Comparative use of active searches and artificial refuges to detect amphibians in terrestrial environments
Abstract
Artificial refuges (cover boards) are commonly used to survey and monitor herpetofauna in many parts of the world. Despite the extensive use of artificial refuges in mesic environments, their effectiveness for detecting amphibians in temperate zones has rarely been examined. We compared amphibian detection probabilities between two survey methods; active searches of natural habitat and artificial refuges of three different types (corrugated steel, roofing tiles and timber railway sleepers). Our study area included five bioregions encompassing a 1180-km latitudinal gradient across a modified, temperate eucalypt woodland vegetation community in south-eastern Australia. We deployed 14 778 artificial refuges in terrestrial environments, within patches of remnant vegetation, and collected presence and abundance data on herpetofauna between 1999 and 2017. We used Bayesian logistic regression to identify the most effective survey method for detecting frog species across all bioregions. We modelled frog detections by fitting survey method, time since refuge deployment and rainfall prior to each survey. We detected 3970 individuals from 18 frog species. Overall, we found active searches and timber substrates most effective for detecting a broad range of species, although detection rates were driven by the numerically abundant spotted marsh frog Limnodynastes tasmaniensis. Timber refuges were effective for detecting several burrowing species, whereas active searches were effective at detecting habitat generalists. Quadratic effects of rainfall prior to survey as opposed to linear effects of time since artificial refuge placement was important in explaining frog detection rates in some bioregions. Active searches, timber railway sleepers and sheets of corrugated steel provide complimentary survey methods for detecting amphibians, although detection rates are influenced by rainfall patterns. Artificial refuges provide a time-effective and standardized method for studying amphibians in their non-breeding terrestrial environment and should be incorporated into future surveys and biodiversity monitoring programmes.
Introduction
Amphibian declines have been reported around the world, and Australia is no exception (Richards et al. 1994; Hines et al. 1999; Hero & Morrison 2004; Laurance 2008; Gillespie et al. 2015; Scheele et al. 2017). More than 30% of Australian amphibian species are recognized as threatened and seven species have become extinct in the past 30 years (Hero et al. 2006, 2014; Scheele et al. 2017). Some of the main causes of amphibian population declines include habitat loss and degradation (Hazell 2003), land use change, climate change, disease (Scheele et al. 2017), environmental contaminants and invasive species (Bower et al. 2017).
Ongoing declines in amphibian populations have highlighted the need for; increased survey effort to define species ranges, the development of effective monitoring programmes to detect changes in population dynamics (Bower et al. 2014; McGinness et al. 2014; Skerratt et al. 2016) and the use of effective survey methods (Wassens et al. 2017). However, amphibian populations often exhibit large spatial and temporal variation in abundance (Toft 1980; Brown & Shine 2016), both within their breeding and non-breeding environment. Natural variation in amphibian abundance can be influenced by seasonal weather patterns (Brown & Shine 2007) and climatic extremes (e.g. droughts and floods; Piha et al. 2007; Scheele et al. 2012; Wassens et al. 2013; Mac Nally et al. 2014), often making it difficult to differentiate between concerning declines and background fluctuations. Thus, decoupling causal influences of threatening processes on amphibian abundance requires long-term datasets gathered under standardized conditions (Dodd 2010), and across spatial scales and environments that are relevant to the target species (Gillespie et al. 2018).
Many amphibians have a biphasic life history, whereby adults migrate to waterbodies to breed and lay eggs before returning to terrestrial habitats (Hazell et al. 2004; Dodd 2010). However, much of the global research on amphibians has focused on aquatic breeding habitats, leaving substantial knowledge gaps on amphibian use of terrestrial environments (Westgate et al. 2018), especially within heavily modified, agricultural landscapes (Hazell 2003; Hazell et al. 2004; Pulsford et al. 2018). The use of artificial refuges (also called cover boards and cover objects) is a well-established method for detecting amphibians in terrestrial environments (Hampton 2007; Willson & Gibbons 2010), and has been used extensively to study salamanders (Hyde & Simons 2001; Houze & Chandler 2002; Marsh & Goicochea 2003; Bailey et al. 2004; Hesed 2012; Gorgolewski et al. 2015; Siddig et al. 2015) and anurans in the Northern Hemisphere (Grant et al. 1992; Wakelin et al. 2003; Hampton 2007).
As many amphibians prefer moist habitats, artificial refuges placed in contact with the ground have the potential to attract a broad range of cover-dependent species. Artificial refuges also have an advantage over labour-intensive trapping methods (such as pitfall traps), because they can yield cost-effective, long-term spatial-recapture data (Sutherland et al. 2016), and reduce disturbance to the environment (Hesed 2012). Artificial refuges also present little risk to the animals being monitored (e.g. from injury), and although there is potential risk of predation (Valdez et al. 2017), large frog predators such elapid snakes are rarely detected beneath small-sized artificial refuges (see Michael et al. 2012). The vast majority of studies on amphibians involving artificial substrates have focused on species located in the Northern Hemisphere (Willson & Gibbons 2010). Only a limited number of studies have used artificial refuges to survey amphibians in Australia (Michael et al. 2004, 2012; Kay et al. 2017), possibly due to the focus on frog breeding habitat.
In this study, we evaluated the effectiveness of using artificial refuges (deployed to also survey a broad range of reptiles) to detect amphibians in their terrestrial environment across a topographically and climatically variable temperate eucalypt woodland ecosystem. We compared amphibian detection rates between active searches of natural habitat and three types of artificial refuges (corrugated steel, roofing tiles and timber railway sleepers), over time and in relation to rainfall patterns. The refuge types were chosen to simultaneously survey other taxa such as reptiles (Michael et al. unpubl. data, 2018). For the purpose of this study, we addressed three main questions: (i) Are artificial refuges and active searches effective methods for detecting amphibian species in terrestrial woodland environments? (ii) Does rainfall and time since refuge installation influence amphibian detection rates? (iii) Are there species-specific differences in detection rates among survey methods and bioregions? We answered these three broad questions using datasets collected from five long-term monitoring programmes which reflect geographically different bioregions in south-eastern Australia.
Methods
Study area
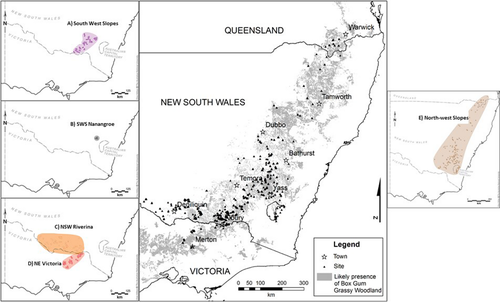
We conducted our study in the temperate eucalypt woodlands of south-eastern Australia, and predominantly within the critically endangered white box Eucalyptus albens, yellow box E. melliodora and Blakely's red gum E. blakelyi grassy woodland and derived native grassland ecological vegetation community (Fig. 1). We included five monitoring programmes in the study, encompassing two water catchment management areas in Victoria and four bioregions in NSW and southern Queensland (Thackway & Cresswell 1997). Thus, our entire study area encompassed five geographically and climatically distinct regions: (i) North East and Goulburn Broken catchment areas in Victoria (hereafter called NE Victoria), (ii) NSW Riverina bioregion, (iii) NSW South-west Slopes bioregion (hereafter called SWS), (iv) a small-scale monitoring programme within the NSW South-west Slopes bioregion (hereafter called Nanangroe) and (v) Nandewar, New England Tablelands and Brigalow Belt South bioregions in northern NSW and southern Queensland (hereafter called the North-west Slopes – NWS; Table 1). The entire region extends from Warwick in southern Queensland (28°01S 152°11E) to Merton in southern Victoria (36°58′ 145°42′) and spans a latitudinal gradient of 1180 km (Fig. 1). The average annual rainfall across the study area ranges from 696 mm in the north, peaking in the summer months (Warwick weather station No. 41525), to 710 mm in the south, peaking in the winter months (Alexandra weather station No. 88001). The average annual minimum and maximum summer temperatures range from 17.9–30.0°C in the north to 11.9–29.3°C in the south. The average annual minimum and maximum winter temperatures range from 2.9–17.9°C in the north to 2.5–11.2°C in the south (BOM 2017).
| Monitoring programme (bio)region | Mean average annual rainfall (mm) | Number of sites | Year of survey | Survey effort (sites × year) |
|---|---|---|---|---|
| NE Victoria | 551 | 40 | 2010, 2011, 2012, 2014, 2016 | 200 |
| NSW Riverina | 375 | 111 | 2008, 2009, 2010, 2012, 2014, 2016 | 666 |
| SWS | 526 | 219 | 2002, 2003, 2005, 2008, 2011, 2013, 2015 | 1533 |
| SWS (Nanangroe) | 548 | 126 | 1999, 2000, 2001, 2003, 2006, 2011, 2013, 2015, 2017 | 1134 |
| NWS (NSW & Qld) | 569 | 325 | 2010, 2011, 2012, 2013, 2014, 2015, 2016 | 2275 |
| Total | 821 | 5808 |
Our study area encompassed a large proportion of the temperate eucalypt woodland ecological vegetation community in south-eastern Australia. This broad vegetation type once formed a relatively continuous band of vegetation on fertile soils west of the Great Dividing Range from approximately 27° S in southern Queensland to the lower south-east of South Australia (Lindenmayer et al. 2005). Currently, more than 95% of the temperate eucalypt woodland has been cleared and converted to agriculture (Yates & Hobbs 2000; Lindenmayer et al. 2010). For this reason, the majority of remnant vegetation on private property in our study area is used for livestock production purposes and remains in a modified condition.
Experimental design and survey protocol
We established 821 survey sites, primarily on private property, across the study area as part of five biophysical monitoring programmes (Table 1). Twenty-eight sites were located in Travelling Stock Reserves in NSW, six sites were located in conservation reserves in Victoria, and 16 sites were located in State Forests in the Nanangroe region near Gundagai in southern NSW. Each site consisted of a 200 × 50 m search area. Grazing management varied at each site and included areas under set stocking, rotational grazing (e.g. spring – summer grazing exclusion) or total grazing exclusion. Between 1999 and 2017, we conducted 5808 site visits across the entire study area.
At each site, we surveyed amphibians using time- and area-constrained (20 min × 1 ha) active searches of natural habitat and inspections of artificial refuges (Fig. 2). Artificial refuges were placed in arrays and consisted of four timber railway sleepers (1.2 m in length), four terracotta or concrete roofing tiles (423 × 265 mm), and one double stack of 1 m2 corrugated steel sheeting (Michael et al. 2012). Two arrays were established at each site within the same 1 ha search area, placed 100 m apart and checked 3 months after deployment. The total amount of time to inspect both refuge arrays at each site was 5 min. Active searches included raking through leaf litter, lifting logs and surface rocks and inspecting exfoliating bark of mature trees.

- Note: In the absence of logs or rocks, timber railway sleepers were used to restrain the sheets of corrugated steel.
For all monitoring programmes, four to six people visited between eight and ten sites per day. In total, we inspected 1642 arrays (consisting of 6568 roof tiles, 6568 timber refuges and 1642 corrugated steel stacks) between five and nine times over a 19-year period (1999–2017), representing a single survey every 2 years. All of the artificial refuges were placed flat on the ground in terrestrial environments without disturbing surrounding vegetation. However, during above average rainfall years (2011 and 2012), many monitoring sites located in the NSW Riverina were inundated due to local flooding. In all regions, many roofing tiles were damaged by livestock and periodically replaced, and in 2010 all of the original timber refuges (fence palings) in the SWS and Nanangroe were replaced with recycled timber railway sleepers for comparison with other monitoring programmes. We completed surveys between August and December and between 09.00 and 16.00 hours on clear, sunny days. To standardize detections, the order in which sites were surveyed was rotated to ensure each site was surveyed at different times of the day, and by different observers.
Statistical analysis
We modelled the probability of detecting any frog species separately for each bioregion using a Bayesian generalized linear mixed model (GLMM) with a Bernoulli distribution and logistic link function. We modelled detection probability rather than abundance due to differences in sampling area between active searches and artificial refuges methods. We completed our analysis in R (Team 2017) using the brms (Bürkner 2016) package. The models we considered included the following terms: Capture method (active search, tile, timber and tin); linear and quadratic effects of rainfall in the 3 months prior to the survey being conducted (termed recent rainfall); linear and quadratic effects of rainfall in the 4–12 months prior to survey (termed early season rainfall); and linear and quadratic effects of time since the artificial substrates were deployed (placement time). All continuous variables were standardized to have zero mean and standard deviation one. Sites were split into northern and southern clusters for the North-west Slopes (NWS) region as there was a large latitudinal gradient.
We used default priors in the brms package and ran the Markov chain Monte Carlo (MCMC) with four chains, for 10 000 iterations with the first 2000 used as burn-in and a thinning factor of 8, giving 4000 MCMC samples for inference. We used standard MCMC convergence diagnostics and all chains showed adequate mixing (Gelman & Rubin 1992). One hundred and sixty-two models were considered for the NWS region and 81 models were considered for the other four regions. We used the leave-one-out cross-validation information criteria (LOOIC) (Vehtari et al. 2016, 2017) to choose the most parsimonious model within two LOOIC units of the best fitting model. We report posterior means and 95% credible intervals.
We also modelled the probability of detecting individual frog species in each bioregion where there was sufficient detections to warrant further statistical modelling using the same terms as described above. We modelled the following species in the corresponding bioregions: spotted marsh frog Limnodynastes tasmaniensis (all bioregions), eastern sign-bearing froglet Crinia signifera (Nanangroe and NWS), inland banjo frog L. interioris (SWS), barking marsh frog L. fletcheri (NSW Riverina), eastern banjo frog L. dumerilii (NE Victoria), Peron's tree frog Litoria peronii (SWS) and smooth toadlet Uperoleia laevigata (NWS).
Results
Summary statistics
We recorded a total of 3970 individuals representing 18 frog species from two families, Myobatrachidae and Hylidae (Appendix S1). The spotted marsh frog L. tasmaniensis was the most abundant species, accounting for 67.75% of all observations and was detected using all four survey methods across all five regions. Four additional species (Crinia parinsignifera, C. signifera, L. dumerilii and Lit. peronii) were detected in all regions using at least one survey method. Frog species richness increased along a latitudinal gradient with the northern bioregion supporting, on average, twice as many frog species as sites in southern regions (Appendix S1).
Effect of survey method on amphibian detections
Table 2 gives the overall detection rates for the presence of any frog species in each of the five regions by capture method. Overall, active searches and timber substrates produced the highest detection rates for the presence of any frog species. Active searches were the most effective method for detecting frogs in Nanangroe, a combination of active searches and timber substrates was most effective for detecting frogs in the SWS and NWS, whereas timber substrates were more effective at detecting frogs in NE Victoria and NSW Riverina (Table 2). Of all survey methods, roofing tiles were the least effective method for detecting frogs in all regions.
| Response | Region | Number of sites | Number of surveys | Active search | Roofing tiles | Railway sleepers | Corrugated steel |
|---|---|---|---|---|---|---|---|
| Any frog species | Nanangroe | 126 | 8 | 5.7 | 1.9 | 1.9 | 3.9 |
| SWS | 219 | 6 | 5.6 | 1.3 | 5.3 | 4.3 | |
| NSW Riverina | 111 | 5 | 7.5 | 4.5 | 12.2 | 8.7 | |
| NE Victoria | 40 | 5 | 13.7 | 5.7 | 20 | 9.7 | |
| NWS | 325 | 6 | 16.7 | 4.5 | 14.8 | 10.3 |
Effect of placement time and rainfall on amphibian detection rates
The best fitting model for the presence of any frog species in Nanangroe, SWS and NWS was characterized by an interaction between capture method and a quadratic effect of substrate placement time. The model for Nanangroe included an interaction between the linear component and capture method, whereby detection rates from active searches increased steadily over time, whereas detections beneath refuges were consistently low (Fig. 3). The models for the SWS and NWS regions revealed both interactions between the linear and quadratic components and capture method. We provide the results of the LOOIC model selection for the each of the five bioregions for the presence of any frog in Appendix S2 and the various temporal trajectories for each region are illustrated in Figure 3.
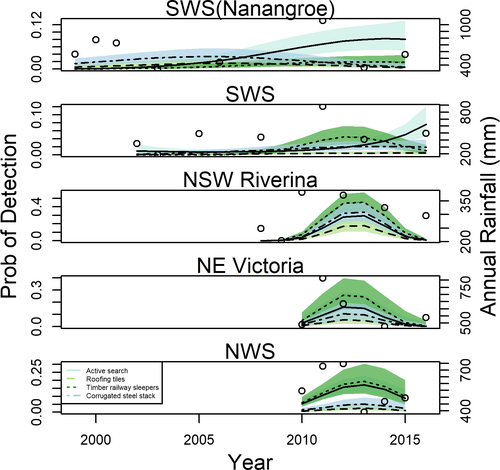
- Note: Two bioregions (NSW Riverina and NE Victoria) have additive effects of time since initiation and capture method and the y-axis values vary across regions. The open circles indicate the year of survey and the amount of rainfall in the prior 12 months.
Early season rainfall (encompassing 4–12 months prior to survey) was positively associated with frog detections in the SWS, coinciding with a lag time in peak frog detections approximately 8 years after refuge deployment. By contrast, we found no rainfall effects for Nanangroe. In NWS, recent rainfall (within 3 months of a survey) and early season rainfall (4–12 months prior to survey) had positive effects on frog detection with peak detections occurring within 2 years after refuge deployment. These results indicate that an optimal amount of rainfall for detecting any frog species in this region is at least 215 mm 3 months prior to conducting a survey, and 704 mm earlier in the season (at least 4–12 months prior to a survey).
In the NSW Riverina and NE Victoria bioregions, we found additive effects of capture method and time since substrate deployment (Fig. 3), indicating that differences among capture methods were constant over time. The ranking of capture methods for NSW Riverina (from best to worst) was timber railway sleepers, corrugated steel, active search and roofing tiles, whereas the ranking for NE Victoria was timber railway sleepers, active search, corrugated steel and roofing tiles. We found a quadratic relationship between detection and recent rainfall (peak = 210 mm) for NSW Riverina and a positive relationship between frog detections and early season rainfall in NE Victoria (Fig. 3). Overall, across most bioregions, frog detections using active searches and several different types of artificial refuge peaked simultaneously, irrespective of when refuges were first deployed.
Species-specific detection rates
We provide the overall detection rates by capture method for seven common frog species in each region in Table 3. Active searches, timber railway sleepers and corrugated steel were most effective at detecting L. tasmaniensis in all regions, except NSW Riverina, where timber and steel substrates yielded the highest detection rates. The probability of occurrence for L. tasmaniensis differed between the northern and southern parts of the NWS region, although capture patterns by method over time were identical. Active searches were most effective for detecting C. signifera and U. laevigata, whereas timber was effective at detecting Lit. peronii and a combination of timber and steel was effective for detecting Lit. interioris, Lit. fletcheri and L. dumerilii (Table 3). Limnodynastes tasmaniensis was the only common species detected in all regions, so for this species we provide the various temporal trajectories by region in Figure 4. We provide the results of the LOOIC model selection for L. tasmaniensis and the six other abundant frog species in Appendices S3 and S4.
| Species | Bioregion | Number of sites | Number of surveys | Active search | Roofing tiles | Railway sleepers | Corrugated steel |
|---|---|---|---|---|---|---|---|
| Limnodynastes tasmaniensis | SWS (Nanangroe) | 126 | 8 | 2.2 | 1.3 | 1.3 | 2.6 |
| SWS | 219 | 6 | 2.4 | 0.9 | 2.8 | 3.1 | |
| NSW Riverina | 111 | 5 | 5.6 | 4.2 | 10.1 | 7.7 | |
| NE Victoria | 40 | 5 | 5.7 | 1.7 | 8.0 | 4.6 | |
| NWS | 325 | 6 | 8.4 | 2.8 | 9.3 | 6.7 | |
| Crinia signifera | SWS (Nanangroe) | 126 | 8 | 3.1 | 0.8 | 0.1 | 0.6 |
| NWS | 325 | 6 | 2.7 | 0.1 | 0.5 | 0.3 | |
| Limnodynastes interioris | SWS | 219 | 6 | 0.9 | 0.4 | 1.1 | 1.4 |
| Limnodynastes fletcheri | NSW Riverina | 111 | 5 | 0.9 | 0.5 | 1.4 | 0.9 |
| Limnodynastes dumerilii | NE Victoria | 40 | 5 | 5.1 | 2.9 | 8.6 | 4.6 |
| Litoria peronii | SWS | 219 | 6 | 0.9 | 0.2 | 1.8 | 0.3 |
| Uperoleia laevigata | NWS | 325 | 6 | 5.4 | 1.6 | 3.0 | 3.5 |
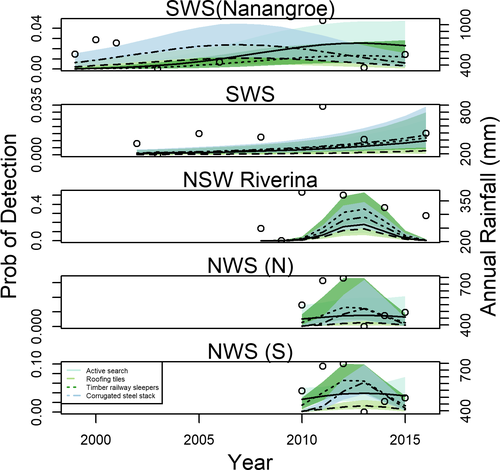
- Note: One bioregion (NSW Riverina) has additive effects of time since initiation and capture method and the y-axis values vary across regions. NE Victoria is not presented as there were no interaction effects with survey method and year. The open circles indicate the year of survey and the amount of rainfall in the prior 12 months. Note the best fitting model for the NWS bioregion had different detections rates between the north and south bioregions.
Discussion
Artificial refuges, or cover boards, are widely used to survey amphibians (predominantly salamanders) in the Northern Hemisphere (Willson & Gibbons 2010; Heyer et al. 2014). Studies using artificial substrates to survey amphibians in the Southern Hemisphere are limited, although they have been used to survey frogs in New Zealand (Wakelin et al. 2003) and parts of southern Australia (Michael et al. 2004, 2012). As far as we are aware, this is the first empirical study to evaluate the use of artificial refuges to survey amphibians in terrestrial environments across an entire ecoregion. Our key findings include: (i) Active searches and timber railway sleepers were effective for procuring records of a broad range of frog species, although detection rates were driven by the widespread and numerically abundant L. tasmaniensis; (ii) The probability of detecting any frog species in some bioregions was influenced by above average rainfall patterns prior to survey; (iii) Species-specific differences in detection rates were evident across survey methods and bioregions, with timber refuges most effective at detecting burrowing species, and active searches most effective for detecting small cryptozoic species. Below, we discuss the merits of using artificial refuges and active searches to detect amphibians in modified landscapes and the application of these survey methods in future studies and monitoring programmes.
Effect of survey method on amphibian detections
Overall, we recorded more than 3000 individuals from 18 species of the 25 frog species predicted to occur within the study area (Cogger 2014). Frogs were detected using all survey methods, although detection rates varied according to method and region. Generally, active searches and timber refuges were equally effective in detecting a variety of frog species, whereas roofing tiles generally performed poorly in all regions. Artificial refuges may provide a more standardized method for detecting amphibians across sites than active searches because levels of natural microhabitats can be highly variable, especially in modified landscapes, but using refuge requires financial resources (e.g. approximately AU$4.00 per railway sleeper) and labour to deploy them before surveys commence. The time between refuge deployment and first survey also needs to be taken into account because detection rates are influenced by rainfall patterns, and this may potentially preclude their use in short-term studies, especially those conducted during droughts or below average rainfall years. Refuges also may be disturbed by livestock, wildlife poachers or damaged by strong winds meaning they may need to be regularly replaced or repositioned.
Our findings suggest that artificial refuges can produce similar if not higher frog detection rates than active searches in some bioregions. Furthermore, the time required to inspect refuge arrays (approximately 5 min/site) is four times less than the time required to obtain similar frog numbers by actively searching natural habitat over a larger search area (1 ha). For example, we recorded a maximum detection rate of 20% beneath timber in the NSW Riverina as opposed to a 16.7% detection rate using active searches in the high rainfall NWS bioregion. Thus, while costs are associated with establishing artificial refuges, once established, they require less survey effort to return similar results to active searches.
Timber substrates, particularly the recycled railway sleepers used in this study, provide amphibians with suitable terrestrial refugia in agricultural landscapes because they are solid, and hence, not easily disturbed or damaged by livestock. Damage caused by livestock trampling and breaking roofing tiles used in this study was evident and a potential reason why detection rates beneath this type of refuge were comparatively low. Weathered timber refuges also provide a variety of microhabitats, including vertical holes and subsurface cracks and cavities, where both terrestrial and arboreal species were often found sequestered. Timber refuges also retain soil moisture and create deep soil cracks as ambient air temperature increases (Michael et al. 2004), providing humid microclimates during dry periods and ideal subterranean conditions for burrowing species. We suggest that future studies and amphibian monitoring programmes consider using timber railway sleepers and sheets of corrugated steel as complimentary methods for obtaining estimates of frog abundance in terrestrial environments because they are durable and provide a standardized method for comparing across sites, although we acknowledge that occupancy patterns are likely to be influenced by a range of biotic and abiotic factors (Hoare et al. 2009; Thierry et al. 2009). Timber refuges also may prove to be more cost-effective in the long-term for detecting frogs in any given area as the time required to inspect refuges is considerably less than the time required to search for frogs in their terrestrial habitat, an important consideration in environmental assessments. Thus, artificial refuges provide a useful tool for understanding amphibian use of terrestrial environments and provide a robust standardized method for evaluating frog occupancy patterns, although frog detection rates using both active searches and artificial refuges are likely to be influenced by natural variation in levels of suitable terrestrial shelter sites in the surrounding landscape.
Effect of time since deployment and rainfall on frog detection rates
A key assumption associated with using artificial refuges is that they provide a reliable and standardized tool for detecting species in their natural habitat. However, factors such as sampling intensity (Marsh & Goicochea 2003), time of sampling (Lettink & Cree 2007), weather variables (Hoare et al. 2009), refuge thermal properties (Thierry et al. 2009) and placement period can influence detection rates (Batson et al. 2015). We are not aware of any studies that have investigated the use of artificial refuges or cover boards to monitor herpetofaunal abundance over timeframes longer than 10 years. In our two longest monitoring programmes (SWS and Nanangroe), we found interactions between survey method and refuge placement period. In Nanangroe, detection rates by any method were low prior to 2010 (during a prolonged drought period), thereafter increasing using actives searches while simultaneously decreasing beneath refuges. Similarly, in the SWS, detection rates by any method were low prior to 2010, increased substantially beneath refuges during 2011 (coinciding with above average rainfall events), whereas detections using active searches steadily increased. In these two bioregions, fence palings were used for the first 9 years, thereafter replaced with railway sleepers due to the rapid rate at which the fence palings degraded. The replacement by a different type of timber refuge may have resulted in improved detection rates in the SWS, although increased detections post 2010 were not evident in Nanangroe. Prior to 2010, the probability of detecting any frog species was low and was likely influenced by the Millennium drought, a decade long period associated with declines in amphibian abundance in south-eastern Australia (Scheele et al. 2012; Mac Nally et al. 2014). During drought years, many frog species also spend long periods sequestered below-ground, thereby reducing detectability.
In NE Victoria, NSW Riverina and NWS regions, detection rates beneath refuges peaked approximately 2 years after deployment and then sharply declined in 2015/2016. These peaks occurred simultaneously across all survey methods suggesting that lag times in detection are influenced by weather-related variables rather than survey method. Thus, by examining frog detection rates between methods, we are able to separate the temporal effects of placement time from the stochastic effects of rainfall and conclude that time since refuge deployment had little influence on frog detections in this study. Several studies have reported increased frog abundance along a rainfall gradient (Woinarski et al. 1999), with increased detection rates at breeding sites attributable to recent rainfall (Paltridge & Southgate 2001; Penman et al. 2006) as well as long-term rainfall patterns (Trenham et al. 2003). Variables such as water levels, water temperature and long-term weather patterns, including drought-flood cycles, can affect breeding activity, community structure and population dynamics (Dostine et al. 2013; Wassens et al. 2013; Mac Nally et al. 2014). However, little is known about the influence of climate patterns on amphibian movements and their use of terrestrial habitats (Hazell 2003; Ocock et al. 2014). As frog movement and dispersal behaviour occur more frequently during the breeding season, and under favourable environmental conditions, (Pittman et al. 2014; Westgate et al. 2018), encounter rates with terrestrial refuges are also predicted to increase during these periods.
Species-specific responses to artificial refuges
The most abundant and widespread species responsible for driving overall frog detection patterns was L. tasmaniensis, a pond-breeding species that utilizes a wide variety of terrestrial microhabitats for over-wintering and foraging (Barker et al. 1995), and for migrating between ephemeral, rain-fed wetlands (Wassens et al. 2013) and farm dams (Hazell et al. 2004). The broad terrestrial habitat requirements of this species (Barker et al. 1995), were reflected in the capture rates beneath both natural (logs and rocks) and artificial refuges across all five regions. Species-specific dispersal ability and dependence on waterbodies for breeding are thus likely to explain differences in capture rates among species with different life-history traits and ecological requirements. For example, stream-dwelling species that were predicted to occur in the study area were rarely or never detected, whereas several burrowing and pond-breeding species (e.g. Notaden bennettii and Pseudophryne bibroni) were only occasionally detected. Furthermore, small cryptozoic species such as U. laevigata and C. signifera were more likely to be detected beneath natural substrates such as surface rocks and logs than artificial substrates. Therefore, species capable of using a wide variety of natural, semi-natural of artificial wetlands are more likely to encounter artificial refuges than species restricted to permanent waterbodies, have poor dispersal ability or specific habitat requirements. This bias towards capturing wide-ranging habitat generalists suggests that artificial refuges may have limited application for procuring records of sedentary, range-restricted or stream-breeding frog species, traits that are shared by many threatened Australian frog species (Hero et al. 2006), unless artificial refuges are placed specifically along wetland or stream margins. The use of artificial refuges to survey and monitor stream-dwelling species in Australia requires further research.
Conclusions
Amphibians are a major component of the biota inhabiting riparian and wetland ecosystems in Australia and are often targeted as indicator species in wetland management and surveillance monitoring programmes (McGinness et al. 2014). Although there are well-established methods for surveying amphibians in aquatic environments (Wassens et al. 2017), labour-intensive methods such as installing pitfall and funnel traps have been the primary method of quantifying amphibian abundance and movement patterns in terrestrial environments (Pulsford et al. 2018; Westgate et al. 2018). This study provides the first empirical comparative assessment of the effectiveness of using active searches and artificial refuges to detect amphibians in terrestrial environments. In low rainfall regions, timber refuges were effective for detecting floodplain species during above average rainfall years, whereas active searches of natural habitat and timber and corrugated steel were equally effective for detecting amphibians in high rainfall environments. Active searches and artificial refuges (such as timber railway sleepers and corrugated steel) should be used in future studies as both methods are complimentary in procuring records of different amphibian species. Artificial refuges also provide a robust standardized method for evaluating frog occupancy patterns, although frog detection rates using both active searches and artificial refuges are likely to be influenced by natural variation in levels of suitable terrestrial shelter sites in the surrounding landscape.
Acknowledgements
The work was funded by the Australian Government's Caring for Our Country Initiative, the National Environmental Science Program's Threatened Species Recovery Hub and the Australian Research Council, and is supported by the Murray Local Land Services, Riverina Local land Services, Central Tablelands Local land Services, North East Catchment Management Authority, Goulburn Broken Catchment Management Authority, NSW Environment Trust, Ian Potter Foundation and the Vincent Fairfax Family Foundation. We thank members of our field team whom have assisted with data collection, Mason Crane, Daniel Florance, Christopher MacGregor, David Blair, Lachlan McBurney, Thea O'Loughlin, David Smith, Clare Crane and Sachiko Okada.
Conflicts of Interest
The authors declare no conflicts of interest.
Ethical Approval
This work was approved by the Australian National University Animal Care and Ethics Committee and was conducted under the following scientific licenses: Department of Environment and Climate Change (No. 13174), Queensland Government Environmental Protection Agency (No. WISP08460910), NSW Office of Environment and Heritage (Nos. S12603, SL101022, SL100969), Department of Sustainability and Environment (No. 10005355) and Department of Environment, Land, water and Planning (No. 10007917).




