, midpoint or spoil heap (P > 0.05). For example, more than double the amount of force was required to break through the undisturbed soil surface (1.3 kg cm−2, 95% CI = 1.0–1.7 kg cm−2) than the soil in the pit base (0.6 kg cm−2, 95% CI = 0.4–1.0 kg cm−2). Interactions between age and microsite, as well as age and habitat, were included in the global model; however, this model received little support (Δi = 33.94).
Population estimate
Based on a population estimate of 116 individuals (95% CI = 107–136), there was a population density of 2.03 bandicoots per hectare, over the 57 hectares.
Soil bulk density
Soil bulk density at Churchill Island was habitat-dependent, changing from 0.94 g cm-3 in the woodland habitat to 0.88 g cm-3 in the open pastures. The overall average soil bulk density used in the final turnover rate equation was 0.91 g cm-3.
Soil turnover
In a single night of the study, the average soil mass displaced by a bandicoot (averaged across habitat type) was 13.15 kg (95% CI = 11.2–14.2 kg). This equates to a monthly soil turnover amount of ~ 400 kg per individual (95% CI = 342–433 kg). Assuming there is no climatic, temporal or spatial variation in foraging activity, the annual turnover rate of an individual eastern barred bandicoot on Churchill Island is 5.27 m3 or 4.8 tonnes (95% CI = 4.1–5.2 tonnes).
Discussion
Ground-dwelling, digging mammals have sustained heavy losses in Australia, and the significance of this for ecosystem function is of considerable conservation and management concern (Fleming et al. 2013). We found that in winter, eastern barred bandicoot diggings were relatively small in size, but numerous. Diggings reduced soil compaction (particularly in pastures) and increased soil moisture content (especially in the drier woodland habitat). Our results suggest eastern barred bandicoots, such as other ecosystem engineers, play an important role in affecting soil properties. This highlights the value of retaining these species within ecosystems and can be used to help inform their return via conservation translocations, which could in turn help promote ecosystem restoration.
Soil turnover
Knowledge regarding the individual digging rates of Australian mammals is limited, providing little opportunity for direct comparisons. However based on the lower confidence interval of 4.1 tonnes of annual soil turnover on Churchill Island, eastern barred bandicoot turnover appears similar to other Australian digging species of similar body size and feeding habit, such as southern brown bandicoots (~45 pits/24 h = 3.9 tonnes annually) or brush-tailed bettongs (38–114 pits/24 h = 4.8 tonnes annually; Garkaklis et al. 2004; Valentine et al. 2013). Comparisons with other mammal species globally show some large differences, but we caution that the potential effects of environmental differences on such observations must be acknowledged. For example, a single heteromyid rodent (Dipodomys spp.) in the Chihuahuan Desert, USA, is capable of displacing 7.2 tonnes of soil annually (Eldridge et al. 2012) and cape porcupines (Hystrix africaeaustralis), a moderately large herbivore (12–24 kg) in South Africa, annually displace a total of 1.6 m3 of soil (Bragg et al. 2005). The similarity in turnover quantity between eastern barred bandicoots and other Australian digging species indicates that impacts on soil caused by their digging activity might be similar to other native species; however, they may differ in part due to unique pit characteristics (i.e. small pits but high in numbers and benefits may be relative to foraging pit volume) (Fleming et al. 2013).
Our turnover estimate assumes no seasonal variation in digging activity, and replicating this study at other sites was beyond the capacity of the project. Therefore, this study’s results are likely to be an overestimation for the species, are only directly applicable to the population at Churchill Island and should be considered with caution and not generalised to other seasons or sites. Rainfall during our study is likely to have affected foraging activity of bandicoots. Given the circumstances on Churchill Island at the time of the study, our turnover estimation appears feasible for this population. Eastern barred bandicoot foraging pit density interacts seasonally with soil compaction, causing pit numbers to increase with decreasing soil compaction as it is less energetically costly to dig in wetter, softer soils (Winnard 2010). The high amount of rainfall that fell at the time of the study likely caused the soil to be soft, enabling easier foraging. Above-ground prey items for greater bilbies (M. lagotis) are known to increase in summer months (Gibson 2001) and seasonal shifts in diet and digging activity has been documented for other soil-disturbing species such as the honey badger (Mellivora capensis) (Begg et al. 2003). Seasonal changes such as these may also occur on Churchill Island with dietary shifts potentially causing significantly less soil turnover in summer months. A reduction in bioturbation in warmer, drier months may also be a result of more compact soils, which are physically harder to turnover.
During our study, it is thought the density of bandicoots on the island was approaching carrying capacity (D. Sutherland, unpublished data, 2017), which might also explain the high soil turnover rate. Southern brown bandicoots at high densities have overlapping home ranges, leaving multiple individuals creating foraging pits in a single home range (Dickman & Broughton 1991). Home ranges of individual eastern barred bandicoots on Churchill Island are also known to overlap, particularly for males who share almost half of their range with other males (Rendall et al. 2018); thus, it is likely that multiple individuals are contributing to the 487 pits per hectare.
Variation in bandicoot foraging activity
Environmental factors appeared to influence variation in foraging and associated pit sizes on Churchill Island. In the woodland habitat, the bigger diggings may be explained by less compact, drier soil which is physically easier to turnover and possibly contains invertebrates deeper in the soil profile (Staley et al. 2007). Pastures may provide easier, more successful foraging opportunities as obstruction from dense vegetation is reduced, allowing easier manoeuvrability and prey detection. This pattern has previously been observed with long-nosed bandicoots (P. nasuta), where foraging pit occurrence increased with decreasing ground cover (Claridge & Barry 2000; Chambers & Dickman 2002). A similar trend in eastern barred bandicoot foraging has previously been highlighted, where digging numbers increased in more open habitat (Winnard et al. 2013). A pattern of smaller pit sizes in the open habitat was detected, similar to the foraging pit characteristics of Tasmanian eastern barred bandicoots (Mallick et al. 1997). Although pits were smaller in the open habitat, disturbance levels in such environments do not appear to have a negative impact on foraging frequency, with digging densities still high in the open pastures that were grazed by stock or regularly impacted by humans (e.g. weed spraying).
Temporal variation in foraging and soil turnover across the year is likely, due to changes in the softness/hardness of soil and the location of prey in or on it (deeper/shallower/surface) (Anderson & Smith 2000). A high rainfall period in late April may have increased prey abundance and availability in our study. Another possibility may be that the beginning of winter, and cooler temperatures, may have triggered the onset of breeding and shifting energy requirements, a correlation previously observed (Winnard 2010). This may explain the fluctuations seen in foraging activity over this short time period. Specific examples of temporal variation in digging activity are limited; however, documenting turnover rates of some species over time has revealed seasonal fluctuations. Brush-tailed bettong soil turnover, over two years, ranged between 2.7 and 9.7 tonnes (Garkaklis et al. 2004), and heteromyid rodents in the Chihuahuan Desert commonly increase soil turnover following high rainfall periods (Eldridge et al. 2012).
Bioturbation affecting soil properties
The extent to which ecosystem engineers influence ecosystems can vary across environmental gradients (Crain & Bertness 2006). In xeric environments, increased water infiltration may make a substantial difference to plant survival and growth, as soil nutrients are more easily absorbed when in solution (Chapin 1980). The subtle effects of bandicoot digging on soil moisture observed were probably due to the mesic conditions of this site, with high initial soil moisture leaving little opportunity for moisture to be further increased. In drought periods or drier years, foraging pits may become more important for plants, soil and invertebrate communities. Bioturbation positively affected soil compaction and soil moisture (particularly in the open grazed pastures), similar to the effect of black-tailed prairie dogs (Cynomys ludovicianus) and northern pocket gophers (T. talpoides; Day & Detling 1994; Butler & Butler 2009). This may play an important role in the agricultural industry, as increased water infiltration via diggings, could assist pasture growth and health, reduce topsoil runoff and be an effective way to help mitigate the effects of stock trampling and soil compaction. Critical weight range mammals could provide considerable ecosystem services to a range of industries, including farming, which highlights the value of maintaining these species and assisting their recovery within the landscape. With more of these conservation translocations, there is the opportunity to preserve and recover many species and to simultaneously restore the health of degraded landscapes, including production areas, potentially conferring a suite of benefits to both natural and more disturbed areas.
Management implications and further research
There is increasing support for the reintroduction of ecological engineers, including digging mammals, as a way to promote ecosystem recovery and health (Fernández et al. 2017). To achieve this, demonstrating how different species and their ecological functions affect ecosystems is required. Re-establishing ecosystem engineers as an approach to rewild (restore ecological functions via species) and restore ecosystems is becoming a key focus for conservation globally (Seddon et al. 2014; Fernández et al. 2017). Our study outlines some of the effects eastern barred bandicoots appear to be having in a mesic, insular system. Like other species, the effects of eastern barred bandicoots on ecosystems will likely vary across environmental gradients (James & Eldridge 2007) and through time (due to seasonal variation), something that requires more examination and comparison geographically and seasonally. This knowledge will assist management decisions regarding translocations elsewhere, as part of this species’ planned recovery. Adverse impacts of eastern barred bandicoot digging activity were not detected, and benefits to soil properties were evident. However, their effects on other environmental features (e.g. invertebrate communities), via foraging, remain poorly understood. We suggest that translocations of ecological engineers, including in some cases beyond their known historical geographic distributions, could be a way to assist ecosystem and species recovery.
Acknowledgements
This project was undertaken in accordance with the regulations of Phillip Island Nature Parks Animal Ethics Committee (Project No. 3.2015) and in accordance with the Department of Environment, Land, Water and Planning (DELWP), Research Permit No. 10007613. We thank Deakin University, Phillip Island Nature Parks and Zoos Victoria for providing financial and logistical support for the project. We are grateful for the field volunteer's assistance during data collection and the support and hospitality recieved from the land managers at Churchill Island. Leonie Valentine was supported by the Australian National Environmental Science Program – Threatened Species Recovery Hub (NESP – TSR).




