Behavioural tactics used by invasive cane toads (Rhinella marina) to exploit apiaries in Australia
Abstract
Behavioural flexibility plays a key role in facilitating the ability of invasive species to exploit anthropogenically-created resources. In Australia, invasive cane toads (Rhinella marina) often gather around commercial beehives (apiaries), whereas native frogs do not. To document how toads use this resource, we spool-tracked cane toads in areas containing beehives and in adjacent natural habitat without beehives, conducted standardized observations of toad feeding behaviour, and ran prey-manipulation trials to compare the responses of cane toads versus native frogs to honeybees as potential prey. Toads feeding around beehives travelled shorter distances per night, and hence used different microhabitats, than did toads from nearby control sites without beehives. The toads consumed live bees from the hive entrance (rather than dead bees from the ground), often climbing on top of one another to gain access to the hive entrance. Prey manipulation trials confirm that bee movement is the critical stimulus that elicits the toads’ feeding response; and in standardized trials, native frogs consumed bees less frequently than did toads. In summary, cane toads flexibly modify their movements, foraging behaviour and dietary composition to exploit the nutritional opportunities created by commercial beehives, whereas native anurans do not.
Introduction
To satisfy the demands of a growing human population, many natural landscapes are being converted to farmland (Lambin et al. 2001; Poschlod et al. 2005), thereby directly impacting biodiversity (van Diggelen et al. 2005; Foley et al. 2005; Poschlod et al. 2005). Where some species decline however, others thrive. Although agricultural activities negatively impact species richness, they may facilitate the establishment of invasive taxa (Marvier et al. 2004; MacDougall & Turkington 2005). With increasing international trade, instances of species being transported across biogeographical barriers are on the rise (Hulme 2009). These invasive taxa may compete with natives (Mooney & Cleland 2001), catalyzing biotic homogenization and amplifying the negative impacts of land-clearing (McKinney & Lockwood 1999; Doherty et al. 2015). To understand the impact of anthropogenic habitat modification on biodiversity, we first need to understand the biology of species that benefit from those changes.
What makes invasive species successful in disturbed habitats, whereas natives typically decline? Several traits facilitate an invader's ability to exploit novel environments (Prentis et al. 2008). For example, invasion success has been linked to traits such as high reproductive rates, rapid sexual maturation and physiological plasticity (promoting tolerance to a wide range of environmental conditions: Pockl 2007; Zerebecki & Sorte 2011). Behavioural flexibility may be equally important. For example, innovative foraging techniques may allow high rates of prey intake (Sol et al. 2002). The ability to exploit a wide variety of nutritional resources is common amongst invasive species (Snyder & Evans 2006), and may increase feeding rates, and thus individual fitness (Baird 1990; Wauters & Dhondt 1995; Mooney & Cleland 2001). Understanding the behavioural tactics that invasive taxa utilize to exploit nutritional resources may clarify reasons for their success, and could therefore inform the control and management of such species.
In this study, we investigated how the presence of commercial beehives in eastern Australia influences the ecology and behaviour of invasive cane toads (Rhinella marina). More specifically, we examined nocturnal movement patterns and microhabitat use by spool-tracking toads, quantified their foraging behaviour by direct observation, and measured their degree of philopatry via a mark-recapture study. We also investigated prey movement as a cue that may elicit feeding responses of toads (and native frogs) towards honey bees (Apis mellifera).
Materials and Methods
Study species
Native to Latin America, the cane toad (R. marina, formerly Bufo marinus) was introduced to Australia in 1935 to control insect pests (Lever 2001). Since then, these large bufonid anurans have rapidly spread across tropical and subtropical Australia (Urban et al. 2007). Cane toads primarily exploit anthropogenically-modified sites (Zug & Zug 1979; González-Bernal et al. 2016). Both in New South Wales (NSW) and in the Northern Territory (NT) – the extreme ends of the cane toad's distribution in Australia – we have consistently observed aggregations of cane toads (but not native frogs) around beehives. All methods involving the study species were approved by The University of Sydney's Animal Ethics Committee Protocol #2015/882.
Study sites
Apiaries (sites containing commercial beehives) and control sites (adjacent natural habitat without beehives) were located in or near Yuraygir National Park and Bungawalbin State Forest in the Northern Rivers region of NSW. Apiaries were in open grassy areas ~600 m2 in size surrounded by thick coastal heath. Each clearing contained 12 or more commercial beehives (apiaries, n = 5 clearings) or lacked hives (control sites, n = 6 clearings; see Table 1).
| Site | Classification | Latitude | Longitude |
|---|---|---|---|
| Site 1 | Hived | −29°34′58″ | 153°19′37″ |
| Site 2 | Hived | −29°36′26″ | 153°19′35″ |
| Site 3 | Hived | −29°37′21″ | 153°18′52″ |
| Site 4 | Hived | −29°34′4″ | 153°18′16″ |
| Site 5 | Hived | −29°27′55″ | 153°13′30″ |
| Site 6 | Control | −29°37′27″ | 153°18′55″ |
| Site 7 | Control | −29°34′50″ | 153°19′23″ |
| Site 8 | Control | −29°33′58″ | 153°18′19″ |
| Site 9 | Control | −29°06′10″ | 153°09′54 |
| Site 10 | Control | −29°20′10″ | 153°13′14″ |
| Site 11 | Control | −29°34′26″ | 153°19′23″ |
- All sites, both those containing commercial honeybee hives (‘hived’) and those containing no hives (‘control’) were located in or near Yuraygir National Park and Bungawalbin State Forest in New South Wales, Australia.
Spool-tracking trials
We collected cane toads from apiaries (n = 35 toads) and control sites (n = 34 toads) over 11 nights between October 2015 and January 2016, weighed and measured the toads, and released them again after affixing a 120-m cotton spool around the waist. Next morning, at 10-m intervals along each spool-line, we measured the distance to the nearest beehive (at apiary sites only), and scored microhabitats (grass height and coverage, percentage of each substrate type, and percentage canopy cover) within 1-m2 quadrats. We also measured straight-line displacement from where the toad was found (or the spool ran out) to the toad's release point, and total distance moved by each animal.
Mark-recapture surveys
We carried out mark-recapture surveys at two apiary sites and two control sites over nine capture sessions (between October 2015 and January 2016). Toads were captured in the defined site areas between 21.00 and 01.00 hours, and processed on site (i.e. we recorded each individual's mass, sex and snout-urostyle length) before we released each individual at their site of capture on the same night. Toads <80 mm snout-vent length were classed as juveniles. Toads (n = 282) were individually marked by toe-clipping, with no more than two toes clipped per limb (i.e. each toad had between one and eight phalanges clipped). If an individual was re-captured on a subsequent night, we recorded its unique identification number before releasing the toad once more. This method of uniquely identifying individuals does not induce significant stress in cane toads (Lampo & Bayliss 1996; Fisher et al. 2013; Hudson et al. 2017).
Behavioural surveys
Over 10 nights between December 2015 and January 2016, we observed toads (and any other anuran species present) at apiaries under red-light illumination, instantaneously scoring the behaviours of each individual within a 1-m radius of the hive entrance for 1 min per individual. We recorded foraging behaviour (i.e. individual toads' physical response to the presence of bees as well as how many bees were consumed by focal toads per 1 min survey) as well as toad sex, and distance of each individual from the hive entrance.
Trials of anuran feeding responses
Over 10 nights between December 2015 and January 2016, we dangled model bees in front of nocturnally active anurans (R. marina (n = 91), and the Australian green tree frog Litoria caerulea (n = 45)) to record their responses. To simulate a bee, we attached a cylindrical bead of black electrical tape to the end of a cotton thread 2 m long, with the other end of the thread held by the observer. Initially we placed the stimulus 10 cm in front of the anuran's snout for 25 s (to simulate dead prey), then dragged the ‘bee’ slowly across the ground back and forth in front of the anuran for 25 s (to simulate a bee walking around the hive entrance). The anuran's behaviour and time taken to react were recorded. Each 50 s trial was performed only once per individual anuran to eliminate potential for learned responses. We also utilized a 1 min acclimation period between first approaching the anurans and initiating the feeding trials to reduce disturbance effects.
Statistical analyses
For the spool-tracking trials, variation amongst toads in dispersal traits was examined using ancova (one factor with two levels; apiary vs. control) for both total distance (against the covariate of toad mass), as well as total displacement (against the covariate of minimum night-time temperature). Site was nested within treatment (apiary vs. control) for all analyses. We used Principal Component Analysis (PCA; SPSS v22; IBM Corporation, Armonk, NY, USA) to reduce the number of intercorrelated microhabitat variables. Three significant principal components (PCs; eigenvalues >1) were used as dependent variables in anovas with hive presence as the factor (apiary vs. control).
For the behavioural surveys we used anova (one factor, three levels; female, male, juvenile) to compare toad sex/age classes with respect to distance to the hive entrance, and to the delay before focal toads seized the model mimicking live bees. Logistic regression was used to examine differences between the sex/age classes of toads in this respect, and if they were observed jumping on the back of another toad near the hive entrance. Data were log-transformed to attain homoscedasticity.
For trials of anuran feeding responses we used anovas to examine predatory responses of toads and frogs to bee models (for toads, one factor with two levels, apiary vs. control; if NS, data were pooled for toads from both treatments to compare them to L. caerulea). If there was a significant effect of site, only toads tested at control sites were compared to L. caerulea.
Results
Use of apiaries by toads versus frogs
During our nocturnal behavioural surveys, we recorded 139 cane toads but only seven native frogs (three L. caerulea and four Litoria nasuta) within 2 m of beehives. Native frogs were broadly distributed through the habitat matrix, but did not aggregate around hives. Numbers of native frogs around beehives were too low for detailed behavioural studies, except for the feeding-response trials where we could test frogs throughout the wider landscape rather than only around the hives.
Spool-tracking trials
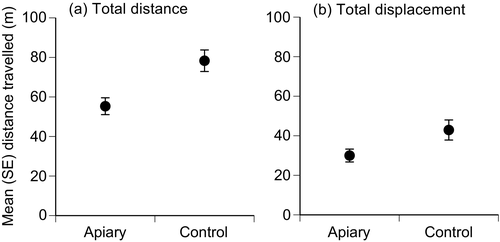
Compared to conspecifics from control sites, total distance travelled by cane toads tracked at apiaries was, on average, 22–23 m less than toads at control sites (F1,55 = 9.04, P = 0.004; Fig. 1a), with larger toads moving on average 21 m further than their smaller counterparts (F1,55 = 7.73, P = 0.007; nested site effect F11,55 = 1.41, P > 0.05). Similarly, total displacement of toads tracked at apiaries was, on average, 13 m less than those at control sites (F1,55 = 9.92, P = 0.003; Fig. 1b), with displacement increasing in warmer weather (F1,55 = 4.68, P = 0.035; nested site effect F11,55 = 2.11, P = 0.035).
From the microhabitat variables scored, PCA calculated three biologically significant PCs. The first PC axis (33% of total variation) was associated with exposed substrate, the second PC axis (26% of variation) with short grass (height ≤30 cm), and the third PC with tall grass (>30 cm high). This third PC axis showed no significant difference in usage between toads at apiary versus control sites (F1,459 = 1.64, P > 0.05; nested site effect F11,459 = 3.33, P < 0.001). However, toads at apiaries differed from conspecifics in nearby control sites with respect to both PC1 (F1,459 = 25.44, P < 0.001) and PC2 (F1,459 = 11.45, P < 0.001). Toads were recorded more frequently in open, grassy habitat at apiaries than at control sites. There was also a significant nested effect of site for both these PC axes (F11,459 = 16.20, P < 0.001 and F11,459 = 3.19, P < 0.001, respectively).
Mark-recapture surveys
Toads were more philopatric around beehives (52% of marked toads were subsequently recaptured at apiaries, versus 38% of toads at control sites: logistic regression, χ2 = 4.63, d.f. = 1, P = 0.032).
Behavioural surveys
Of 139 toads observed over 10 nights at two apiaries, 83 were adult females, 23 were adult males, and the remaining 33 were classified as juveniles. We observed predation of a live bee 30 times (i.e. on 22% of the 1-min observation sessions). When bees leaving the hive walked along the hive entrance, or upwards on the hive box, nearby toads often reacted by raising their heads and lunging towards the bee. Of these successful predation attempts, 57% were by female toads, 13% by males and 30% by juveniles. Over the observation period (1 min per toad), most toads ate just one live bee (or none); but on six occasions, focal toads ate two bees in quick succession. Toads were never seen eating dead bees, although bee carcasses often littered the ground near the hive entrance. There was no significant difference in mean rate per capita of seizing bees amongst sex/age class of toads (F2,133 = 0.25, P > 0.05; mean bees consumed by female toads 0.25, males 0.20, juveniles 0.30; nested site effect F3,133 = 0.98, P > 0.05). Similarly, there was no significant effect of sex/age class on the toads’ mean distance to the hive entrance (F2,133 = 0.18, P > 0.05; mean distance female toads 7 cm, males 8 cm, juveniles 9 cm), although sites differed in this respect (F3,133 = 4.24, P = 0.007).
Another common behaviour (observed 22 times, or 16% of observation records) was for a toad to jump onto the back of another toad to access the hive entrance (Fig. 2). Of the toads manifesting this behaviour, 13 were female (59%), 5 were male (23%), and 4 were juveniles (18%). Therefore, adult female toads, adult males, and juveniles were equally likely to exhibit this behaviour (χ2 = 0.05, d.f. = 2, P > 0.05).

Trials of anuran feeding responses
There was no significant effect of treatment (apiary vs. control) on the rate that cane toads attacked the stimulus object, nor a significant nested effect of site (all P > 0.05; summarized in Table 2; Fig. 3a). There was, however, a significant effect of treatment on reaction time to first predation attempt: cane toads from control sites reacted faster to the presence of simulated live prey than did toads from apiaries (P = 0.005; Fig. 3c). Time to first predation attempt decreased with increasing temperature (P = 0.001).
| Species | Response | d.f. | F | P |
|---|---|---|---|---|
| Rhinella marina | Predation rate | 1, 85 | 0.68 | 0.41 |
| Site | 4, 85 | 1.22 | 0.31 | |
| Minimum temperature | >0.05 | |||
| Rhinella marina | Reaction time | 1, 63 | 8.51 | 0.005 |
| Site | 4, 63 | 1.82 | 0.14 | |
| Minimum temperature | 1, 63 | 12.03 | <0.001 | |
| Litoria caerulea vs. Rhinella marina | Predation rate | 1, 127 | 7.56 | 0.007 |
| Site | 1, 127 | 0.99 | 0.44 | |
| Minimum temperature | >0.05 | |||
| Litoria caerulea vs. Rhinella marina | Reaction time | 1, 36 | 0.6 | 0.44 |
| Site | 1, 36 | 2.89 | 0.049 | |
| Minimum temperature | >0.05 |
- Boldface font indicates a significant effect (P < 0.05).

Cane toads were more likely to launch an attack on the stimulus object than were native frogs (L. caerulea, P = 0.007; Fig. 3b; site effect P > 0.05). Although R. marina was faster to react to the prey stimulus than L. caerulea, this effect was not statistically significant (P > 0.05; nested effect of site P = 0.049; Fig. 3d). Interestingly, neither species reacted to the simulated dead prey; prey movement was needed to initiate feeding responses in both cane toads and green tree frogs.
Discussion
The presence of commercial beehives in eastern Australia has had multiple impacts on the behaviour of invasive cane toads. In this study, apiaries reduced the rate of movement of toads, with toads remaining close to this point source of food at night and over a period of weeks. Total displacements were also reduced at apiary sites, with toads selecting diurnal refugia close to the hives. Commercial beehives not only attract cane toads, but also induce sedentary behaviour. Although habitats were similar in all sites used for spool-tracking (open, grassy areas surrounded by coastal heath vegetation), toads spent their time in different habitats in apiary versus control sites. Beehive-associated toads stayed in open areas close to apiaries rather than the surrounding denser vegetation, whereas toads from control sites rarely used such open habitat.
Similar phenomena occur around anthropogenically-disturbed habitats (buildings) compared to bushland settings in tropical Australia. González-Bernal et al. (2016) reported that cane toads near buildings congregated under artificial light sources (that attract insects), thus inducing a decrease in nocturnal movement. Similarly, toads congregate at bovine feces piles (‘cowpats’), not only for food (dung beetles), but also for moisture and warmth (González-Bernal et al. 2012). These accounts underscore the behavioural plasticity by which cane toads exploit anthropogenic resources. A preference for human-modified habitats is evident in cane toads even within their native range (Zug & Zug 1979), suggesting that behavioural flexibility is a preadaptation to invasion success, rather than a trait that has evolved in response to invasion of novel environments.
Cane toads captured at apiary sites exhibited greater philopatry than did conspecifics that were tracked in control sites of natural habitat. This difference suggests that toads recognize beehives as a reliable source of nutrition within the landscape, and exploit them by revisiting these sites on subsequent nights or weeks; the use of diurnal refuges close to the hives facilitates repeated access to this resource.
Philopatry of cane toads around a nutritional resource has also been reported by earlier studies. For example, Brattstrom (1962) recorded that most of the native-range cane toads he translocated returned to their original capture sites within a few days. Similarly, when Boland (2004) moved cane toads away from the nesting burrows of rainbow bee-eaters, the toads soon returned to their original point of capture. Experimental trials confirm that species within the genus Rhinella can navigate using geometric information (Sotelo et al. 2015). Therefore, toads clearly have the ability to navigate back to sites that provide nutritional resources. Individuals of many invasive species remain philopatric in nutrient-rich hotspots to exploit abundant resources, with extended site fidelity promoting locally-adapted populations (Huey et al. 2005).
Although our red-light surveys indicated that different age/sex classes of cane toads are disproportionately represented around beehives (female toads are most prevalent), we did not observe any differences in foraging tactics between toads in these categories. Mean distance from the hive entrance, as well as foraging success (rate per capita of seizing live bees), were similar amongst all sex/age classes. This null result suggests that all cane toads (both male and female toads, juvenile and adult) were equally likely to recognize and utilize hives as a prey resource.
The specific cues that attract these invasive anurans to beehives remain unclear. Movement by individual bees evokes foraging responses in toads, as shown by our feeding response trials as well as red-light observations (where toads responded to movement at the hive entrance). Previous research into the ‘snap zones’ of anurans suggests that movement is the primary mechanism by which toads locate (and subsequently consume) prey (Heatwole & Heatwole 1968; Ingle 1971). In accordance with this idea, toads may actively select pale-coloured substrates that provide contrast with dark-coloured prey (González-Bernal et al. 2011). All beehives used in our surveys were white (as are most commercial hives), potentially increasing their appeal to foraging toads. However, other cues may play a role also, with leopard frogs found to prey on insects based on chemical cues (Shinn & Dole 1978). Similarly, some anurans use auditory cues for prey-location, with predatory cane toads detecting tungara frogs via their calls (Jaeger 1976; Zug & Zug 1979). Honey bee colonies produce strong odours, as well as acoustic and vibrational stimuli arising from nest ventilation and communication signals (Kirchner 1993). Auditory communication signals are unlikely at night, whereas ventilation is ongoing (Jones & Oldroyd 2007). Cane toads may therefore locate bee colonies via visual, olfactory and/or auditory cues. Future research could usefully explore the mechanisms by which these invasive anurans locate anthropogenically-provided resources.
Behavioural surveys also illustrated the eagerness of toads to gain access to hives, by climbing on top of conspecifics. Clearly, the bees’ defensive strategies (venomous stings: Schmidt 1990) do not deter cane toads from exploiting this nutritious resource. Cane toads are more willing to ingest chemically-defended prey than are many other anuran taxa (Grant 1948), a trait that could enhance competitive superiority (Zug & Zug 1979). We saw no evidence of toads defending food resources against other toads; instead, they used conspecifics as platforms to obtain better access to the hive entrance (Fig. 2). Higher densities of cane toads around a beehive thus may increase total offtake of bees on a per-toad basis, because toads exploit each other's presence to forage more effectively.
Cane toads are often abundant near commercial beehives, and benefit from the food resource that colonies provide, so why don't native frogs also exploit this resource? Our prey manipulation trials indicate that cane toads more readily attack model ‘live bee’ prey than do native green tree frogs. Native frogs recognize honey bees as potential prey, but may avoid areas containing commercial hives due to the presence of larger vertebrate predators (such as snakes) that are also attracted to the hives, as observed during surveys (Silvester et al. 2017). Thus, the benefits of exploiting an abundant and easily accessible prey source may be offset by a higher risk of predation (MacArthur & Pianka 1966). Alternatively, frogs may avoid hives because of the presence of toads. Direct competitive impacts of invasive toads on native frogs are minimal (Freeland & Kerin 1988; Greenlees et al. 2007; Shine 2014), but frogs tend to avoid toads (Greenlees et al. 2007; Pizzatto & Shine 2009; Mayer et al. 2015). Competitive exclusion of L. caerulea by cane toads in apiaries is unlikely, but warrants future research. The final (and most likely) explanation for the scarcity of native frogs around apiaries involves habitat use. Most commercial beehives in northern NSW are situated in open, artificially-maintained grassy areas. Green tree frogs are primarily arboreal, and hence are most often found in woodland settings (Tyler & Knight 2011), reducing resource overlap with cane toads in the open areas around commercial hives. The cane toad's preference for open areas (unlike native frogs) may be a key factor that enhances cane toad utilization of commercial beehives.
In summary, cane toads prefer the open habitats created by apiarists, and exhibit the behavioural plasticity needed to exploit these novel nutritional resources. This plasticity has contributed to the species’ invasion success in Australia (see also González-Bernal et al. 2016). Anthropogenically-modified sites offer resources to invasives that enhance their competitive superiority, and hence facilitate their establishment and spread (MacDougall & Turkington 2005). Making these human-modified sites less appealing to invasive taxa could therefore limit the invader's success in novel environments (Simberloff et al. 2005). Every invasive species is different however, so we need to understand specific invasive taxa in this respect if we are to develop and implement appropriate control programs to target them.
Acknowledgements
We thank staff of Yuraygir National Park (including Russel Jago), David Cowling and Northern Rivers beekeepers for their assistance in data collection. We also thank Lachlan Pettit and Melanie Elphick for their assistance.
Funding
This work was supported by the Australian Research Council (grant number FL120100074).
Data Accessibility Statement
Analyses reported in this article can be reproduced using the data provided by Silvester et al. (2018). Data will be uploaded to Dryad and a DOI provided for the final version of the manuscript.




