Understanding predator densities for successful co-existence of alien predators and threatened prey
Abstract
The high failure rate of threatened species translocations has prompted many managers to fence areas to protect wildlife from introduced predators. However, conservation fencing is expensive, restrictive and exacerbates prey naïveté reducing the chance of future co-existence between native prey and introduced predators. Here, we ask whether two globally threatened mammal species protected in fenced reserves, with a history of predation-driven decline and reintroduction failure, could co-exist with introduced predators. We defined co-existence as population persistence for at least 3 years and successful recruitment. We manipulated the density of feral cats within a large fenced paddock and measured the impact on abundance and reproduction of 353 reintroduced burrowing bettongs and 47 greater bilbies over 3 years. We increased cat densities from 0.038 to 0.46 per square km and both threatened species survived, reproduced and increased their population size. However, a previous reintroduction trial of 66 bettongs into the same paddock found one red fox (Vulpes vulpes), at a density of 0.027 per square km, drove the bettong population extinct within 12 months. Our results show that different predator species vary in their impact and that despite a history of reintroduction failure, threatened mammal species can co-exist with low densities of feral cats. There may be a threshold density below which it is possible to maintain unfenced populations of reintroduced marsupials. Understanding the numerical relationships between population densities of introduced predators and threatened species is urgently needed if these species are to be re-established at landscape scales. Such knowledge will enable a priori assessment of the risk of reintroduction failure thereby increasing the likelihood of reintroduction success and reducing the financial and ethical cost of failed translocations.
Introduction
Reintroductions of threatened species aim to reverse declines and reduce extinction risk but many programs fail due to predation by introduced predators (Fischer & Lindenmayer 2000; Towns et al. 2001; Johnson 2006; Clayton et al. 2014; Woinarski et al. 2014). Introduced predators have had significant impacts on Australian fauna (Dickman 1996) and have also decimated native bird populations in countries such as Japan (Arcilla et al. 2015) and the United States (Young et al. 2013). Introduced predators often have greater impacts on wildlife than native predators due in part to evolutionary naïveté, which occurs when native prey lack adequate adaptions to detect and avoid novel predators (Saul & Jeschke 2015; Banks et al. 2018).
Conservation fencing to protect reintroduced and remnant wildlife populations is one technique to reduce vulnerability and is used in many countries including New Zealand, Japan, USA and Australia (Moseby & Read 2006; Short 2009; Burns et al. 2011; Young et al. 2013). On mainland Australia, conservation fences protect reintroduced populations of critical weight range (35–5500 g) mammals that have been extirpated from large areas of the continent due to predation by introduced predators, red foxes (Vulpes vulpes) and feral cats (Felis catus; Burbidge & McKenzie 1989; Hayward et al. 2014).
Whilst conservation fences are highly effective in the short term (Moseby et al. 2011), they create a series of novel challenges for conservation managers because they limit dispersal, increase chances of inbreeding, cause overpopulation in the absence of predators and may exacerbate issues of prey naïveté (Blumstein & Daniel 2005; Carthey & Banks 2014; Moseby et al. 2018). Co-existence with introduced predators is unlikely to evolve and may be lost following relaxed selection (Lahti et al. 2009). Nevertheless, the high failure rate of reintroductions into areas where introduced predators are present (Fischer & Lindenmayer 2000) has encouraged increased investment in conservation fencing. It is conceivable that increasing reliance on predator-proof fencing could generate a feedback-loop, whereby native species become reliant on fences as anti-predator behaviour is lost, and fewer wildlife agencies are willing to undertake broad scale reintroductions because of the high risk of failure.
For many practitioners, the ultimate aim is to re-establish threatened species to their former range and enable them to survive in the wild with minimal management intervention (IUCN/SSC, 2013). Thus, a fundamental understanding of the numerical relationship between populations of co-existing introduced predators and native prey will optimise predator control to maximise reintroduction success. Currently, aside from modelling exercises (e.g. Southgate & Possingham 1995), there is little empirical understanding of the threshold predator density below which populations of reintroduced threatened mammals can persist. This means that despite the IUCN Guidelines for Reintroduction (IUCN/SSC 2013) recommending that the causes of a species’ decline be addressed prior to translocation, there are rarely evidence-based targets for introduced predator control in wildlife protection programs beyond the aim of complete eradication or maximising predator reduction (Moseby et al. 2011; but see Armstrong et al. 2006).
Furthermore, little evidence exists to inform such targets due to an absence of standardised data collection methods for translocation programs. In some cases, managers set arbitrary targets (Morris et al. 2004) or have suggested that targets do not exist. Clayton et al. (2014) suggest that for some threatened macropodid species there may be no safe levels of introduced predators.
A study comparing the vulnerability of threatened Australian mammals to introduced predators ranked species from low to extreme (Radford et al. 2018). An extreme rating suggests that the species is ‘unable to persist where at least one of the introduced predators occur’ (Radford et al. 2018). Here, we describe short-term reintroductions of the burrowing bettong (Bettongia lesuer, 1.6 kg) and greater bilby (Macrotis lagotis, 1–2.5 kg) two omnivorous, fossorial, IUCN-listed mammal species listed by Radford et al. (2018) as high and extremely susceptible to the presence of introduced predators respectively. The ranges of bettongs and bilbies have drastically declined due to predation by introduced cats and foxes (Southgate 1994; Short & Turner 2000), and previous reintroduction attempts outside fenced exclosures have failed due to predation by introduced predators (Christensen & Burrows 1995; Moseby et al. 2011). We exposed these species to low densities of cats or foxes to determine whether short-term co-existence with these predators is possible. We defined co-existence as population persistence and successful recruitment in the presence of predators for at least 3 years. Although other studies suggest a 5-year time period as a criterion for reintroduction success (e.g. Clayton et al. 2014), our study aimed to determine if at least short-term survival was possible when predator sensitive species were exposed to predators. A further aim of our study was to quantify the densities of reintroduced predators below which the reintroduced mammals could co-exist. Knowledge of this threshold density would allow for a priori assessment of the risk of reintroduction failure, thereby reducing the financial and ethical cost of failed reintroductions. Furthermore, such knowledge could be used to set target population densities for introduced predator control programs. While these targets are likely to be specific to reintroduced prey-introduced predator combinations, the idea of developing targets has broad application that may assist with future reintroduction trials into areas where introduced predators are present and it is unfeasible to eradicate them.
Materials and Methods
Study species and susceptibility to introduced predators
Burrowing bettongs are bipedal marsupials that live communally in burrows (Van Dyck & Strahan 2008). They are extinct in the wild on mainland Australia but survive on three offshore islands and have been successfully reintroduced to cat- and fox-free, fenced mainland sanctuaries (Short & Turner 2000; Moseby et al. 2011). Bettongs are considered to be highly susceptible to predation by cats and foxes (Radford et al. 2018) and unlikely to survive in the presence of one or more introduced predators. There have been numerous attempts to release bettongs into areas with predators. Three attempts to release them into areas with predators have failed due to cat predation (Christensen & Burrows 1995) or a combination of cat, fox and dingo predation (Moseby et al. 2011; Bannister et al. 2016). Twelve per cent of 50 carcases found in a reintroduction at Heirisson Prong Sanctuary in Western Australia were attributed to cat predation, 64% to fox predation and 24% were unknown (Short & Turner 2000). Fox incursions into fenced sanctuaries resulted in surplus killing and high mortality of bettongs before foxes were removed (Short et al. 2002). Reintroductions of the similar-sized, Bettongia pencillata failed due to predation by foxes (Bellchambers 2001) and cats (Priddel & Wheeler 2004).
Greater bilbies are nocturnal, solitary marsupials that live in simple burrows and dig extensively for seeds, roots and invertebrates (Van Dyck & Strahan 2008). Bilbies have declined by more than 80% since European settlement but are considered to be less sensitive to introduced predators than bettongs since they are still found in the wild in parts of arid Australia (Woinarski et al. 2014; Radford et al. 2018). Ongoing declines are attributed to predation pressure from foxes and cats and land degradation from domestic stock and changed fire regimes (Pavey 2006). Four failed bilby reintroductions into Australian deserts were attributed to cat predation (Moseby et al. 2011) or a combination of cat, fox and/or dingo predation (Southgate & Possingham 1995). Feral cats were responsible for significant predation on wild bilbies in South West Queensland with 119 confirmed kills in 12 months (Rich et al. 2014), and bilbies reintroduced to the Currawinya fenced reserve in Queensland were decimated by cat incursions (Lollback et al. 2015).
Study site
This study was conducted at Arid Recovery in South Australia (30 29′S, 136 53′E) between 2008 and 2017 (Fig. 1). Arid Recovery is a 123 km2 reserve that has been fenced to exclude introduced rabbits (Oryctolagus cuniculus), cats and foxes. Mean annual rainfall at the site is 166 mm per year.
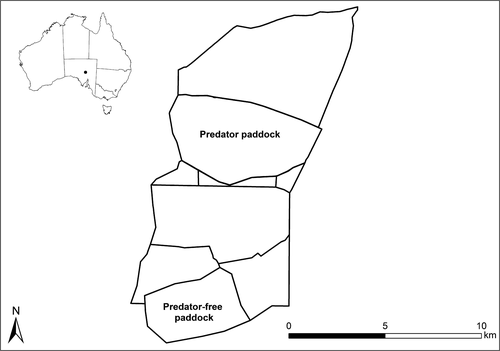
The study was conducted in two sections of the reserve, a 26 km2 Predator Paddock where rabbits and eutherian predators were present and a 14 km2 Predator-free Paddock where they were absent for the entire study period. Both paddocks were surrounded by a 1.8 m high mesh fence with an external floppy overhang preventing access by other feral cats and foxes (Moseby & Read 2006). Bettongs and bilbies were reintroduced to the Predator-free Paddock in 1999 and 2000. Within the Predator Paddock, bettongs were reintroduced in 2008, became extinct in 2013 and were reintroduced again in 2014, this time with bilbies.
Predator Paddock first reintroduction 2008–2014
In September 2008, 66 burrowing bettongs (28 F, 38 M) were moved to the Predator Paddock from Predator-free Paddocks in Arid Recovery. Bettongs were captured at warrens in cage traps, weighed, sexed, measured, checked for body and reproductive condition, given a unique tag and released into the Paddock on the same night of capture. Details of the reintroduction can be found in Moseby et al. (2011).
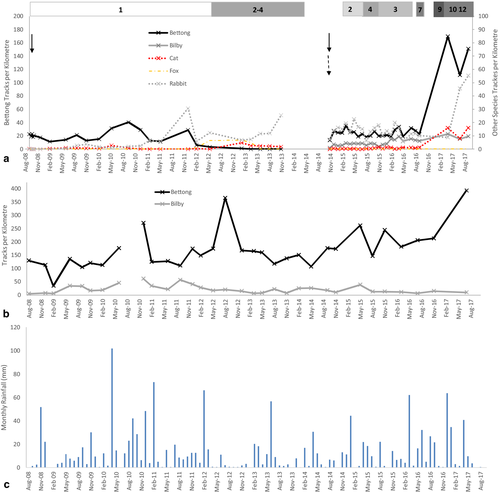
During the first reintroduction of bettongs to the Predator Paddock in September 2008, one cat was present within the Predator Paddock. From 2008 to 2013 the number of cats within the paddock was unknown but estimated using track count data (method described below) and the later removal of cats. Foxes were not deliberately added to the Predator Paddock but a fox gained access to the Predator Paddock sometime between December 2011 and February 2012 through a hole in the fence (Fig. 2). Bettongs went extinct in the paddock by March 2013. The fox was present within the paddock until November 2013 when it was removed.
Predator Paddock second reintroduction 2014–2016
After the failure of the first release, bettongs were again released into the Predator Paddock between October and December 2014, this time with bilbies. A total of 353 burrowing bettongs (146 F, 207 M) and 47 bilbies (27 F, 20 M) were moved to the Predator Paddock from Predator-free Paddocks. Bettongs were captured as per the 2008 release methods outlined above. All bettongs were released at the same central release point. Bilbies were captured using hand nets during nocturnal spotlighting, processed and released over 15 nights as per bettong methods outlined above.
At the start of the 2014 bilby and bettong release, camera trap, spotlighting and track data indicated that there was one cat of unknown sex present within the Predator Paddock. Five additional feral cats (four sterilised males and one female, Appendix S1) were captured in the surrounding area and added between 6 and 8 months after bettong/bilby release and their continued presence within the paddock monitored using radio telemetry and camera traps. Cats were given a uniquely placed ear tag to enable identification on camera. Cat density was estimated through different combinations of ear tag placement, sex and coat patterns (Bengsen et al. 2012) recorded on 20 remote cameras set continuously in a grid pattern through the paddock from August 2014 until September 2017 (Appendix S2). Camera traps were placed more than 800 m apart along roads that crossed through dune (n = 10) and swale habitat (n = 10). Large male cats were targeted for introduction to the Paddock because previous research suggested that large male cats are disproportionately responsible for predation on threatened species of this size (Moseby et al. 2015). After October 2016, cat densities increased through incursions and breeding.
Predator-free Paddock reintroduction
In order to provide an experimental control for potential prey fluctuations due to climatic conditions, we monitored prey activity over the same time period in a Predator-free Paddock located in the southern section of the Arid Recovery Reserve. This paddock was approximately 5 km south of the Predator Paddock but both paddocks were within the Arid Recovery Reserve, within the same land system and dominated by longitudinal sand dunes supporting Acacia shrubland and interdunal clay swales supporting chenopod shrubs. The Predator-free Paddock contained bettongs and bilbies that were reintroduced in 1999 and 2000 respectively. No introduced predators were detected in the Predator-free Paddock over the study period.
Monitoring
Abundance of the bettong and/or bilby populations in the Predator-free and Predator Paddocks was indexed using a combination of camera trapping, track counts, radiotracking and/or cage trapping. Track counts were conducted during both Predator Paddock reintroductions and also in the Predator-free Paddock 3–4 times a year from September 2008 to September 2017 but were not conducted between November 2013 and November 2014 due to the extinction of the bettong population within the Predator Paddock during this time. Track counts have been successfully used to monitor relative abundance of both species within the Arid Recovery Reserve for 17 years (Moseby et al. 2011) and are known to be significantly associated with bettong population abundance estimated via capture mark recapture through a power relationship (Moseby et al. 2018). Transects traversed five to eleven kilometres of dune habitat within each Paddock and followed methods described in Moseby et al. (2011). The average tracks per km were calculated for each species. Bilby track counts also included gait length measurements in order to determine population demography (Southgate 2005). Although track counts can only be conducted on sandy substrate which is not present throughout all areas of the Predator-free and Predator Paddock, this habitat type represents preferred habitat for both species (Moseby & O'Donnell 2003; Finlayson & Moseby 2004) and is thus likely to reflect broader population trends within the paddocks.
Additional methods were used to estimate population trends during the second reintroduction to the Predator Paddock between 2014 and 2017. We counted cats, bettongs, bilbies, rabbits and small mammals recorded on the previously described camera grid established in the Predator Paddock using a new detection interval of >10 min.
Bettongs released during the second reintroduction to the Predator Paddock in 2014 were also monitored using radiotracking for the first 20 months after release. Twenty-six bettongs were fitted with brass loop VHF collars (25 g; Sirtrack Ltd). Upon detecting a mortality signal, the collar and carcass were retrieved, examined for causes of mortality, and DNA swabs taken for forensic analysis (Moseby et al. 2015).
We estimated bettong population size in April 2016 and April 2017, 18 months and 30 months after the second 2014 release into the Predator Paddock. We set 140 Sheffield cage traps for four nights, evenly spaced along the 34.8 km road network within the Paddock. Traps were cleared each morning at dawn and animals identified using microchips or eartags, weighed and checked for body and reproductive condition. We calculated a density and population estimate for bettongs by fitting likelihood-based spatially explicit capture mark recapture (SECR) models (Borchers & Efford 2008) in the package ‘secr’ (Efford 2012) in R version 3.3.2. Each night of trapping was included as a session and we selected multi-trap likelihood (Efford & Fewster 2013). A habitat mask was created, with the boundary fence of the Paddock set as the outer limit. The mask was divided into two zones to represent the area of dense dunes in the south east of the Paddock and the remaining area of clay interdunal swale. We considered males and females as two separate groups (within the same model) and ran an initial test of the best detection function for home-range (half-normal, hazard or exponential; Efford & Fewster 2013) and selected the best model (based on Akaike's Information Criterion (AIC). The initial test results suggested that the hazard detection function (Hayes & Buckland 1983) was the top model, with an AIC weight of 1, suggesting bettongs have core home ranges, but sometimes move large distances outside them. We then ran a model selection process by fitting models with the hazard detection function and including co-variates of sex and zone (dune or swale). Again, we used the lowest AIC value to select the best model. Using the density estimation of bettongs within the Predator Paddock provided by the best model (bettongs ha−1) we then estimated the population size within the Paddock 18 months and 30 months after release by multiplying the density by 2600 (area of the Paddock in ha).
Results
Predator-free Paddock
Bettongs and bilbies were present in the Predator-free Paddock during the entire study period from 2008 to 2017 (Fig. 2b). Track activity fluctuated but generally remained between 100 and 200 tracks per km for bettongs and 10–50 tracks per km for bilbies. Bettong tracks increased to over 350 per km in late 2016 possibly due to an increase in rainfall at this time (Fig. 2c). These trends are supported by a previous study using capture mark recapture data which also reported stable or increasing bettong populations over this period (Moseby et al. 2018).
Predator Paddock first reintroduction 2008
Bettong track counts initially increased after the first bettong reintroduction in 2008 but declined to extinction 5 years later. At the start of the 2008 reintroduction 22.6 bettong tracks per km were recorded reaching a peak of 40.4 tracks per km 24 months later (Fig. 2a). At 41 months post-reintroduction a dramatic decline was observed when bettong tracks fell from 28.6 tracks per km in December 2011 to just 6.0 tracks per km during the next track count in February 2012. This coincided with the first detection of fox tracks in February 2012. Rainfall records indicate that this decline occurred at the end of an average rainfall year and during a large rainfall event that triggered an increase in bettong activity in the Predator-free Paddock (Fig. 2). Over the next 12 months bettong tracks continued to decline while fox track detections increased until no bettong tracks were detected in March 2013 (Fig. 2a). During this period cat tracks also increased from an average of 0.39 tracks per km across the first 41 months of the reintroduction, to 2.0 tracks per km during the decline of the bettong population. Track counts and later removal of cats suggested that the number of cats in the paddock fluctuated between one and four cats at a density of 0.038 and 0.154 per km2 (Fig. 2a).
Predator Paddock second reintroduction 2014
All methods used to monitor population trends after the 2014 release into the Predator Paddock suggested that abundances of bettongs and bilbies increased along with cats and that both prey species successfully recruited young (Table 1). Bettongs dispersed throughout the Paddock after release in 2014 and more than 90 warrens were located during opportunistic searches over the 18 months after release (Appendix S2). In January 2015, immediately after reintroduction, bettong and bilby tracks averaged 24.6 and 2.45 tracks per km respectively (Fig. 2). By September 2017, track counts for both species had increased, bettong tracks were 150 tracks per km and bilbies were 9.6 tracks per km.
| Date | Bilby | Bettong | Cat | ||||
|---|---|---|---|---|---|---|---|
| Tracks | Camera | Tracks | CMR-no. per km2 | Tracks | Camera | MKTBA-no. per km2 | |
| December 2014 | 3.6 | 27 | 13.5† | 0.45 | 0.04 | ||
| April/August 2015 | 3.82 | 2.5 | 25 | n/a | 0.18 | 2.0 | 0.08 |
| April 2016 | 9.45 | 5.5 | 33 | 19 (CI = 14–25) | 0.36 | 1.6 | 0.12 |
| April 2017 | 11.37 | 20 | 169 | 33 (CI = 23–49) | 15.89 | 18 | 0.35 |
- †No confidence intervals as density is based on number released. CI, confidence intervals; CMR, capture mark recapture; MKTBA, minimum known to be alive.
Cameras, cat introductions and radiotelemetry data suggest cat density was 0.04 from October 2014 to April 2015 and then fluctuated between 0.08 and 0.12 cats per km2 from July 2015 until September 2016 (Fig. 2). Breeding and incursions then occurred and the number of cats identified on camera ranged from 9 to 12 over this period equating to a minimum density of 0.35–0.46 per square km. Track transects indicated similar increases in activity, cat tracks averaged 0.5 ± 0.1(SE) tracks per km until September 2016, ranging from 0 to 1.45 tracks per km (Fig. 2). After September 2016 cat track density increased dramatically to 16 tracks per km.
Several factors suggested that cats were regularly interacting with bettongs and bilbies inside the Paddock. Eight of the 26 (31%) radio-collared bettongs translocated to the Paddock in 2014 died throughout the study and two of these tested positive for cat DNA. Some of the other carcasses were unable to be swabbed immediately following death. In addition, bilby remains were detected in one of 10 cat scats analysed during the study, radio-collared cats were found to regularly traverse the entire paddock (Appendix S2) and cats were detected on every kilometre of track transects and more than 50% of camera traps.
During trapping in April 2016, a total of 211 burrowing bettongs (82 F, 129 M) and two newly recruited bilbies (1 of each sex) were caught in cage traps. Of the bettongs, 102 were originally translocated animals and 109 were newly recruited. Of the 77 adult female bettongs, 79.2% had pouch young. During trapping in April 2017, a total of 228 burrowing bettongs (92 F, 136 M) were captured. Of these captures, 137 were newly recruited animals and 91 were previously trapped animals. Of the 86 adult females, 65.1% had pouch young.
Spatially explicit capture mark recapture model selection identified that the best model for both years was a model with hazard rate detection function including sex and where home range varied with zone (dune or swale). This model estimated bettong density in April 2016 at 0.19 per ha (95% CI 0.14–0.25) with a total population size in the paddock of 494 individuals compared with 353 at the time of release. Bettong density in April 2017 was estimated at 0.33 per ha (95% CI 0.23–0.49) with a total population size of 858 bettongs in the Paddock. The estimated bettong density in April 2016 was 19 bettongs per km2 whilst the known cat density was 0.12 cats per km2. In April 2017, the estimated bettong density was 33 bettongs per km2 and the known cat density was 0.35 per km2.
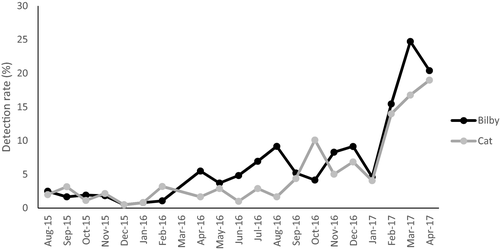
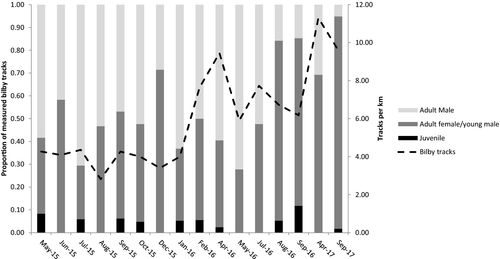
Bilby trapping success was very low due to high trap saturation from bettongs so we focus on results from other survey techniques. Camera trapping data revealed an increase in bilby detections from 2.5 in August 2015 to a peak of 24 detections per 100 camera trap nights in April 2017 (Fig. 3, Table 1). Track counts also recorded an increase from 2.8 to 9.6 tracks per km after release, and bilby gait measurements revealed that juveniles were recruited into the population (Fig. 4). Populations of both species were still extant in September 2018 almost 4 years after release (K. Moseby pers. obs., September 2018).
Discussion
The positive growth rates, successful recruitment and survival of bettongs and bilbies for 4 years in the presence of feral cats provides tangible evidence that these threatened species can co-exist with feral cats under certain conditions. The failure of previous bettong reintroductions (Christensen & Burrows 1995; Priddel & Wheeler 2004; Moseby et al. 2011), bilby reintroductions (Southgate 1994; Moseby et al. 2011) and similar-sized Australian mammals (Hardman et al. 2016) has been attributed, wholly or in part, to cat predation. Cats are also known to significantly impact wild populations of bilbies (Rich et al. 2014) and bettongs (Marlow et al. 2015), causing population decline. However, our study suggests that even extremely susceptible species such as burrowing bettongs, extinct on the mainland with a history of failed reintroductions from predation, are able to increase their population and successfully recruit with cats present at low densities. Although the rate of bettong increase in the Predator Paddock was lower than that in the Predator-free Paddock over the same period and indicated that cats were likely suppressing the population, the persistence of the populations for at least 4 years after release is encouraging. These findings suggest that the extreme susceptibility category stated in Radford et al. (2018) for bettongs may be too pessimistic and contradict Clayton et al. (2014) who suggested that for some threatened macropodid species, no safe levels of introduced predators may exist.
Our findings are significant because they suggest that long-term co-existence of native prey with introduced cats may be possible if appropriate predator densities can be reached and sustained. Cat densities within the Predator Paddock increased more than 10 fold from 0.038 to 0.46 cats per km2 over the 3 years, densities which are higher than the average Australian arid zone cat density (0.18 cats per km2 95% CI 0.13–0.28); and within the lower end of the range reported at arid sites during dry conditions (range 0.01–2.4 cats per km2, Legge et al. 2017). These results suggest that, in the absence of other mammalian predators, these threatened species may be able to co-exist with cats at some unfenced sites with little or no control effort, at least in the short term. The challenge will be maintaining these low, tolerable densities of cats through drought and high rainfall years when predator densities fluctuate significantly, suggesting that ongoing monitoring and control of predators will still be required.
Whilst both species were able to tolerate cat densities of up to 0.46 cats per km2, the addition of a single fox at a density of 0.038 per km2 was enough to cause population extinction of bettongs in less than 12 months. No population declines were recorded in bettongs within the Predator-free Paddock over the same time period strengthening the conclusion that the decline was due to a fox incursion. These results support the contentions made in previous studies, that foxes pose a more significant threat to Australia's native fauna than cats (Burrows et al. 2003; Possingham et al. 2004; McKenzie et al. 2007) and can cause high mortality within short time frames (Short et al. 2002). Globally, the red fox is one of the most common exotic species targeted for control and impacts threatened species in some parts of the USA (Harding et al. 2001). These results suggest that reintroductions into areas where foxes are present, even at low densities, are likely to cause accelerated population decline and/or extinction in burrowing bettongs. Our results also suggest that when determining prey susceptibility to predators, combining prey susceptibility for cats and foxes as per Radford et al. (2018) may be overly simplistic and that prey species may have different susceptibilities to feral cats and foxes.
The success of the bettong and bilby reintroductions into the Predator Paddock in 2014 may be due in part to the large release groups used and the fact that prey species were reintroduced first before adding feral cats. Although one cat was resident in the paddock at the time of release, other cats were not added until several months later, possibly giving prey a chance to establish shelter sites and home ranges, reducing predation pressure during the important establishment phase. This is in direct contrast to most reintroductions conducted outside islands or fenced reserves where predators were already resident (Jones et al. 1997; Clarke et al. 2002; Priddel & Wheeler 2004; Wimberger et al. 2009; Moseby et al. 2011) giving predators the advantage and possibly increasing their impacts. Many reintroduced individuals are killed by predators in the first few weeks or months after release (Moseby et al. 2011; Bannister et al. 2016) and intensive predator control may be most important to implement during this establishment phase. Large release groups are generally more successful than smaller ones (Wolf et al. 1996; Fischer & Lindenmayer 2000), and the larger release group used in 2014 compared with 2008 may have contributed to the success of this second reintroduction.
A framework for understanding co-existence
Understanding the predator densities required to facilitate co-existence would greatly assist threatened species reintroduction and recovery programs but the challenge is determining how the per capita kill rate of predators on prey may fluctuate according to prey density (Holling 1966), age structure of prey (Sand et al. 2012), vegetation cover (Hopcraft et al. 2005; McGregor et al. 2014) and individual hunting ability and preferences of predators (Moseby et al. 2015). Large male cats were purposely chosen for introduction based on previous research suggesting that large male cats are disproportionally responsible for prey deaths in prey of this size (Moseby et al. 2015). Under normal wild conditions young male and/or female cats would be present, suggesting that tolerable predator densities may be higher in the wild. Cats can also learn to hunt difficult prey (Moseby et al. 2015) and implement accelerated hunting (Hardman et al. 2016). These factors may change predator densities required for co-existence over time.
The abundance of alternative prey has been shown to influence bait uptake by feral cats (Christensen & Burrows 1995) and other mammalian predators (Hebblewhite et al. 2003), implying that predation rates are also likely to be influenced by the availability of other prey species. Introduced rabbits and native rodents were present in the Paddock throughout the study and both species are consumed by feral cats (Read & Bowen 2001). Predation rates on bilbies and bettongs may have been much higher if these alternative prey species had been absent, however, rabbits and native rodents are widespread throughout arid Australia and would likely be present at most unfenced reintroduction sites. Predators’ prey preferences may also be an important factor influencing their impacts on reintroduced species. Cats, for example, often exhibit a preference for small rodents (Pearre et al. 1998). Although both predator species prey on bettongs (Short et al. 2002; Moseby et al. 2011) and rabbits (Read & Bowen 2001), it is not known whether these predators differ in their prey preferences which may also have contributed to the variation in impact on the bettong population.
Conservation applications
When designing fenced reserves, cost estimates assume that feral predators must be completely excluded and that any predator that gains access to fenced reserves must be immediately removed (Bode et al. 2012; Helmstedt et al. 2014). However, our results showing that reintroduced mammals can co-exist with low to moderate densities of feral cats suggest that complete predator exclusion may not always be necessary.
A management implication of our findings is that fence designs that deter as opposed to completely exclude introduced cats may also be suitable for threatened species reintroductions. Although thought to be inferior by some practitioners (e.g. Norbury et al. 2014) such fences would be less expensive to construct and maintain than completely predator-proof fences.
More importantly, our results suggest that there may be threshold densities of introduced predators below which maintenance of threatened species populations is possible. This implies that some threatened species, including bettongs, may be suitable candidates for reintroductions into unfenced areas with low cat densities provided that foxes are effectively controlled. It should be noted, however, that cats can have a substantial effect on bettongs and other native mammal populations when foxes are suppressed (e.g. Risbey et al. 2000; Marlow et al. 2015). We encourage practitioners to conduct similar experiments using other native prey/exotic predator combinations to determine if such threshold densities exist because this knowledge could be used to set targets for introduced predator population reductions prior to conducting threatened species reintroductions. Having such knowledge is likely to significantly advance reintroduction science (Seddon et al. 2007), increase the success of threatened species reintroductions and allay financial and ethical concerns during the pre-release phase of reintroduction programs.
Acknowledgements
This study was conducted at Arid Recovery, an independent conservation and research initiative supported by BHP Billiton, the South Australian Department for Environment and the University of Adelaide. We would like to acknowledge D Williams, R Pedler, L Steindler, Z Richardson, R Shepherd, H Crisp and E Griffith for significant field assistance, and H McGregor for guidance on mark recapture analyses. Funding was provided by the Australian Research Council. Ethics approval was obtained from the South Australian Wildlife Ethics committee, approval no. 1/2014M2. Upon acceptance, data presented within this paper will be archived in the Dryad data repository.
Author Contributions
KM and RW conceived the idea and designed the methodology. KM and RW collected the data, RW analysed the data with input from KM, DB and ML, KM and RW led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.




