Ythdf2 Ablation Protects Aged Retina From RGC Dendrite Shrinking and Visual Decline
Funding: Shenzhen Municipal Natural Science Fund Key Project, Grant/Award Number: JCYJ20241202125401003; Shenzhen Medical Research Fund, Grant/Award Number: B2302040; National Natural Science Foundation of China, Grant/Award Number: 32371040; Shenzhen-Hong Kong Institute of Brain Science-Shenzhen Fundamental Research Institutions, Grant/Award Number: 2022SHIBS0002. Key-Area Research and Development Program of Guangdong Province, Grant/Award Number: 2023B0303010001.
Fugui Niu and Gaoxin Long contributed equally to this work.
ABSTRACT
Aging-related retinal degeneration and vision loss have been severely affecting the elderly worldwide. Previously, we showed that the m6A reader YTHDF2 is a negative regulator for dendrite development and protection of retinal ganglion cells (RGC) in mice. Here, we further show that conditional ablation of Ythdf2 protects the retina from RGC dendrite shrinking and vision loss in aged mice. Additionally, we identify Hspa12a and Islr2 as the potential YTHDF2 target mRNAs mediating these effects. Together, our results indicate that the m6A reader YTHDF2 regulates retinal degeneration caused by aging, which might provide important therapeutic potential for developing new treatment approaches against aging-related vision loss.
Abbreviations
-
- cKO
-
- conditional knockout
-
- OMR
-
- optomotor response
-
- RGC
-
- retinal ganglion cells
Vision loss and blindness in the elder are affecting hundreds of millions of people worldwide, which needs to be addressed as a public health issue with the aging global population (Blindness et al. 2021; Flaxman et al. 2017). Vision loss in old patients is mostly attributed to aging-related macular degeneration, glaucoma, cataracts, and ocular complications of diabetes mellitus (Pelletier et al. 2016). The retina, as the fundamental structural tissue to encode and transmit visual signals into the brain, is organized by diverse cell types mediating the signal transduction cooperatively (Masland 2012). The degenerations in the aging retina are associated with such diseases as the progressive degeneration of photoreceptors in aging-related macular degeneration and retinal ganglion cells (RGCs) degeneration in glaucoma (Fleckenstein et al. 2021; Weinreb et al. 2014). In addition, disease-free vision decline is also relevant to structural and physiological changes in the retina, including RGC dendrite shrinking, retinal pigment epithelium degeneration, and photoreceptor dysfunction (Datta et al. 2017; Esquiva et al. 2017; Jackson et al. 2002; Owsley 2016; Samuel et al. 2011; Spear 1993). The dendrite arbor is the primary information receptive site in a neuron. The geometrical structure of its dendritic arbor determines the receiving region of input, synaptic density, numerous presynaptic partners, and certain physiological properties (Lefebvre et al. 2015). Rather than neuronal loss, alterations in the dendrite arbors, axonal collaterals, and synaptic density are anatomically detectable in the aged brains (Koch et al. 2021).
Previously we discovered that the m6A reader YTHDF2 negatively regulates dendrite development and injury of RGCs (Niu et al. 2022). The expansion of RGC dendrite arbors and more synapses in the inner plexiform layer after conditional knockout (cKO) of Ythdf2 in the retina modestly improve the visual acuity of mice in an optomotor assay (Niu et al. 2022). In the glaucoma models, the m6A writers METTL3 and WTAP, and its reader YTHDF2, are upregulated, and the loss-of-function of YTHDF2 has a neuroprotective role (Niu et al. 2022; Qu et al. 2021). Besides, m6A modification and METTL3 expression are upregulated under hypoxic and diabetic stress, which governs retinal angiogenesis and pericyte dysfunction of retinal vascular complication (Suo et al. 2022; Yao et al. 2020). However, it remains unknown whether m6A modification and its reader YTHDF2 regulate the degeneration of RGCs in the aged retinas.
We kept Six3-cre+/−,Ythdf2fl/fl (Ythdf2 cKO) and Ythdf2fl/fl control mice and had them grow to 23–25 months old, which is equivalent to approximately 70 years old for humans. We first checked the dendrite morphology of ipRGCs with anti-melanopsin immunostaining in adult and aged mouse retinas. The dendritic area of melanopsin+ ipRGCs was significantly decreased in the aged control mice (23–25 months old, P24M) compared with adult control mice (3.5 months old, P3.5M) (a1 versus a3 in Figure 1a, and quantification in Figure 1b), which is consistent with the previous studies (Samuel et al. 2011).
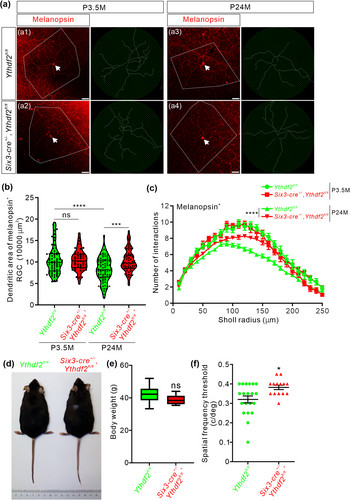
Although the dendrite branching of ipRGCs appeared denser in the young Ythdf2 cKO mice compared to the young control mice at P20 (postnatal 20 days old) (Niu et al. 2022), there are no significant differences in dendritic area or branching in the adult Ythdf2 cKO mice compared with control at P3.5M (a1 versus a2 in Figure 1a, and quantification in Figure 1b,c). These data suggest that the developmental phenotype of Ythdf2 cKO in RGC dendrites is not maintained to adulthood.
Next, we continued to examine the dendrite morphology of ipRGCs in the aged Ythdf2 cKO and control mice at P24M. The dendritic area of ipRGCs was significantly larger in the aged Ythdf2 cKO mice compared to the age-matched controls (Figure 1a,b). The ipRGCs of the aged Ythdf2 cKO mice exhibited more dendrite branches and higher complexity compared to the age-matched controls (Figure 1a,c). All these results suggest that the shrinking of dendrite area and complexity of ipRGCs caused by aging is dramatically alleviated in the aged Ythdf2 cKO mice.
The aging-related declines in spatial contrast sensitivity and visual acuity are attributed to neuronal changes, such as degeneration of RGC dendrites or axons (Samuel et al. 2011). The better RGC dendrite maintenance in the aged Ythdf2 cKO mice inspired us to further explore whether the visual responses of the aged Ythdf2 cKO mice were improved or not. The aged Ythdf2 cKO mice showed similar body size and weight as controls (Figure 1d,e). We carried out the optomotor test on these aged mice. The aged control mice revealed significantly decreased visual acuity with the spatial frequency threshold as 0.32 ± 0.018 c/deg (Figure 1f), compared with the young control mice measuring 0.43 ± 0.0085 c/deg (Niu et al. 2022). However, the aged Ythdf2 cKO mice showed significantly better visual acuity (0.38 ± 0.011 c/deg) compared with the aged control (Figure 1g). These data suggest that the ablation of Ythdf2 in the retina improves the visual acuity of the aged mice.
By proteomic analysis and anti-YTHDF2 RNA immunoprecipitation sequencing, we have identified Hspa12a and Islr2 as the YTHDF2 targets in a glaucoma model (Niu et al. 2022). We further investigated whether Hspa12a and Islr2 mediate RGC dendrite shrinking in the aged retinas. We first performed RT-qPCR to check the expression levels of Hspa12a and Islr2 by comparing the aged Ythdf2 cKO and control retinas. Upregulation of their expression levels was detected in the aged Ythdf2 cKO retina (Figure 2a), implying that the improved visual function in the Ythdf2 cKO mice is likely mediated by the neuroprotective YTHDF2 targets Hspa12a and Islr2. Next, we continued to explore this pathway in the normal aging process. We found that the expression of Mettl14 and Ythdf2 was upregulated in the aged mouse retina compared with the young adults, although Mettl3 expression was not changed (Figure 2b). In line with this, Hspa12a and Islr2 mRNA levels were downregulated with aging (Figure 2c). The upregulation of the m6A writer Mettl14 and reader Ythdf2 in the aged retinas might account for the downregulation of Hspa12a and Islr2.
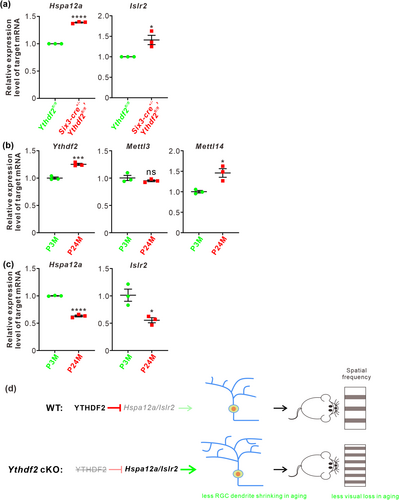
Together, these data suggest that Ythdf2 cKO protects the aged retina from aging-related RGC dendrite shrinking and visual loss, possibly through avoiding downregulation of its neuroprotective targets, Hspa12a and Islr2.
In sum, in the previous work, we revealed that YTHDF2 is a negative regulator for dendrite development and injury of RGCs. Loss of function of YTHDF2 results in increased RGC dendrite branching, more synapses, improved visual acuity, and more resistance for glaucoma model (Niu et al. 2022). In this study, we further explored the function of the m6A reader YTHDF2 to mediate m6A modification in RGC degeneration and vision loss by aging. In the aged retinas, Ythdf2 ablation disrupts the de-stabilization of its target mRNAs and thus increases the levels of its target mRNAs including Hspa12a and Islr2, which results in less RGC dendrite shrinking and less visual loss (Figure 2d). The precise mechanisms of how the aging upregulates m6A modification and how the YTHDF2 target mRNAs protect neurodegeneration in retina still require further investigation. Nevertheless, the epitranscriptomic regulation through m6A modification and its reader proteins in gene expression can be evaluated as possible therapeutic targets for aging-related vision decline.
1 Materials and Methods
1.1 Animals
Ythdf2fl/fl mice were reported previously (Yu et al. 2021), and Six3-cre (Furuta et al. 2000) (The Jackson Laboratory, #019755) were from Jackson Laboratory. Genotyping primers are as follows: The first Ythdf2-loxp site: 5′-GCTTGTAGTTATGTTGTGTACCAC-3′ and 5′-GCAGCTCTGACTATTCTAAAACCTCC-3′; the second Ythdf2-loxp site: 5′-CTCATAACATCCATAGCCACAGG-3′ and 5′-CCAAGAGATAGCTTTCCTAATG-3′. Six3-cre site: 5′-CCTTCCTCCCTCTCTATGTG-3′ and 5′-GAACGAACCTGGTCGAAATC-3′. All experiments using mice were carried out following the animal protocols approved by the Laboratory Animal Welfare and Ethics Committee of Southern University of Science and Technology.
1.2 Immunostaining
For anti-melanopsin retinal wholemount staining, the process was described previously (Niu et al. 2022). All images were captured on a Zeiss LSM 800 confocal microscope with identical settings for each group in the same experiment.
1.3 RT-qPCR
Total RNA was extracted from retinas with TRIzol Reagent (Life) and then used for reverse transcription by PrimeScript RT Master Mix (TaKaRa). Synthesized cDNA was performed with 2× ChamQ Universal SYBR qPCR Master Mix (Vazyme) on BioRad CFX96 Touch Real-Time PCR system. Primers used for qPCR are as follows: mouse Gapdh: 5′-TTGTCAGCAATGCATCCTGCACCACC-3′ and 5′-CTGAGTGGCAGTGATGGCATGGAC-3′ (Niu et al. 2022); mouse Ythdf2: 5′-GAGCAGAGACCAAAAGGTCAAG-3′ and 5′-CTGTGGGCTCAAGTAAGGTTC-3′ (Niu et al. 2022); mouse Hspa12a: 5′-GGGTTTGCACAGGCTAAGGA-3′ and 5′-TCTGATGGACGGTCAGGTCT-3′ (Niu et al. 2022); mouse Islr2: 5′-GAAGCTCCCTTAGACTGTCACC-3′ and 5′-CCCCATCGTGACTCCTGCTG-3′ (Niu et al. 2022). Mouse Mettl3: 5′-AACATCTGTGGCCCCTGAAC-3′ and 5′-AGGTGCATCTGGCGTAGAGA-3′; mouse Mettl14: 5′-TATGCTTGCGAAAGTGGGGT-3′ and 5′-CATCAGGCAATGCTCCTTTGT-3′.
1.4 Optomotor Response (OMR) Assay
Ythdf2 cKO and control mice aged about 24 months were applied for OMR assay as previously reported (Niu et al. 2022). Using the Matlab program, 0.075, 0.1, 0.2, 0.25, 0.3, 0.35, 0.4, 0.45, and 0.5 c/deg (30s per direction of rotation) were used in the recording process. Mouse behaviors were analyzed in real time during the experiment and re-checked with the video recordings. The clockwise maximum spatial frequency that could drive head tracking to the rotation was defined as left eye spatial frequency threshold, and the counterclockwise one was defined as right eye spatial frequency threshold. Finally, the spatial frequency thresholds of the two eyes for each mouse were recorded and analyzed.
1.5 Sholl Analysis
Sholl analysis based on anti-melanopsin retinal wholemount staining was described previously (Niu et al. 2022).
1.6 Dendritic Area Quantification
Quantification of dendritic area followed the previously reported protocols (Milosević et al. 2009). Briefly, the area of the convex polygon joining the outermost distal tips of each terminal dendrite was quantified with traced melanopsin signals.
1.7 Quantification and Statistical Analysis
All experiments were conducted at a minimum of three independent biological replicates in the lab. Statistical analysis was performed using GraphPad Prism 9.0. When comparing the means of two groups, an unpaired t-test was performed on the basis of experimental design. The settings for all box and whisker plots are: 25th–75th percentiles (boxes), minimum and maximum (whiskers), and medians (horizontal lines). Data for all other graphs are mean ± SEM. A p value less than 0.05 was considered statistically significant: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Author Contributions
Conceptualization: F.N., S.-J.J.; methodology: F.N., G.L.; investigation: F.N., G.L., J.Z., J.Y.; data analysis: F.N., G.L.; manuscript writing: F.N.; manuscript revision: F.N., G.L., S.-J.J.; funding acquisition: S.-J.J.; resources: S.-J.J.; supervision: S.-J.J.
Acknowledgements
We thank other members of Ji laboratory for technical support, helpful discussions, and comments on the manuscript. We thank the technical support from the Laboratory Animal Center and the Core Research Facilities of Southern University of Science and Technology.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that supports the findings of this study are available in the Supporting Information of this article.