Phenological modularity in amphibian calling behaviour: Geographic trends and local determinants
Abstract
Phenology of species, the coupling of vital activities to specific times of the year, plays a main role in ecosystem functioning and is expected to be affected by global change. We analysed the temporal structure of 52 amphibian communities in South America encompassing a latitudinal range from 7º to 34º south. Phenological modularity – species tendencies to aggregate along the months – is here introduced as a ubiquitous property of biodiversity architecture. Further, we identified an increase in phenological modularity with species richness, available energy and in communities with lower thermal dependence (i.e. the rate of change in the number of species active along the year associated with the environmental temperature). These patterns are in agreement with predictions derived from several ecological hypotheses: complexity-stability, species-energy and metabolic ecology. However, no direct association between modularity and the phylogenetic structure of communities was observed. A structural equation model that outperformed all the plausible alternative models considered supports these results. Modularity is reported here as a main feature of the phenology of communities that depends on environmental conditions. Here, we report for the first time a putative connection between community species richness and the degree of temporal structure – phenological modularity; the thermal dependence shows that communities at low latitudes are more vulnerable to climate change; energetic environments also promote communities with phenological modularity; and latitudinal patterns of phylogenetic community structure can give us clues of which species would be important to the conservation of community processes. These results call for further theoretical analyses to support the connection between phenological modularity, community stability and vulnerability to global change.
Introduction
The need for a better understanding of the interplay between environmental conditions and the architecture and functioning of biodiversity is a pressing challenge in ecology (McCann 2007; Naeem et al. 2012; Rohr et al. 2014). The temporal dimension has been early recognised as an axis of community structuring (Schoener 1974a,b; Jaksic 1982; Kronfeld-Schor & Dayan 2003). In this vein, a central component of the biodiversity architecture is the phenological structure (Visser et al. 2010). Phenology of species involves the temporal coupling of vital activities – that is reproduction, migration – to environmental conditions (Visser et al. 2010). Phenology is closely associated with climatic variables, which makes it vulnerable to global climate change processes (Scheffers et al. 2016; Kronfeld-Schor et al. 2017; Vázquez et al. 2017). Temperature has a main role in the functioning of all biological systems (Angilletta 2009). The thermal dependence of metabolic rate determines changes in behaviour of individuals and physiology that scale up to community structure and function (e.g. Gillooly et al. 2001; Allen et al. 2002; Dell et al. 2011). Consequently, seasonal trends in temperature are the main determinants of phenological patterns (Angilletta 2009; Steen et al. 2013; Tonkin et al. 2017). Therefore, unravelling the connections among thermal dependence and community structure is emerging as a key objective in different areas of ecology (Dell et al. 2011, 2014; Araújo et al. 2013; Pawar et al. 2016).
The thermal dependence of amphibian species phenology was recently associated with environmental gradients. The activities of individuals become more dependent on the temperature at lower latitudes, with higher local productivity, and are also related to local species richness (Canavero et al. 2018). These results and the evidence for an association between the phylogenetic composition of communities and thermal dependence indicate its connection with other features of community structure. The representation of phenology as a bipartite network of species registered at different times was proposed as a fruitful approach for the analysis of the time dimension of community architecture (e.g. Canavero et al. 2009; Aizen & Rovere 2010; Forrest & Miller-Rushing 2010; Biella et al. 2017). A modular organisation of ecological networks has been identified as a recurring feature of mutualistic, host-parasite, trophic and phenological networks, closely related with the stability of communities (Olesen et al. 2007; Tur et al. 2015; Biella et al. 2017). Modular structure implies the existence of link-dense regions representing a group or module of species that interact more strongly among themselves than with the rest of the network. The degree of this organisation type is named the modularity of the network (Olesen et al. 2007). The opposite pattern, antimodular, could be also expected when the interactions are more frequent between groups than within groups (Hintze & Adami 2010; Grilli et al. 2016). A modular organisation in amphibian phenology would indicate the existence of groups of species with a higher tendency to be active at the same time in comparison with the other species in the community (Canavero et al. 2009). Niche conservatism and phylogenetic signal in the breeding period could determine that related species are active under similar conditions (Canavero et al. 2018). Consequently, the phylogenetic composition of communities may be also be related to the strength and nature of its modular organisation (e.g. Rezende et al. 2009; Krasnov et al. 2012; Peralta 2016).
In this context, it is expected that thermal dependence, modularity and phylogenetic structure should be three main and interrelated components of the phenology of amphibian species (Peralta 2016; CaraDonna et al. 2017; Canavero et al. 2018). Specifically, the following hypotheses were considered: latitudinal gradients in climatic conditions and community structure are pervasive in nature (Brown 2014), and despite multiple attempts to relate single environmental variables with biological trends, large-scale geographic gradients typically involve changes on several abiotic and biotic variables, mutually correlated (Storch 2012).
Consequently, the emerging prediction from this hypothesis is that community structure may be related to this general latitudinal gradient of abiotic and biotic variables (Fig. 1 H1) (Canavero et al. 2009). Statistically, this covariation can be represented as a ‘latent variable’, a variable that is not directly measured but it is associated to several observed variables – similar to the axis of a principal component analysis (Shipley 2016). Phenological modularity may be part of this general gradient and/or be directly affected by independent variables.
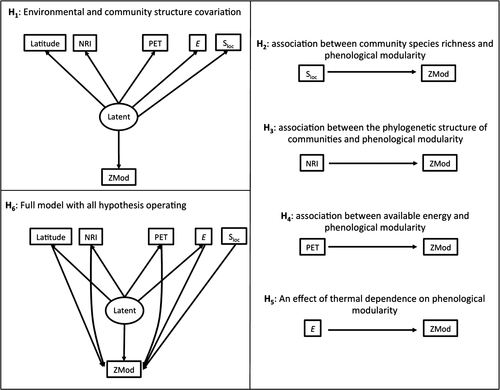
The next hypotheses refer to these direct connections. The second hypothesis points to the expected association between community species richness and modularity (Fig. 1 H2). Since the seminal works of May (1972), it was stated that the stability of communities that increase in species richness might depend on the increase in modularity (see also Stouffer & Bascompte 2011).
The third hypothesis predicts an association between the phylogenetic structure of communities and phenological modularity (Fig. 1 H3). The phylogenetic similarity among species may reflect trait similarity and consequently similar response to environmental variables and species interactions (Cadotte & Tucker 2017). This may determine the temporal aggregation of species activity reflected in a modular organisation of community phenology (Canavero et al. 2009).
The fourth hypothesis predicts an association between available energy and modularity (Fig. 1 H4). Available energy may foster or preclude species coexistence (Hawkins et al. 2003; Steen et al. 2013; Leibold & Chase 2017) and also affects the calling behaviour amphibians (Ziegler et al. 2016). These effects may determine aggregation or overdispersion of species activity along months, and consequently, the strength of the modular organisation of amphibian phenology.
Finally, the fifth hypothesis predicts the effect of thermal dependence on modularity (Fig. 1 H5). In the phenology of amphibian species, thermal dependence on species richness mainly describes the metabolic response to the seasonal changes in temperature (Allen et al. 2002; Dell et al. 2014; Canavero et al. 2018). This response involves a combination of nested diversity and species replacement between months (Canavero et al. 2009). Both nestedness and species turnover are directly connected with the strength of modularity (Fortuna et al. 2009; Ortiz & Arim 2016).
These hypotheses were translated to casual determinants of phenological modularity on amphibians’ calling activity (Fig. 1 H6). Alternative causal connections were evaluated with structural equation models using 52 local communities from tropical to temperate locations (see Appendix S1: Fig. S1). A single structure that outperforms the entire set of alternative hypotheses was identified.
We focus on amphibian phenologies, which present a number of advantages to approaching this aim (Wiens et al. 2010; Schleuning et al. 2014). First, herpetologists have reported annual trends in activity for several locations, covering a wide biogeographic range in the Neotropics (see Canavero et al. 2009, 2018). Second, the evolutionary history of amphibians in this region is relatively well-known (Pyron & Wiens 2011) allowing for quantification and comparison of the phylogenetic community structure (e.g. Wiens et al. 2006). Third, amphibian activity and performance are closely related to environmental conditions. Specifically, amphibians are ectotherms with moist and naked skin, and aquatic reproduction, which is consequently coupled with weather conditions (Wells 2007); calling behaviour of anurans has a thermal dependence, an order of magnitude larger than that reported for other activities or taxonomic groups (Canavero et al. 2018); and the global decline in amphibian species was related to local and global environmental changes more than other taxa (Wake & Vredenburg 2008; Grant et al. 2016).
Methods
Phenological modularity and its relationship with environmental conditions and community structure were analysed across 52 local communities in the Neotropics (compiled in Canavero et al. 2009, 2018). The latitude, potential evapotranspiration, total species richness (the sum of all species that called at least once during the study period in a local community), an index of phylogenetic community structure and a normalised modularity index were calculated for each local community.
We considered two local environmental variables: latitude and potential evapotranspiration (PET). The first could better represent climatic conditions and biotic variables than any other single variable (Rohde 1992; Willig et al. 2003). We obtained the latitude directly from the papers of each database (Table 1). The PET is considered as a proxy of the environmental available energy in the context of species-energy theory (e.g. Hawkins et al. 2003); it represents the local relationship between heat and relative humidity (Currie 1991). We obtained the local values of PET using the database presented with the software Spatial Analysis in Macroecology (Hijmans et al. 2005; Rangel et al. 2006).
| Locality | S loc | E | ZMod | NRI | PET | |
|---|---|---|---|---|---|---|
| Abrunhosa et al. (2006) | 22º50′S; 42º27′W | 19 | 2.319 | 1.64 | 0.481 | 82.002 |
| Afonso and Eterovick (2007) | 20°05′S; 43°29′W | 12 | 0.623 | 3.51 | 0.848 | 79.910 |
| Arzabe (1999) | 07°17′S; 37°21′W | 11 | 3.524 | −1.05 | −0.687 | 103.820 |
| Arzabe (1999) | 07°11′S; 37°19′W | 16 | 3.257 | −0.13 | −1.260 | 103.820 |
| Arzabe et al. (1998) | 11°20′S; 37°25′W | 17 | −1.033 | 5.72 | −0.512 | 115.073 |
| Ávila and Ferreira (2004) | 18°58′S; 57°39′W | 15 | 2.562 | −0.80 | −0.823 | 126.480 |
| Bernarde and dos Anjos (1999) | 23°27′S; 51°15′W | 18 | 0.754 | 2.24 | 0.457 | 81.373 |
| Bernarde and Kokubum (1999) | 21°16′S; 50°37′W | 19 | 1.735 | 0.14 | −0.186 | 98.930 |
| Bernarde and Machado (2000) | 25°27′S; 53°07′W | 20 | 0.997 | −1.57 | −1.647 | 69.233 |
| Bernarde (2007) | 11°35′S; 60°41′W | 33 | 0.354 | 6.63 | 0.033 | 87.138 |
| Bertoluci and Rodrigues (2002) | 23°38′S; 45°52′W | 28 | 0.951 | 4.23 | 2.462 | 77.917 |
| Bertoluci (1998) | 24°15′S; 48°24′W | 26 | 0.978 | 3.54 | 1.974 | 73.162 |
| Blamires et al. (1997) | 16°39′S; 48°36′W | 13 | 1.476 | 2.57 | 0.443 | 85.403 |
| Borges and de Freitas Juliano (2007) | 17º47′S; 49º23′W | 25 | 2.318 | 4.14 | −1.032 | 97.730 |
| Both et al. (2008) | 29°32′S; 53°47′W | 18 | 0.859 | −0.78 | −0.335 | 77.418 |
| Canavero et al. (2008) | 34°47′S; 55°22′W | 10 | 0.674 | 2.31 | −1.325 | 68.460 |
| Candeira (2007) | 20º20′S; 49º11′W | 24 | 3.181 | −2.81 | −1.293 | 95.515 |
| Canelas and Bertoluci (2007) | 20°05′S; 43°28′W | 32 | 1.606 | 6.80 | 1.321 | 79.910 |
| Cardoso and Haddad (1992) | 21°48′S; 46°35′W | 19 | 1.657 | −0.76 | −0.650 | 74.593 |
| Cardoso and Souza (1996) | 10°08′S; 67°35′W | 31 | 2.598 | −0.72 | 1.848 | 119.998 |
| Conte and Machado (2005) | 25°57′S; 49°13′W | 21 | 1.682 | 0.50 | 1.883 | 66.385 |
| Conte and Rossa-Feres (2006) | 25°41′S; 49°03′W | 31 | 0.847 | 2.51 | 0.836 | 66.385 |
| Conte and Rossa-Feres (2007) | 25º39′S; 49º16′W | 29 | 1.550 | −1.58 | 0.690 | 66.385 |
| Filho (2009) | 20°05′S; 56°36′W | 15 | 2.156 | 4.32 | −0.220 | 108.575 |
| Forti (2009) | 24°02′S; 47°53′W | 20 | 0.482 | 0.87 | 1.330 | 76.278 |
| Grandinetti and Jacobi (2005) | 20°07′S; 43°52′W | 11 | 0.167 | 1.33 | 1.300 | 79.910 |
| Heyer et al. (1990) | 23°38′S; 45°52′W | 35 | 2.972 | 0.76 | 1.343 | 77.917 |
| Kopp and Eterovick (2006) | 20°06′S; 43°29′W | 20 | 1.612 | 2.08 | 0.896 | 79.910 |
| Kopp et al. (2010) | 17°49′S; 52°39′W | 25 | 2.589 | −1.93 | −0.694 | 94.135 |
| Maffei (2010) | 22°48′S; 48°55′W | 39 | 1.345 | 7.74 | 0.188 | 83.813 |
| Moreira et al. (2007) | 29°42′S; 50°59′W | 15 | −0.721 | −4.13 | −0.737 | 72.685 |
| Narvaes et al. (2009) | 24°31′S; 47°16′W | 11 | 0.248 | −0.51 | 3.023 | 86.390 |
| Nascimento et al. (1994) | 20°00′S; 43°50′W | 9 | −0.455 | 2.72 | 0.147 | 79.910 |
| Nomura (2008) | 23º10′S; 46º31′W | 29 | 0.452 | 1.09 | 2.073 | 70.257 |
| Nomura (2008) | 20º21′S; 49º16′W | 23 | 1.898 | 4.57 | −0.745 | 95.515 |
| Nomura (2008) | 20º12′S; 50º29′W | 23 | 2.033 | 1.03 | −0.816 | 94.618 |
| Oda et al. (2009) | 14°09′S; 48°20′W | 21 | 4.958 | −5.07 | −0.628 | 90.233 |
| Papp (1997) | 22°52′S; 46°02′W | 13 | 1.211 | 4.13 | 1.883 | 74.198 |
| Pombal and Gordo (2004) | 24°25′S; 47°15′W | 23 | 0.92 | −0.12 | 1.752 | 86.390 |
| Pombal (1997) | 24°13′S; 48°46′W | 19 | 1.02 | 3.40 | 0.783 | 73.162 |
| Prado et al. (2005) | 19°34′S; 57°00′W | 23 | 1.574 | 10.00 | −0.372 | 125.963 |
| Prado and Pombal (2005) | 20°16′S; 40°28′W | 17 | 0.604 | 0.66 | 2.730 | 93.137 |
| Rossa-Feres and Jim (1994) | 22°59′S; 48°25′W | 25 | 0.481 | 4.09 | −0.342 | 83.813 |
| Santos (2009) | 08º43′S; 35º50′W | 28 | −0.221 | 0.86 | 2.377 | 93.912 |
| Santos et al. (2007) | 20°11′S; 50°53′W | 13 | 2.956 | 0.73 | −0.419 | 94.618 |
| Santos et al. (2008) | 29º42′S. 53°42′W | 24 | 0.535 | 4.70 | −0.977 | 77.418 |
| São Pedro and Feio (2010) | 20º31′S. 43º41′W | 28 | 0.676 | 0.70 | 0.789 | 79.910 |
| Silva (2007) | 20°20′S. 49°11′W | 18 | 2.437 | −0.53 | −0.914 | 95.515 |
| Teixeira (2009) | 22º59′S; 48°30′W | 15 | 1.094 | −0.48 | 1.572 | 83.813 |
| Toledo et al. (2003) | 22°25′S; 47°33′W | 19 | 1.825 | −0.35 | −0.716 | 80.825 |
| Vieira et al. (2007) | 07º25′S; 36º30′W | 15 | 3.184 | 2.25 | −2.938 | 95.245 |
| Zina et al. (2007) | 22°22′S; 47°28′W | 22 | 2.172 | 0.08 | −0.166 | 80.825 |
- Sloc, total number of species that call at least once in the study period. Included is the fit of the metabolic parameter E, calling activation energy (Canavero et al. 2018); NRI, net relatedness index; PET, potential evapotranspiration; ZMod, modularity index. Dark and light grey cells indicate significant Z values at P < 0.05 and marginal Z values at P < 0.1, respectively.
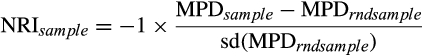
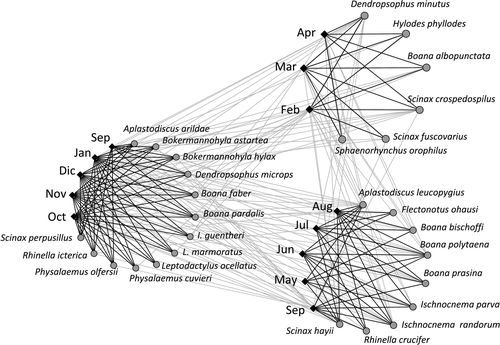
We constructed presence (1) – absence (0) matrices for each anuran community with months in columns and species in rows. These matrices represent bipartite networks wherein one level is represented by months – temporal dimension – and the other by anuran species that present calling activity (Canavero et al. 2009). At the temporal dimension, modularity identifies the existence of subsets of species whose probability of co-occurring across months is higher when compared with the probability of co-occurring with other species of the community (Olesen et al. 2007). In terms of the performance of the index, it was identified as a novel and robust method for estimating modularity in different networks, and its use has expanded in theoretical and applied ecology (e.g. Thébault & Fontaine 2010; Scheffer et al. 2012; Clune et al. 2013; Borthagaray et al. 2014; Takemoto et al. 2014; Grilli et al. 2016).
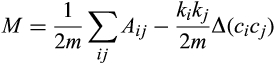
Structural equation modelling
Having evaluated the strength of modularity among 52 local communities, we focused on the interplay between responses to temporal gradients in local temperature (activation energy, E), available energy (PET), species richness (Sloc), phylogenetic community structure (NRI) and latitude. Understanding the effects of climate change on the community structure has been considered a challenge due to the lack of appropriate methodological approaches and databases (Vázquez et al. 2017). In this sense, communities with thermal dependence (i.e. significant thermal dependence, E) are the ones that could be directly affected by climatic change (Dillon et al. 2010). Specifically, we explore the putative connections between the strength of thermal dependence and the other components of community structure, in particular, phenological modularity. Towards this aim, we focused on the 34 communities reported by Canavero et al. (2018) that showed significant relationships between species richness and temperature (e.g. with significant calling activation energy, E). The putative causal connection between community structure with the thermal dependence of amphibians’ activity and the environmental characteristics was studied with path analysis (Shipley 2016).
We first estimated a latent variable (i.e. unobserved variable that is correlated with observed ones) related with a subjacent gradient of environmental conditions and with ecosystem structure. This latent variable was related to latitude, PET, phylogenetic species relationship (NRI), local Sloc, phenological modularity (ZMod) and calling activation energy (E). We used maximum likelihood methods and standardised coefficients in our path analysis. The overall path diagram and the significance of every single connection between a couple of variables were tested using the structural equation modelling (SEM). To assess the significance of the overall path model, we used a χ2 statistic computed from the departure between the observed and expected covariance matrix from the proposed path model (see Canavero & Arim 2009; Shipley 2016). A significant χ2 (P < 0.05) indicates that the model is not supported by the data. The explained variance for each endogenous variable is estimated as one minus the path coefficient between its associated error variable (Shipley 2016). Because our data matrix does not present multivariate normality based on kurtosis (using the mvnorm.kur.test function of the ‘ICS’ R-package, W = 27.4605, w1 = 0.48, d.f.1 = 35.00, w2 = 0.80, d.f.2 = 1.00, P < 0.05), we used the Satorra–Bentler robust estimation of the chi-squared statistic and standard error (Shipley 2016). This method corrects excessive kurtosis, problems in which the errors are not independent of their causal non-descendants, which is important for models with latent variables (Rosseel 2012). To identify plausible causal models, we considered 16 models covering the potential range of connections that could be expected in the study system (see Appendix S1: Fig. S1). All SEM models were fitted using the ‘lavaan’ R-package (Rosseel 2012) and compared by the Akaike's Information Criterion corrected for small samples (AICC); the lowest AICC values were selected (see Appendix S1: Table S1). Analyses were performed using R version 3.03 (R Core Team 2017).
Results
Modularity is observed as a recurrent feature in the organisation of amphibians’ phenology (Fig. 2). It was detected in 22 communities but covering a range from antimodular in three communities to significantly modular (see Table 1). This type of range from negative to positive deviation indicates that even non-significant modularities are part of a biological gradient, which requires attention (Ulrich & Almeida-Neto 2012). Similarly, of the 34 time series of calling anurans with significant thermal dependence, 17 yielded a ZMod modularity index that deviated from null expectations (P < 0.05), 16 on the positive side (i.e. significant phenological modularity) and one on the negative side (Table 1). Four communities presented significant deviations (P < 0.05) from null expectations of NRI and six presented marginal deviations (P < 0.10). Of the ten communities that presented marginal and significant deviations, seven showed phylogenetic attraction and three showed repulsion (Table 1). High variability is detected covering the possible extremes of indices, which is well accounted for by the path analysis. In fact, along with the 16 path analysis models considered (see Appendix S1: Fig. S1a,b), one presented a significantly better performance and no discrepancy with the observed covariation matrix (wiAICC = 0.571). When we only consider the models supported by data, the weighted wiAICC was 0.82 for the best model (Fig. 3). This model considered a latent variable directly related with a positive connection with latitude (r = 0.81) and NRI (r = 0.52), and a negative connection with E (r = −0.82) and PET (r = −0.70) (Fig. 3). This result partially supports H1 (Fig. 1) in terms of the expected covariation of several components of the environment, biotic and abiotic. Further, the path model also indicated an effect over ZMod from Sloc (r = 0.36) (Fig. 1 H2, a connection between complexity and community structure), PET (r = 0.56) (Fig. 1 H4, the energy availability foster the time partitioning) and E (r = −0.60) (Fig. 1 H5, communities with less thermal dependence will structure time dimension, species could use a wider time window along the year without restricting its activity to the warm period). This model accounted for 27% of the variation in phylogenetic relatedness (NRI), 42% of the ZMod variance and 68% of the variation in thermal dependence of the activity in amphibian communities (Fig. 3).
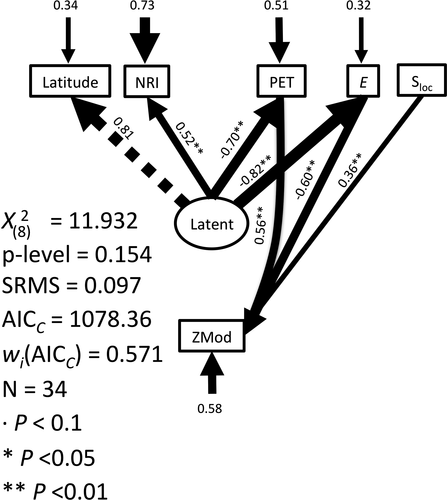
Discussion
Phenological modularity was identified here as a main feature of amphibians’ phenology. The anuran species may be interacting, adapting and evolving, modularly using the time as structuring axis on which the complexity of the system is expressed (Canavero et al. 2009). A temporally segmented organisation of amphibians’ activity is expected from classic and recent theories (Schoener 1974a,b; Jaksic 1982; Kronfeld-Schor et al. 2017). Specifically, the increase in modularity in interaction networks with species richness – that is complexity – is a long-standing prediction in ecology (May 1972; Stouffer & Bascompte 2011). Recent results further indicate that modularity may enhance species adaptability, improving long-term community stability (Scheffer et al. 2012; Clune et al. 2013). However, these theoretical expectations were not previously supported on empirical patterns of amphibians’ phenology. Here, we report the pervasive occurrence of modularity in the study system within a range from negative to positive deviation, which could indicate that even non-significant modularities are part of a biological gradient (Ulrich & Almeida-Neto 2012), and also its dependence on environmental conditions (Takemoto et al. 2014).
We advance on the connection between phenological modularity with other components of community organisation, such as available energy, thermal dependence of calling activity and species richness. The increase in modularity with available energy may be related with an increase in the number of active species with similar environmental requirements – modules – and/or an expansion of the time window of activity allowing different modules to be represented along the year (Currie 1991; Hawkins et al. 2003; Leibold & Chase 2017). We also found that thermal dependence of phenology – the rate of change in diversity with temperature along months – is related to the modular organisation of the amphibians’ phenology. Communities with large thermal dependence have lower phenological modularity. This finding may connect community phenology with climate change (Kronfeld-Schor et al. 2017; Vázquez et al. 2017). In this sense, it is noted that at higher latitudes, communities have lower thermal dependence but are more segmented in time use. Consequently, it may be expected that a rise in thermal variability and average temperature will produce a larger impact across lower latitudes. It may be highlighted that, on the basis of individuals’ thermal dependence, previous studies suggested a similar trend in the vulnerability of amphibian communities with latitude (Dillon et al. 2010). If modularity is positively related to stability (May 1972), the lower modularity associated with high thermal dependence may exacerbate the effect of climate change. However, the direct connection between modularity and stability may demand more theoretical and empirical analyses (Thébault & Fontaine 2010). The final direct connection with modularity involves a direct effect of species richness. The increase in species richness is associated with larger segregation of species along months, promoting a rise in phenological modularity (May 1972; Kronfeld-Schor & Dayan 2003; Canavero et al. 2009; Leibold & Chase 2017). This, in turn, may be related with the species segregation in time likely reducing interspecific competition (Schoener 1974a,b; Jaksic 1982; Scheffer & van Nes 2006).
Understanding the interplay among the organisms’ energetics, their thermal dependence and their consequences at higher levels of organisation is a pressing issue for ecology in a global warming scenario (Dillon et al. 2010; Dell et al. 2011, 2014; Vázquez et al. 2017; Canavero et al. 2018). The interpretation of activation energy in thermal biology is not restricted to a direct connection between temperature, metabolism and species coexistence (Allen et al. 2002; Dell et al. 2014; Segura et al. 2015; Canavero et al. 2018). Indeed, it represents a useful description of the association between temperature and community structure. This association was here related to phenological modularity, indicating that understanding the connection between environmental temperature organisms’ physiology and community assembly has to also consider the effect of temperature on the modular organisation of communities.
Although we did not find a direct connection between communities’ phylogenetic structure and phenological modularity, there is an indirect association probably mediated by the common response to a latitudinal trend in other variables. The existence of a non-random phylogenetic, as well as a modular organisation of local communities, is emerging as frequent properties of natural communities (e.g. Rezende et al. 2009; Krasnov et al. 2012; Peralta 2016). However, the connection between these two structural properties is just starting to be explored and deserves further attention. On the other hand, we found that communities with phylogenetically related species are observed at high latitudes where the environment is less productive and thermal dependence is lower. This result could be showing a connection between these species with the ability to be active with low activation energy and in environments that provide low energy (Wiens et al. 2006, 2010; Peralta 2016). Untangling which species are these and which roles they play on the phenological community structure is a relevant issue to develop conservation measures of community processes.
Phenology is a key component of community structure, shaping the strength and nature of biotic interactions, biodiversity and ecosystem functioning (Forrest & Miller-Rushing 2010; Biella et al. 2017). In a scenario of global change in climatic conditions and species diversity, understanding the interplay between environmental temperature, phenology, species richness and community structure has become a pressing issue (Dillon et al. 2010; Scheffers et al. 2016; Kronfeld-Schor et al. 2017; Vázquez et al. 2017). Here, we found a significant association among three main components of community structure: species richness, thermal dependence and phenological modularity. As is the case for most elements of community structure, available energy and thermal dependence directly foster the modular organisation of amphibians’ activity. Indeed, thermal dependence was found to be a parameter that can be used to track the effects of climate change on community structure (Dillon et al. 2010; Vázquez et al. 2017). If anything, our results reinforce the idea that the connection between modularity and environmental conditions is a ‘key area for active investigation’ (Takemoto et al. 2014).
Acknowledgements
We thank Arley Camargo for his contributions to the construction of the phylogenetic tree of the Neotropical anurans used in this work; we thank AnnelieseLaten for her contribution on English editing. We also thank two anonymous reviewers, whose suggestions have significantly improved this manuscript.AC received a fellowship from the Vicerrectoría Adjunta de Investigación y Doctorado-PUC, Chile; from the Programa de Desarrollo de las Ciencias Básicas (PEDECIBA, Uruguay); and from the Agencia Nacional de Investigación e Innovación (ANII, Uruguay). AC and MA thank the support of CSIC-grupos (Comisión Sectorial de Investigación Científica-UdelaR). MA thanks CSIC I+D 2016-id 333-Universidad de la República. AC and FMJ also received support from CONICYT PIA/BASAL FB0002. PAM acknowledges support from grant ICM-MINECON P05-002 and PFB-CONICYT P-023.




