Negative or positive density-dependence in movements depends on climatic seasons: The case of a Neotropical marsupial
Abstract
One of the major challenges in animal ecology is to understand the factors and processes driving movement behaviour. Although density may influence movement patterns, the occurrence and nature of density-dependence in animal movements are still unclear, particularly whether it may vary among populations of a species, or across time within a population. Here, we evaluate the occurrence and nature of density-dependence in the movements of a Neotropical marsupial, the Grey four-eyed opossum Philander frenatus (Didelphidae, Didelphimorphia). We quantified fine-scale path tortuosity of individuals inhabiting continuous forest areas and forest fragments, in different climatic seasons (humid vs. super-humid). We also determined the relative importance of population size compared to sex and body mass on movements, using a model-selection approach. In forest fragments, path tortuosity increased with population size in the super-humid season, but decreased in the humid season. In the continuous forest, path tortuosity was affected only by sex and body mass, being slightly higher in males and negatively related to body mass. The occurrence of density-dependence on movements only in forest fragments is likely to reflect the higher overall density of P. frenatus in small forest fragments. The variation in the nature of density-dependence between climatic seasons is likely to reflect a trade off between foraging over large areas (humid season, low resource availability) versus avoiding agonistic encounters (super-humid season, high resource availability). Our results show that (i) density-dependence in movements may be context-dependent occurring only in areas of relatively high overall population density; and (ii) density may affect movements in different ways at different climatic seasons.
Introduction
One of the major challenges in animal ecology is to understand the factors that drive individual movement patterns (Nathan et al. 2008). Animal movement may be affected by several factors extrinsic to individuals, such as luminosity (Zollner & Lima 1999), interspecific interactions (Nathan et al. 2008) and food availability and quality (e.g. Vedder 1984). In addition, movements may also be determined by intrinsic factors, such as body mass and diet type (e.g. Carbone et al. 2005), sex and age (e.g. Austin et al. 2004; Åkesson & Weimerskirch 2014) and memory (Fagan et al. 2013). Recent development of statistical methods and modelling of movements have allowed better description and new inferences based on movement paths (Schick et al. 2008), but it is still unclear which factors are the main determinants of the diversity of movement behaviours found in nature.
Population density is one of the factors that can influence animal movements. Density-dependent mechanisms, such as competition for food and suitable habitat, can regulate species abundance, indirectly affecting how individuals move (e.g. Rodenhouse et al. 2003). Movements may also be directly affected by density, either if individuals avoid or are attracted by co-specifics, which determine, for example, the tortuosity of movement paths: more spatially restricted movements imply higher tortuosity of movement paths, whereas more linear and longer movements imply low path tortuosity (Almeida et al. 2015). If movement paths become less tortuous with increasing density (negative density-dependence in tortuosity), movements may ultimately contribute to population regulation, by spreading individuals over a larger area, thus reducing density when it is high. The occurrence of positive density-dependence in tortuosity (movements becoming more tortuous with increasing density) imply more spatially restricted use of space in high densities, which may generate aggregation patterns independent of patchiness in resources (Liu et al. 2016).
The occurrence and nature of density-dependence in routine or daily movements are uncertain because most studies that investigated density-dependence in movements focused on dispersal only (e.g. Matthysen 2005; Rodrigues & Johnstone 2014). The few studies that evaluated density-dependence on movement found species-specific responses. A positive density-dependence in tortuosity was observed for the Black-eared opossum, Didelphis aurita, which was attributed to intraspecific competition, with individuals exploring the habitat more intensively (i.e. with higher tortuosity) during high-density periods (Almeida et al. 2015). However, no density-dependence effects were found on daily home range sizes for another didelphid in the same study area, the Brown four-eyed opossum, Metachirus nudicaudatus (Ferreira et al. 2017).
The overall density of a species varies among habitats and landscapes (e.g. Umetsu & Pardini 2007; Martin et al. 2012), and thus density-dependence may vary in direction and strength across different areas. In fragmented landscapes, for example, overall density or abundance may increase or decrease compared to large stretches of remaining habitat (e.g. Pardini et al. 2005). In addition, the density of a species in an area may vary in time, for example between different climatic seasons or years (e.g. Ferreira et al. 2016). Thus, to test the generality of density-dependence in animal movements, it is essential to quantify both density and movement patterns in different populations of a species and at different time periods. As this is logistically challenging, it is still unclear whether density-dependence in movements is context-dependent, in both magnitude and direction.
Hypothesis 1: As population size of P. frenatus is higher in the studied forest fragments than in the continuous forest (Vieira et al. 2009), we hypothesized that density-dependence in movements (either positive or negative) would be stronger or more detectable in forest fragments.
Hypothesis 2: Once detected, the direction (positive or negative) of the density-dependence in movements would vary with climatic seasons (humid versus super-humid), as seasonality affects food availability (e.g. Bergallo & Magnusson 1999; Develey & Peres 2000; Naxara et al. 2009), thus probably affecting both population sizes and movement behaviour of individuals (Almeida et al. 2015; Ferreira et al. 2017).
We also determined the relative importance of population size compared to sex and body mass on movement tortuosity. Our empirical data sets and analyses allowed testing for the occurrence and nature of density-dependence in movement patterns in different ecological contexts, thus contributing for a better understanding of the factors affecting animal density and movement.
Methods
Study area
The study was conducted in the mountain range of Serra dos Órgãos, Rio de Janeiro state, Brazil, in a continuous forest within the Serra dos Órgãos National Park, and in two forest fragments located at the base of the mountain. The climate of both areas is mild-humid-mesothermic (Nimer 1989), with a humid season from May to September and a super-humid season from October to April (Loretto & Vieira 2005; Aisengart-Menezes 2010).
The Serra dos Órgãos National Park (22°28′34″S, 42°59′41″W) was created in 1939 and has approximately 11 000 ha (Rocha 2007). The study was conducted in the locality of Garrafão, municipality of Guapimirim, which has about 100 houses sparsely distributed along a dirt road that crosses this stretch of the park (Rocha 2007). The vegetation is classified as ombrophilous dense forest belonging to the Atlantic Forest biome (IBGE 2012). The canopy of the forest is higher than 25 m, with abundant presence of lianas, palm trees, epiphytes, ferns and bromeliads (Vieira & Cunha 2008), and a lower abundance of the brejaúva palm Astrocaryum aculeatissimum, which is considered an indicator of early to intermediate secondary succession establishing after forest disturbance (Sanchez et al. 1999).
The two forest fragments (Forest fragment A: 22°31′8.1″S, 42°50′3.2″W and Forest Fragment B: 22°31′18.5″S, 42°50′12.1″W) are located in Carrazal Farm, in the Macacu River basin, municipality of Cachoeiras de Macacu, and are surrounded by a heterogeneous matrix of pasture and plantations (see Delciellos et al. 2017 for details). Forest fragments A and B have 7.6 ha and 8.7 ha respectively, and are 210 m apart. The vegetation of both forest fragments is also classified as ombrophilous dense forest (IBGE 2012), but with a history of disturbances resulting in a discontinuous canopy about 20 m high, a relatively open understory, and increased presence of A. aculeatissimum, Cecropia spp., vines and lianas (details in Cabral & Fiszon 2004; Freitas et al. 2011).
Sampling of non-volant small mammals
In the continuous forest, three 0.64 ha grids were established at 748, 652 and 522 m a.s.l. Each grid had a 5 × 5 design, totalising 25 trap stations 20 m apart. Each trap station received one Tomahawk trap and one Sherman trap. From August 2000, thirteen trap stations in each grid also received two traps, one of each type, placed between 7–20 m above the ground. Forty-one bimonthly trap sessions were carried out from October 1998 to June 2005, totalling a sampling effort of 42 450 trap-nights.
In each forest fragment, four 340 m transects were established, each with 15 trap stations inside the forest fragment 20 m apart, and five trap stations in the matrix 10 m apart. Each trap station received two live traps, one Tomahawk and one Sherman. In each transect, one of the traps at six of the trap stations inside the forest fragments was placed in the understory 1–2 m above ground. Thirteen bimonthly sampling sessions were carried out from July 2007 to July 2009, totalling a sampling effort of 20 800 trap-nights.
Live traps were kept open and baited for five consecutive nights, and were checked every day in the morning for the removal of individuals captured and bait replacement. The bait was composed of a mixture of peanut-based butter, banana, oats and bacon. In the continuous forest area, pieces of meat and bacon were also used as bait in the Tomahawks. Animals trapped were identified at the species level, measured for head-body length and weighed using spring scales. Sex and reproductive condition were also determined. All individuals were marked with two ear tags before release (National Band and Tag Co, Newport, Kentucky). Trapping and handling conformed to guidelines sanctioned by the American Society of Mammalogists (Sikes & Animal Care and Use Committee of the American Society of Mammalogists 2016). This study was approved by the IBAMA/MMA (Authorization numbers 02001/004671/98-51, 13861-1, and 13861-2).
Spool-and-line tracking
In both areas (continuous forest and forest fragments), adult specimens of P. frenatus, apparently healthy and without pouch young, received a spool-and-line device for movement tracking. The devices consisted of bobbinless cocoons of nylon thread wrapped in a polyvinyl chloride film (Boonstra & Craine 1986; Cunha & Vieira 2002; Loretto & Vieira 2005, 2008; Mendonça et al. 2010; Delciellos et al. 2017). Each device weighed 4.8 g and had 480 m of thread and was attached to the fur between the shoulders of each individual by using an ester-cyanoacrylate-based glue (Henkel Loctite Adesivos Ltda., Manaus, Brazil; Delciellos et al. 2006, 2017). The thread released by the device allowed mapping the animals` path, obtaining movement data on a fine scale (e.g. Delciellos et al. 2017). The direction of the path was measured by aligning a compass with the direction of the thread to the next point of change of direction greater than 5°, and the linear distance between points was measured with a tape measure (Delciellos et al. 2006, 2017).
Data analysis
Path tortuosity was measured using the fractal dimension (D), which ranges from 1 (a straight line) to 2 (a line tortuous enough to completely cover a plane) (Nams & Bourgeois 2004), in 5.18.0 Fractal software, using the Cartesian coordinates of each mapped path. This software uses the method of divisors for calculation of D, which consists in measuring the total path travelled by the animal with dividers of different predefined sizes, and plotting divider size against total path length as a power function (Almeida & Vieira 2008). Some individuals had more than one path tracked, either in the same trapping session (month) or in different trapping sessions. To maximize the independence of the paths for analyses, however, we used only one tortuosity value per individual. If the multiple paths of a given individual were tracked at the same trapping session, the tortuosity value used was the average tortuosity for the different paths. If the multiple paths of the individual were tracked in different months, we used only the data from the month with more line tracked. These choices ensured that the most reliable data were used for each individual.
Population sizes of P. frenatus were estimated at the same trapping sessions (months) of spool-and-line tracking, using the Minimum Number Known to be Alive method (MNKA; Krebs 1966). For the continuous forest, we grouped captures of the three sampling grids to estimate population size for each month, as the grids were embedded within the same large block of forest. For the forest fragments, we estimated population sizes separately for the two forest fragments, as they were separated by a relatively inhospitable matrix, thus most likely representing two different populations. Unfortunately, the low number of captured individuals in the continuous forest precluded using probabilistic estimators that take capture probability into account (as in Ferreira et al. 2016). For the forest fragments, we also obtained population size estimates from the Huggins closed population model (Huggins 1989), which were made by Aisengart-Menezes (2010) for comparison with MNKA (Appendix S1). Estimates from this probabilistic estimator were highly correlated with the MNKA (Pearson r = 0.86, P < 0.001) and showed very similar results regarding density-dependence in movements (Appendix S1). As the MNKA was the only method applicable to the continuous forest, we used MNKA for both continuous forest and forest fragments, to facilitate comparisons of patterns among these areas.
To investigate how population size and other explanatory factors affected path tortuosity, we adopted a model-selection approach based on the Akaike Information Criterion corrected for small sample sizes (AICc; Burnham & Anderson 2002). We built a total of 27 alternative mixed-effect models (Zuur et al. 2009), which included as potential fixed explanatory factors population size, body mass, sex and climatic season (humid vs. super-humid), and also potential interactions between population size (the main variable of interest) and either body mass, sex or climatic season. Models also included random intercepts for the sampling site (i.e. the grid or forest fragment in which the individual was tracked), to control for potential site differences. Among the 27 models, we included a null model containing only intercept and error as fixed parameters (see Appendix S2 for full model list).
We fit the models separately for continuous forest and forest fragments, considering the different sampling designs between areas. This approach allowed testing for density-dependence separately in continuous versus fragmented forests, as well as quantifying the relative importance of population size versus body mass, sex and climatic season in each type of forest. To quantify the relative importance of each variable, we summed Akaike weights (wi) for each variable over all models (Burnham & Anderson 2002). We used log(D-1) (Caldwell & Nams 2006) and log(body mass) in models, as these transformations normalized values as confirmed by visual inspection of histograms.
Results
We successfully tracked 79 paths from 50 P. frenatus individuals in forest fragments (37 paths from 21 males, and 42 paths from 29 females), and 38 paths from 27 individuals in the continuous forest (22 paths from 20 males, and 16 paths from seven females). Population sizes (MNKA) varied from 1 to 6 in the continuous area, and from 4 to 22 individuals in each of the two forest fragments.
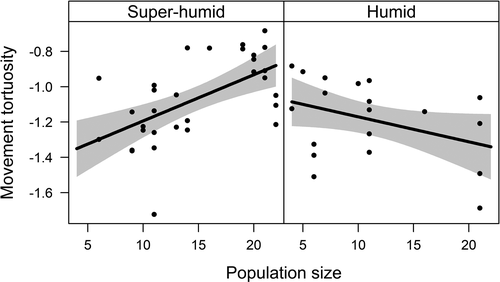
In forest fragments, path tortuosity was affected by population size, climatic season and their interaction (Table 1a). The model including these variables was clearly the most plausible (∆i = 0.00, wi = 0.70), whereas the null model was relatively implausible (∆i = 10.96, wi < 0.01). Path tortuosity increased with population size in the super-humid season, but decreased in the humid season (Fig. 1).
| Variables | k | logLik | AICc | ∆i | w i |
|---|---|---|---|---|---|
| (a) Forest fragments | |||||
| Population size*season | 5 | 10.52 | −7.08 | 0.00 | 0.70 |
| Population size*season + sex | 6 | 10.52 | −4.37 | 2.71 | 0.18 |
| Population size*season + sex + mass | 7 | 10.94 | −2.38 | 4.70 | 0.07 |
| Population size*sex | 5 | 6.35 | 1.25 | 8.33 | 0.01 |
| Population size | 3 | 2.97 | 2.94 | 10.02 | 0.00 |
| Population size*sex + season | 6 | 6.77 | 3.12 | 10.20 | 0.00 |
| Season | 3 | 2.78 | 3.33 | 10.41 | 0.00 |
| Population size*sex + mass | 6 | 6.53 | 3.61 | 10.69 | 0.00 |
| Null | 2 | 1.32 | 3.88 | 10.96 | 0.00 |
| (b) Continuous forest | |||||
| Sex + mass | 4 | 10.61 | −8.36 | 0.00 | 0.17 |
| Sex | 3 | 8.76 | −7.71 | 0.65 | 0.13 |
| Null | 2 | 7.27 | −7.50 | 0.86 | 0.11 |
- k = number of parameters of the model; logLik = log-likelihood; AICc = corrected Akaike Information Criterion; ∆i = AICci – AICcminimum; wi = Akaike's weight of evidence.
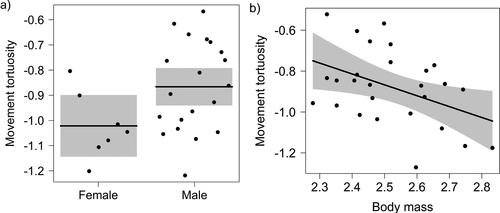
In the continuous forest, the two most plausible models explaining path tortuosity included sex and body mass as explanatory factors (Table 1b). However, the null model was also relatively plausible (∆i = 0.86, wi = 0.11), and the models including population size were less plausible than the null model. Path tortuosity was slightly higher in males than in females (Fig. 2a), and was negatively related to body mass (Fig. 2b).
Discussion
Here, we detected density-dependence on movement tortuosity only in forest fragments, supporting Hypothesis 1. The occurrence of density-dependency in forest fragments, but not in the continuous forest, is probably explained by the marked differences in population size of P. frenatus between these areas (Vieira et al. 2009). Overall, population sizes of P. frenatus are much higher in forest fragments. Thus, the occurrence of density-dependence on movements only in forest fragments probably reflects the higher overall density, as these forest fragments represent small and relatively confined habitat areas, considering that P. frenatus rarely uses the matrix or moves between forest fragments (Aisengart-Menezes 2010; Delciellos et al. 2017).
The nature of density-dependence in the movements of P. frenatus within forest fragments varied between climatic seasons, supporting Hypothesis 2. Tortuosity could only be either positive or negative density-dependent, but the results indicate that the response to co-specific encounters changes between seasons. This pattern is likely the result of a trade off between foraging over large areas versus avoiding agonistic encounters, which differ between seasons. In Atlantic Forest areas, food availability is generally lower in the humid compared to the super-humid season (e.g. Bergallo & Magnusson 1999; Develey & Peres 2000; Naxara et al. 2009). As a result, daily home ranges tend to be larger in the humid season, as previously reported for P. frenatus (Delciellos et al. 2017) and also for other didelphid marsupials (Almeida et al. 2015; Ferreira et al. 2017). Considering that food availability is lower in the humid season, a higher population density in this season may result in stronger competition for the scarce food (Laundré et al. 2014). To acquire the necessary resources for survival, maintenance and reproduction, individuals may need to forage over larger areas, as population density increases. Because straighter paths allow covering larger areas in less time (Zollner & Lima 1999), movement tortuosity decreases with increasing density in the humid season.
Conversely, in the super-humid season, individuals of P. frenatus may forage over smaller areas, with a more tortuous path, because food resources are more abundant, even when population density is high. With this behaviour, individuals may minimize overlap in home ranges, thus reducing the probability of agonistic encounters. In fact, in the super-humid season, movements become more tortuous – and thus restricted to smaller areas – as population density increases, probably reflecting this tendency of restricting movements to avoid agonistic encounters. The same positive association between movement tortuosity and population density was detected in a previous study with D. aurita (Almeida et al. 2015), in the same sites of continuous forest that we studied. Interestingly, however, this previous study detected consistently positive density-dependence irrespective of the climatic season considered. Didelphis aurita is more abundant in the continuous forest (Vieira et al. 2009) and has a more generalist diet than P. frenatus (Moraes et al. 2003). Thus, movements of D. aurita are less likely to be driven by food availability than by co-specific interactions, thus resulting in a positive density-dependence in movement tortuosity even in the humid season.
In the continuous forest, movements of P. frenatus were unaffected by population density, but a minor influence of sex and body mass on movement tortuosity was detected. In Neotropical marsupials, females and males seem to have different behavioural patterns of movements, driven mostly by (i) food acquisition to deal with the high costs of lactation, and (ii) finding mates for reproduction respectively (Loretto & Vieira 2005; Almeida et al. 2015). Previous studies with D. aurita found higher movement tortuosity in the reproductive compared to the nonreproductive season for males, and higher tortuosity in the humid season compared to the super-humid season for females (Loretto & Vieira 2005; Almeida et al. 2015). In our study, path tortuosity was slightly higher in males than in females, with climatic season having no detectable effects. As males and females of P. frenatus have similar daily home ranges (Delciellos et al. 2017), the slightly higher tortuosity of males indicates a more intensive use of similarly sized areas, which may be related to both food acquisition and mate finding. In addition, path tortuosity had a negative relationship to body mass in P. frenatus, probably because large individuals face less obstacles in their paths (Prevedello et al. 2010) and have larger home ranges (McNab 1980; Carbone et al. 2005).
Our results indicate that, at least for P. frenatus, (i) density-dependence may vary in strength and nature among local populations; and (ii) within the same population, density-dependence may have positive or negative effects on movements, depending on climatic seasons and resource availability. Therefore, to better understand the influence of population density on animal movements, it is necessary to test for density-dependence in different populations, varying in overall density and during different years or seasons. These findings contribute to better understanding the ecological contexts under which density-dependence is more likely to affect animal movements.
Acknowledgements
We thank students and staff of the Laboratório de Vertebrados (Universidade Federal do Rio de Janeiro), particularly to Antonio Aisengart-Menezes and Diogo Loretto; and landowners of Carrazal Farm who allowed access to their lands for scientific research. We also thank two anonymous reviewers for valuable comments on the manuscript. Financial support was provided by grants from Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ; to JAP and MVV), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; to JAP and MVV), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and PROBIO II (MCT/MMA/GEF/CNPq). ACD was supported by scholarships from CAPES/FAPERJ (PAPD-E-26/202.144/2015) and SER from CAPES/PROEX (1343229).




