Harnessing Immune Rejuvenation: Advances in Overcoming T Cell Senescence and Exhaustion in Cancer Immunotherapy
Funding: The authors received no specific funding for this work.
ABSTRACT
Immunotherapy has transformed the landscape of cancer treatment, with T cell-based strategies at the forefront of this revolution. However, the durability of these responses is frequently undermined by two intertwined phenomena: T cell exhaustion and senescence. While exhaustion is driven by chronic antigen exposure in the immunosuppressive tumor microenvironment, leading to a reversible state of diminished functionality, senescence reflects a more permanent, age- or stress-induced arrest in cellular proliferation and effector capacity. Together, these processes represent formidable barriers to sustained anti-tumor immunity. In this review, we dissect the molecular underpinnings of T cell exhaustion and senescence, revealing how these dysfunctions synergistically contribute to immune evasion and resistance across a range of solid tumors. We explore cutting-edge therapeutic approaches aimed at rewiring the exhausted and senescent T cell phenotypes. These include advances in immune checkpoint blockade, the engineering of “armored” CAR-T cells, senolytic therapies that selectively eliminate senescent cells, and novel interventions that reinvigorate the immune system's capacity for tumor eradication. By spotlighting emerging strategies that target both exhaustion and senescence, we provide a forward-looking perspective on the potential to harness immune rejuvenation. This comprehensive review outlines the next frontier in cancer immunotherapy: unlocking durable responses by overcoming the immune system's intrinsic aging and exhaustion, ultimately paving the way for transformative therapeutic breakthroughs.
1 Introduction: Immunosenescence
Aging profoundly impacts the immune system through a process known as immunosenescence, which is characterized by a gradual decline in both innate and adaptive immune function. This decline is closely linked to inflammaging—a state of chronic, low-grade sterile inflammation that develops with age (Lian et al. 2020). Immunosenescence and inflammaging are driven by multiple factors such as persistent antigenic load, cellular damage, metabolic stress, and thymic involution (Liu et al. 2023). As early as young adulthood, the thymus—a key organ for generating naïve T cells—begins to shrink. Across age, thymic epithelial tissue is gradually replaced by adipose tissue, impairing the production of naïve T cells (Liang et al. 2022; Kousa et al. 2024). Additionally, there is an age-related shift in hematopoietic stem cells (HSCs) toward myeloid cell differentiation at the expense of lymphoid lineages (Mejia-Ramirez and Florian 2020). As a result, older individuals exhibit a diminished pool of naïve T cells, compromising the immune system's ability to mount effective responses (Ostrand-Rosenberg and Sinha 2009; Ponnappan and Ponnappan 2011) (Figure 1). Importantly, cellular senescence represents a hallmark of aging and plays a pivotal role in driving these immune alterations. Senescent cells in the thymus and bone marrow secrete SASP factors, which exacerbate inflammaging and disrupt the microenvironment required for T cell development and renewal. These changes underscore the intricate connection between aging, cellular senescence, and immunosenescence.
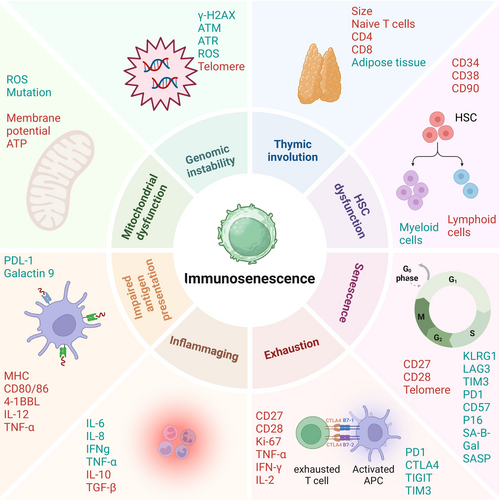
2 T Cell Senescence
T cell senescence represents a specific aspect of immunosenescence, focusing on the functional decline of T cells. Following their release from the thymus, naïve T cells continuously patrol the body, scanning for antigens through their T cell receptor (TCR). Upon antigen recognition, T cells become activated through TCR engagement and co-stimulation by CD28, which binds to B7-1/B7-2 on antigen-presenting cells (APCs) (Han et al. 2023; Mellman et al. 2023). Once activated, T cells can follow one of three primary fates: apoptosis following antigen clearance, exhaustion after repeated low-dose stimulation, or differentiation into long-lived memory T cells (Turner et al. 2021). These memory cells include central memory T (Tcm) cells, which reside in lymphoid tissues and retain self-renewal capacity, and effector memory T (Tem) cells, which reside in peripheral tissues and retain cytolytic functions despite reduced proliferative capacity (Martin and Badovinac 2018). Over time, repeated antigen exposure and aging lead to the accumulation of terminal effector T (TE) cells, known as TEMRA cells. These cells are characterized by the loss of CD27, CD28, and CCR7, along with the re-expression of CD45RA (Wherry and Kurachi 2015). Although TEMRA cells are often considered as senescent T cells, their precise role in immunosenescence remains under investigation (Henson et al. 2015). In addition to the decline in naïve T cells, aging promotes the accumulation of senescent and exhausted T cells. Senescent T cells are characterized by an irreversible cell-cycle arrest and the secretion of SASP factors, contributing to a pro-inflammatory environment. Exhausted T cells, on the other hand, arise from chronic antigen stimulation and exhibit diminished effector functions such as reduced cytokine production and cytotoxicity. Both cell types contribute to immune dysfunction.
In conclusion, while immunosenescence encompasses the broader decline in immune function associated with aging, T cell senescence specifically highlights the deterioration within the T cell compartment. These age-related changes contribute to the progressive deterioration of T cell-mediated immunity and create a permissive environment for the growth of tumors. Therefore, interventions targeting T cell senescence and exhaustion hold promise for improving immune function and enhancing responses to immunotherapies in the context of cancer.
3 Senescent Cells in the TME
The TME is composed of tumor cells and several categories of non-tumor cells such as fibroblasts, endothelial cells, and a variety of immune cells. From the immune cells, CD8+ T cells, CD4+ T cells, natural killer (NK) cells, M1 macrophages, and DCs have an anti-tumor effect. On the other hand, MDCSs, Treg cells, and TAM have a protumor effect and suppress tumor immunity (Gajewski et al. 2013). Senescent cells in the TME have a contradictory role in tumor progression, which can either promote or suppress cancer. Cellular senescence acts as a tumor-suppressive mechanism by restricting the proliferation of damaged cells, including those with activated oncogenes, which are known to drive tumorigenesis (Coppé et al. 2010; He and Sharpless 2017). Spontaneous and therapy-induced senescence (TIS) inhibits tumor growth and stimulates anti-tumor immune responses through several mechanisms, including immune cell recruitment (DCs, NK cells, and T cells) to the TME, activation of TILs, and antigen presentation (Ruscetti et al. 2018, 2020). Similarly, SASP factors such as IL-6, IL-8, CCL5, and CXCL1 are known to promote immune surveillance (Faget et al. 2019) by recruiting M1-like macrophages, NK cells, and CD8 T cells to the TME (Ruscetti et al. 2020). Senescent cells can also present antigens to the immune cells. They are characterized by an elevated expression of MHC-I together with the molecular machinery required for the processing and presentation of antigens, rendering senescent cells highly sensitive to recognition and killing by T cells (Marin et al. 2023; Chen et al. 2023a; Gilioli et al. 2022). Indeed, senescent cancer cells produced a stronger immunization than cancer cells undergoing immunogenic cell death. In addition to directly boosting the immunogenicity of target cells, the induction of senescence in neoplastic cells also enhances T cell-dependent surveillance by promoting the recruitment and maturation of DCs (Marin et al. 2023).
However, in other settings, senescent cells have been shown to contribute to the generation of a protumor and immune-suppressed microenvironment, thereby promoting tumorigenesis (Figure 2). Senescent cells can be tumor-promoting through the secretion of immune suppressive factors, the attraction of immune suppressive cell types, as well as the production of angiogenic and other growth factors (Coppé et al. 2010; Ruscetti et al. 2020; Demaria et al. 2017; Gonzalez-Meljem et al. 2018). For example, senescent endothelial cells create an immunosuppressive TME by favoring tumor infiltration of Tregs and MDSCs and inhibiting infiltration of CD8 T cells, DCs, and NK cells. The SASP factors may also be important mediators of the pro-tumorigenic effects of senescent cells by creating a chronic inflammatory microenvironment that supports cancer growth (Coppé et al. 2008; Lasry and Ben-Neriah 2015). For example, IL-6 secreted by senescent cells in the TME increases the number of tumor-infiltrating MDSCs and suppresses the function of T cells (Faget et al. 2019; Ruhland et al. 2016).
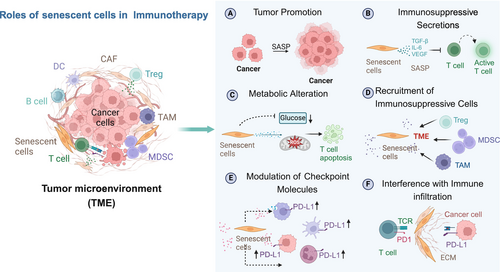
Further, senescent cells in the TME are known to express inhibitory ligands and are resistant to immune-mediated clearance. For example, therapy-induced senescent cells have upregulation of PD-L1 and PD-L2 (Reimann et al. 2021; Chaib et al. 2022; Shahbandi et al. 2022) and anti-PD-L2 treatment is strongly synergistic in combination with standard senescence-inducing chemotherapy in mice, acting as a senolytic immunotherapy (Wang et al. 2022a). Recent works also showed that senescent cells with high levels of PD-L1 inhibit the cytotoxic function of CD8 T cells (Wang et al. 2022a; Onorati et al. 2022). These contradictory effects of senescent cells illustrate the complexity of their functions in the TME and the need to dissect the roles of specific senescent cell subsets and their network of interactions.
The standard cancer treatments—radiation, chemotherapy, and surgery—often result in TIS, contributing to the accumulation of senescent cells that can influence immunotherapy outcomes. Evidence from studies in several types of tumors suggests that the accumulation of senescent cells interferes with immunotherapy, and their removal can reverse this phenotype. For example, recent studies in mice have demonstrated that TIS causes resistance to immunotherapy by reducing CD8+ T cell infiltration and activation within tumors, leading to poor therapeutic outcomes (Demaria et al. 2017; Ruhland et al. 2016). Senescent cells can also synergize with the immunosuppressive microenvironment of tumors. Specifically, they may interact with myeloid cells, which limits the accumulation of CD8+ T cells in the TME and diminishes the efficacy of immunotherapies (Weber et al. 2018; Milanovic et al. 2018). Furthermore, even when a large number of TILs are present in the TME, tumors remain aggressive, suggesting that senescent cells impair effective anti-tumor responses and immunotherapy (Rao and Jackson 2016; Kazemi et al. 2022). Additionally, senescent cells in the TME may promote T cell senescence through SASP factors or direct contact (Rao and Jackson 2016; Hernandez-Segura et al. 2018). Therefore, the presence of senescent T cells poses significant challenges for tumor immunotherapy, which will be discussed next.
Notably, these mechanisms do not manifest uniformly across all cancers and can vary depending on the organ system or disease context. For instance, in the CNS, the immune-privileged environment may dampen senescence-associated immune activation, altering the outcomes of senescent cell accumulation. Similarly, primary tumors and metastatic lesions often exhibit distinct TME compositions, which may influence the role of senescent cells in shaping immune responses and immunotherapy outcomes.
4 Mechanism of T Cell Senescence in the TME
Immunosenescence associated with aging reflects a gradual decline in T cell function due to thymic involution and metabolic stress. In contrast, pathological senescence in the TME is driven by tumor-specific factors such as DNA damage, oxidative stress, telomere attrition, chronic antigen stimulation, and inflammatory signals. Particularly in solid tumors, T cell senescence may be exacerbated by the immunosuppressive nature of the TME, which is characterized by inhibitory immune checkpoints, metabolic constraints, and the presence of immunosuppressive cell populations such as Tregs, TAM, and MDSCs (Woroniecka et al. 2018).
The primary suspects for driving T cell senescence in the TME are nutritional competition, dysregulation of nutrient signaling, and the metabolic reprogramming of T cells (Zhao et al. 2020). While naïve T cells rely on oxidative phosphorylation and fatty acid oxidation to provide energy, upon recognizing an antigen, naïve T cells are activated and differentiate into effector T cells (Sasidharan Nair et al. 2021; Zhang et al. 2024). During this process, mTOR signaling enables a metabolic switch from oxidative phosphorylation to aerobic glycolysis (Fox et al. 2005; Vander Heiden et al. 2009). This shift supports the increased glucose demand in the TME, where competition for glucose among T cells and tumor cells can impact T cell activation, proliferation, and effector functions. Interestingly, SCs are also known to be highly dependent on glycolysis, which all leads to metabolic competition and stress. This metabolic competition results in insufficient energy for T cells and impairs T cell function, proliferation, survival, and promotes senescent phenotypes (Liu et al. 2018; Xia et al. 2021). In association with this, inadequate blood supply to the tumor leads to hypoxic conditions and an acidic environment. Hypoxia and acidic pH conditions are hallmarks of the TME, playing crucial roles in tumor development. Hypoxia and acidic pH are known to cause senescence in different types of cells, including immune cells (Schito and Semenza 2016; Jing et al. 2019).
The other main cause of T cell senescence in the TME is Telomere Shortening. According to the tumor immune cell cycle, T cells, once they recognize their antigen, undergo extensive proliferation, which may lead to telomere attrition (Mellman et al. 2023; Zhao et al. 2020). However, it is important to note that T cells differ from other somatic cells in the expression of the enzyme telomerase. When a T cell recognizes its antigen, TCR activation leads to a transient upregulation of telomerase (Hodes et al. 2002) which enables clonal expansion of T cells and long-lived immunity (Akbar and Vukmanovic-Stejic 2007; Rudolph et al. 1999). However, loss of CD28, which is required for telomerase induction, results in lower telomerase activity and telomere shortening. The most striking finding in this regard is that T cells can elongate their telomeres, even when they do not express telomerase themselves, by acquiring telomeres from vesicles released by APC when they form an immunological synapse (Lanna et al. 2022). Nevertheless, TERT-transduced T cells are still susceptible to telomere-independent senescence, which possibly results from unrepaired cumulative DNA damage that ultimately leads to growth arrest (Scharping et al. 2016). This brings us to the third cause of T cell senescence: DNA damage. As T cells migrate toward sites of TME, they are likely to be subjected to high amounts of ROS generated by innate immune cells such as neutrophils and macrophages. This may cause DNA damage and subsequently senescence. Further, chronic antigenic stimulation induces DNA damage, activating tumor suppressor pathways p53 and p16 to halt the cell cycle and prevent mutation propagation. However, continuous proliferation increases mutation risk, driving genomic instability and senescence (Liu et al. 2023; Campisi 2013).
The TME is characterized by a high presence of immunosuppressive cells, such as TAMs and Tregs. Additionally, in the early stages of tumor formation, adaptive immune cells migrate to the tumor site, leading to dysregulation of cytokines and chemokines, which in turn promotes an immunosuppressive phenotype and contributes to the induction of senescence (Hambardzumyan et al. 2016). Aged macrophages cause paracrine senescence through the secretion of extracellular vesicles (Hou et al. 2024). Similarly, TAMs release reactive oxygen species (ROS), nitric oxide (NO), interleukin (IL)-10, and transforming growth factor (TGF)-β, all known to drive senescence (Dehne et al. 2017). Furthermore, TAMs facilitate the conversion of T cells into Tregs within the TME and enhance the recruitment and immunosuppressive activity of Tregs, which not only impair cytotoxic T cell function but also induce senescence through factors like IL-10 and TGF-β (Gabrilovich and Nagaraj 2009). In a first-in-human study of epidermal growth factor receptor variant III (EGFRvIII)-specific CAR-T cells for glioblastoma (GBM), analysis of tumor specimens from patients who underwent surgery post-treatment revealed an influx of Treg cells, potentially limiting the anti-tumor effects of CAR-T therapy (Rourke et al. 2017). Therefore, elevated levels of TAMs and Tregs may synergistically inhibit T cell-mediated anti-tumor immunity by inducing senescence in the TME.
The other important cause of T cell senescence in the TME is cancer and other senescent cells. Almost all types of cancer therapies, including chemotherapy, radiation, and surgery, are known to cause senescence in the TME known as TIS (detailed review elsewhere; Schmitt et al. 2022; Sullivan et al. 2024). TIS cells accumulated after chemotherapy may induce paracrine senescence in the immune cells in the TME through direct contact or by releasing SASP factors. For example, in mice, CAF senescence limits the presence of activated CD8 + T cells in the TME, and the removal of senescent CAFs increases activated CD8+ T cells and potentiates immune checkpoint therapy (Assouline et al. 2024). Further depletion of CAF reduces the number of Treg cells and reduces tumor growth (Mhaidly and Mechta-Grigoriou 2021). Another study showed that the transfer of senescent T cells to young mice causes paracrine senescence in the recipient mice T cells caused by direct contact with the injected senescent cells or through SASP factors secreted by the injected senescent cells (Callender et al. 2018; Gasek et al. 2021).
5 Dissecting T Cell Senescence and Exhaustion
Both exhausted and senescent T cells share functional deficits and exhibit overlapping molecular features, making these two states difficult to distinguish (Figure 3). However, while exhausted and senescent T cells are often confused, recent findings indicate that they are distinct states of dysfunction (Slaets et al. 2024) and the fate of T cells exhaustion or senescence may be determined by a combination of intrinsic factors and signals from the TME. For example, aged CD8+ T cells intrinsically tend to be more prone to exhaustion, but the aged TME can induce these cells to adopt a senescence-like state (Chen et al. 2024). Interestingly, the aged TME has been shown to push even young, transferred CD8+ T cells toward senescence (Chen et al. 2024), underscoring the critical role of signals from the TME in determining T cell fate.
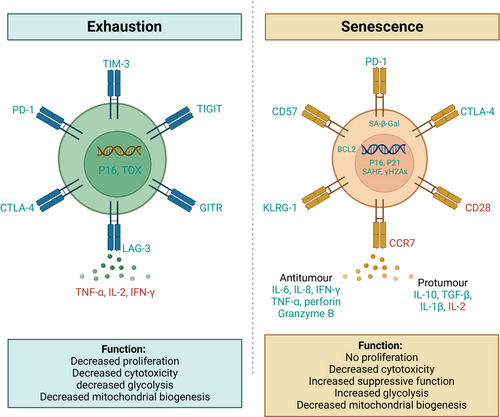
Due to chronic antigen exposure in persistent tumors, T cells undergo a state of exhaustion. T cell exhaustion is characterized by impaired effector functions, reduced cytokine production, diminished cytotoxicity, expression of inhibitory receptors, including PD-1, LAG-3, TIM-3, CTLA-4, and TIGIT, and loss of proliferative capacity (Saleki and Rezaei 2022; Chow et al. 2022). Unlike senescent T cells, exhausted T cells do not show high levels of senescence-associated β-galactosidase (SA-β-Gal) activity (Janelle et al. 2021) and have decreased glycolysis (Han et al. 2023; Cao et al. 2023) (Figures 1 and 3). Further, exhausted T cells are not in a state of irreversible arrest and can potentially regain function with ICIs.
Senescent T cells are characterized by the loss of proliferative capacity, high levels of SA-β-Gal activity, and upregulated cell-cycle regulators like p16 and p21 (Hernandez-Segura et al. 2018). These cells may also upregulate NK cell-like receptors, including killer cell lectin-like receptor subfamily G Member 1 (KLRG1) and CD57, and lose cytotoxic capabilities, such as decreased production of granzyme B and perforin (Liu et al. 2023; Zhang et al. 2021). They also exhibit loss of C-C chemokine receptor type 7 (CCR7), re-expression of CD45RA, and the absence of costimulatory molecules such as CD28 or CD27 (Huang et al. 2024; Kunert et al. 2019). These cells exhibit impaired effector functions, including cytokine production and cytotoxicity, while paradoxically contributing to the pro-inflammatory milieu (Covre et al. 2020; Liu et al. 2020a). Additionally, they often accumulate dysfunctional mitochondria and resist apoptosis through the upregulation of BCL-2 (Hu et al. 2022).
The prevalence of exhausted and senescent T cells varies across tumor types and patient demographics. Exhausted T cells are more commonly associated with solid tumors due to chronic antigen stimulation, whereas senescent T cells are frequently observed in hematological malignancies (Jiang et al. 2015; Kasakovski et al. 2018). Hot tumors, such as melanoma, are characterized by higher infiltration of exhausted T cells. In contrast, cold tumors like GBM exhibit fewer T cells but a higher proportion of senescent immune cells (Woroniecka et al. 2018; Jiang et al. 2015; Miller et al. 2019; Liu et al. 2020b). Evidence suggests that advanced age is associated with higher levels of both exhausted and senescent T cells in tumors, likely due to immunosenescence and cumulative antigen exposure. However, senescence markers appear more pronounced than exhaustion in older patients (Kasakovski et al. 2018). Older patients exhibit phenotypic differences compared to younger patients, including an increased proportion of late-differentiated T cells expressing markers of exhaustion, such as PD-1, and senescence, such as the loss of CD28. These age-related changes in T cell subsets and functionality impair the immune response to tumors and may influence the efficacy of immunotherapies, highlighting the importance of considering patient age in therapeutic strategies (Han et al. 2023; Fernandes et al. 2022; Yu et al. 2025). Although these trends are informative, they are based on limited evidence from small-scale studies and tumor-specific investigations. Variability in methodologies and markers used further complicates comparisons, highlighting the need for broader studies to clarify how the TME, treatment history, and patient age influence these T cell subsets. Further, despite these insights, the precise mechanisms driving T cell exhaustion versus senescence remain poorly understood.
A critical question that remains is whether therapies designed to target T cell exhaustion, such as ICIs, are effective against T cell senescence. Emerging evidence suggests that while ICIs can reverse exhaustion, they may not be effective in counteracting senescence. For instance, a subset of senescent-like CD8+ T cells that are associated with aged TME has been shown to resist ICIs (Chen et al. 2024), indicating that these senescent-like cells represent a unique challenge in the context of immunotherapy. While ICIs can restore the function of exhausted T cells, the persistence of senescent T cells may limit the overall efficacy of these treatments. To improve immunotherapy outcomes, particularly in older patients, it is essential to unravel the specific molecular mechanisms that drive T cell exhaustion and senescence in the aged TME. Therapeutic strategies must be developed to target both dysfunctional T cell states, thereby enhancing the overall immune response and improving the long-term efficacy of cancer immunotherapy.
6 Cancer Immunotherapy
Cancer immunotherapy represents a paradigm shift approach that leverages the immune system's ability to recognize and eradicate tumors (Chen and Mellman 2017). Unlike conventional modalities such as chemotherapy and radiotherapy, which indiscriminately target both tumor and healthy cells, immunotherapies modulate immune pathways to enhance the immune system's ability to discriminate between self and tumor antigens (Mellman et al. 2011). A prominent class of these therapies involves T cell-based strategies, which include immune checkpoint inhibitors (ICIs), CAR-T cell therapy, TCR therapy, tumor-infiltrating lymphocyte (TIL) therapy, oncolytic virus therapy, and Cytokine therapy (Figure 4). Together, these diverse T cell therapies have significantly improved outcomes in various cancer types, including melanoma, non-small cell lung cancer (NSCLC), and renal cancer (Chen and Mellman 2013; Pardoll 2012; Sharma and Allison 2015a; Topalian et al. 2015).
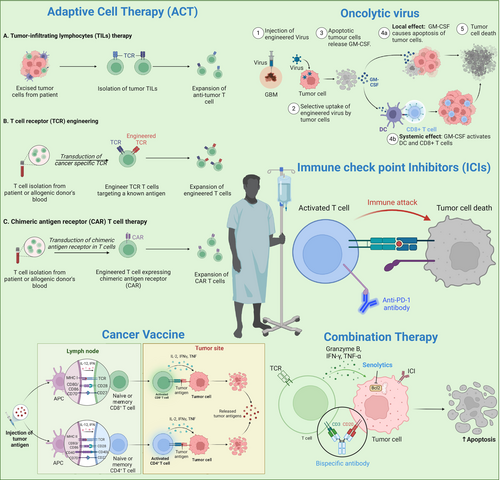
However, the application of immunotherapy to solid tumors has been limited by the immunosuppressive characteristics of the tumor microenvironment (TME). The TME in solid tumors is characterized by multifaceted immune evasion mechanisms, including the accumulation of myeloid-derived suppressor cells (MDSCs), regulatory T cells (Tregs), and tumor-associated macrophages (TAMs) (Wherry 2011; Quail and Joyce 2013). Further, chronic exposure to tumor antigens within this hostile environment leads to T cell exhaustion, a state of progressive functional decline marked by the upregulation of inhibitory receptors such as TIGIT (T-cell Immunoreceptor with Ig and ITIM domains), LAG-3 (Lymphocyte-Activation Gene 3), and TIM-3 (T-cell Immunoglobulin and Mucin-domain containing-3) (Wherry 2011). T cell exhaustion impairs the efficacy of immunotherapies by reducing T cell proliferation, cytokine production, and cytotoxicity of T cells (Pauken and Wherry 2015). In addition to exhaustion, senescence is another key mechanism contributing to immune dysfunction in the TME (Fu et al. 2023). Cellular senescence, originally described as a tumor-suppressive mechanism, is increasingly recognized for its complex and multifaceted roles in tumor biology (Takasugi et al. 2022). Senescent cells are not merely passive bystanders but actively contribute to tumor progression and resistance to therapy (Woroniecka et al. 2018). Unlike exhausted T cells, senescent T cells undergo irreversible cell-cycle arrest wherein they secrete pro-inflammatory secretory phenotypes called senescence-associated secretory phenotypes (SASP) (Fane and Weeraratna 2020). Senescent cells and the SASP factors further contribute to the immunosuppressive nature of the TME, exacerbating the challenge of achieving sustained anti-tumor immunity (Takasugi et al. 2022). The other phenomenon is T cell quiescence, which is a state of reversible dormant state in naïve and memory T cells (Hamilton and Jameson 2012).
Addressing T cell exhaustion and senescence has become a focal point of recent immunotherapy developments. For example, dual checkpoint blockade, such as the combined inhibition of PD-1 and LAG-3, is being investigated to overcome multiple layers of immune suppression (Hodi et al. 2010). Advances in CAR-T cell design, including dual-targeting constructs and engineered resistance to exhaustion, are pushing the boundaries of adaptive cell therapy (ACT) in solid tumors (Bagley et al. 2024). Senolytic agents that selectively eliminate senescent cells offer the potential to dismantle the immunosuppressive signals within the TME and restore effective immune surveillance (Xu et al. 2018). In summary, while immunotherapy has revolutionized cancer treatment, understanding and targeting the complex mechanisms of immune evasion—particularly T cell exhaustion and senescence—within the TME is crucial for its success (Zingoni et al. 2024). This review will focus on the emerging prospects of tumor immunotherapy, with a particular emphasis on the roles of T cell exhaustion and senescence in modulating therapeutic outcomes.
7 Current Landscape of Tumor Immunotherapy
The key immunotherapeutic strategies under investigation include immune ICIs, cancer vaccines, ACT, oncolytic viruses, and combination therapies (Thomas et al. 2012) (Figure 4). Tumor immunotherapy continues to evolve, with an emphasis on overcoming immune evasion mechanisms, such as T cell exhaustion and cellular senescence, which impair the immune system's ability to eliminate tumors (Rong et al. 2022; Sharma et al. 2023).
7.1 ICIs
One of the landmark breakthroughs in cancer immunotherapy came with the development of ICIs, which target regulatory pathways in T cells to restore their anti-tumor activity (Pardoll 2012). Tumors often exploit immune checkpoints like PD-1 and CTLA-4 to suppress immune responses and avoid detection (Wherry and Kurachi 2015). ICIs, such as nivolumab (anti-PD-1) and ipilimumab (anti-CTLA-4), have revolutionized cancer treatment by blocking these pathways, leading to the rejuvenation of exhausted T cells and enhanced anti-tumor immunity (Topalian et al. 2015; Hodi et al. 2010; Sharma and Allison 2015b). However, the efficacy of ICIs is limited especially in some solid tumors due to factors like low neoantigen load and an immunosuppressive TME characterized by Tregs, MDSCs, and TAMs (Hodi et al. 2010; Zhao et al. 2019; Zamora et al. 2018).
7.2 Oncolytic Viruses
Oncolytic viruses are another innovative approach to tumor immunotherapy. These are genetically engineered or naturally occurring viruses that selectively infect and lyse tumor cells while simultaneously stimulating an anti-tumor immune response (Kaufman et al. 2015). Oncolytic viruses can enhance immune recognition of tumors by releasing tumor-specific antigens (TSAs) or tumor-associated antigens (TAAs) and inducing the expression of immune-stimulatory molecules (Asija et al. 2023; Russell et al. 2012). For example, T-VEC expresses the immune-stimulatory cytokine GM-CSF, which promotes the activation of dendritic cells (DCs) and enhances the immune response (Andtbacka et al. 2015). These viruses can modulate the TME, reduce immune checkpoints, and promote immune cell infiltration (Ribas et al. 2017; Desjardins et al. 2018). However, challenges such as pre-existing immunity to viral vectors and ensuring efficient delivery to tumor sites remain.
7.3 Cancer Vaccines
Cancer vaccines aim to train the immune cells to recognize and attack tumors by targeting specific antigens. These vaccines can be peptide-based, DNA-based, or DCs to present antigens to T cells. DC vaccines, in particular, hold promise due to their ability to induce strong anti-tumor immune responses (Yamanaka et al. 2003). For example, the double-blind randomized phase II trial of ICT-107, a peptide-pulsed DC vaccine targeting six GBM-associated antigens, demonstrated a significant improvement in progression-free survival (PFS) by 2.2 months compared to the control group receiving unpulsed DC. In this trial, HLA-A2+ patients exhibited a stronger immune response and greater clinical benefit, particularly in the MGMT-methylated subgroup (Wen et al. 2019). These findings highlight the potential of antigen-specific DC vaccination in GBM treatment, warranting further investigation. The peptide-based vaccine targeting EGFRvIII and a combination of a vaccine targeting survivin with temozolomide (TMZ) have shown extended survival benefits in early-phase trials (Fenstermaker et al. 2016). Personalized mRNA vaccines offer a novel approach by tailoring the vaccine to the unique mutational profile of a patient tumor. These vaccines enable rapid and precise targeting of neoantigens, potentially overcoming challenges of antigen heterogeneity (Wilkinson 2023; Rojas et al. 2023). Despite some successes in early-stage trials, challenges such as antigen heterogeneity, low immunogenicity, and antigen loss variants remain as barriers (Saxena et al. 2021; Grimmett et al. 2022; Katsikis et al. 2024; Lin et al. 2022).
7.4 Adaptive T-Cell Therapy (ACT)
ACT involves the ex vivo expansion and reinfusion of tumor-specific T cells into patients (Dudley and Rosenberg 2003; Rohaan et al. 2019; Posey et al. 2024). This approach includes TILs, T-cell receptor (TCR)-engineered T cells, and chimeric antigen receptor (CAR) T cells (Mohanty et al. 2019; Klebanoff et al. 2023). CAR-T therapy, which engineers T cells to express receptors that recognize specific tumor antigens, has shown remarkable success in hematological malignancies. CAR-T cells targeting TSAs such as EGFRvIII, IL-13Rα2, and HER2 have also shown promising results in preclinical and early-phase clinical trials in solid tumors (Morgan et al. 2012; Durgin et al. 2021; Brown et al. 2024). However, their application in solid tumors presents challenges due to antigen heterogeneity, poor T cell trafficking, and an immunosuppressive TME (Pant and Lim 2023). To address these challenges, strategies such as dual-targeting CARs, armored CARs that secrete cytokines, and locally delivered CAR-T cells are being developed (Bagley et al. 2024). Preclinical studies suggest that these next-generation CAR-T cells may overcome some limitations observed in previous trials (Young et al. 2022; Akhavan et al. 2019). TCR-engineered T cells targeting NY-ESO-1 and other TAA are under evaluation in clinical studies, with the potential to enhance T cell specificity and function against tumor cells (Thomas et al. 2018; Everson et al. 2016). Lastly, trials on TILs indicate that the expansion of TILs isolated from solid tumors is less robust due to the inherently low immune cell infiltration. Further, enhancing the functionality and persistence of TILs within the TME remains a significant challenge (Liu et al. 2017; Verma et al. 2022; Watowich et al. 2023).
7.5 Combination Therapies
Combination therapy aims to target multiple aspects of tumor biology and immune evasion to mitigate resistance mechanisms, improve T cell persistence, and modulate the TME to overcome immunosuppressive barriers. Combining ICIs with conventional treatments (e.g., radiation, chemotherapy) or other immunotherapies (e.g., oncolytic viruses, CAR-T cells) has shown promise in enhancing therapeutic efficacy (Rong et al. 2022; Acosta et al. 2013; Migliorini and Dutoit 2016). Radiation can increase tumor antigen release, while chemotherapy can reduce tumor burden, creating a more favorable environment for immune responses (Bausart et al. 2022; Maity et al. 2018). This effect is exemplified by the abscopal effect, where localized radiation not only induces tumor cell death at the treated site but also triggers systemic immune responses, leading to regression of distant, untreated tumors (Nabrinsky et al. 2022). For example, the combination of TMZ with the IMA950/Poly-ICLC vaccine is currently being explored to leverage chemotherapy-induced immunogenic cell death along with vaccine-induced immune activation (Migliorini and Dutoit 2016; Todo et al. 2022; Migliorini et al. 2019). In addition, ICIs can reinvigorate T cells, while cancer vaccines can enhance local tumor lysis and stimulate a broader immune response (Antonios et al. 2016; Aghajani et al. 2024). For example, DCVax-L, a personalized DC vaccine combined with standard therapies, and ATL-DC, which is being tested in combination with ICIs, have shown promising results in recurrent GBM (Gatto et al. 2023; Everson et al. 2024). In a Phase III trial of DCVax-L, the median overall survival (OS) was 23.1 months for newly diagnosed GBM patients and 13.2 months for recurrent GBM patients, as compared to historical controls. The lack of contemporaneous controls impacts the validity of this trial. Nevertheless, a favorable safety profile was also observed. ATL-DC also boosted T cell activation and infiltration into the TME, countering GBM's immunosuppressive milieu. Its combination with ICIs has led to a favorable OS and increased tumor shrinkage compared to ICIs alone, highlighting its potential to synergistically enhance anti-tumor immunity (Everson et al. 2024). Further, a multi-epitope DC vaccine demonstrated a progression-free survival benefit in a randomized placebo-controlled trial in newly diagnosed GBM patients (Phuphanich et al. 2013).
In summary, recent clinical trials highlight both the promise and challenges of tumor immunotherapy (Weller and Le Rhun 2019). The field is moving toward combination strategies (Bausart et al. 2022), innovative vaccine platforms (Liau et al. 2023), and a deeper understanding of the TME (Sharma et al. 2023) to improve patient outcomes. Ongoing research and future trials will continue to refine these approaches, develop reliable biomarkers, and explore novel combinations to fully realize the potential of immunotherapy.
8 Addressing T Cell Exhaustion and Senescence to Enhance Immunotherapy
Overcoming T cell exhaustion and senescence is essential for improving outcomes in immunotherapy. ICIs have shown limited efficacy in solid tumors, partly due to the high prevalence of senescent T cells within the TME (Maggiorani et al. 2024). Similarly, ACT can be compromised by T cell senescence, leading to decreased therapeutic effectiveness (Amor et al. 2020). Oncolytic viruses and cancer vaccines, designed to boost T cell-mediated anti-tumor immunity, may also face challenges in the presence of these dysfunctional T cells (Chen et al. 2023a). Eliminating senescent cells has shown promise in restoring immune homeostasis and improving survival in preclinical models (Wang et al. 2022b; Prasanna et al. 2021). Therefore, understanding the role of T cell senescence in immunotherapy is crucial for developing more effective treatment strategies. In this section, we explore therapeutic approaches aimed at targeting these dysfunctional T cells.
8.1 Immunomodulatory Interventions
Immunomodulatory interventions, including ICIs, cytokine therapies, and vaccination strategies, aim to reverse T cell exhaustion and reinvigorate anti-tumor immunity. Although ICIs revolutionized cancer treatment, single-agent ICIs often offer limited benefits in highly immunosuppressive tumors like GBM (Chow et al. 2022; Rafiq et al. 2020). Consequently, combining ICIs with agents targeting inhibitory receptors expressed by senescent T cells, such as TIM-3, LAG-3, and TIGIT, has emerged as a promising approach to overcome resistance and enhance T cell rejuvenation (Lu and Tan 2024; Anderson et al. 2016). Recent studies have highlighted the potential of targeting PD-L1/PD-L2-expressing senescent cells to enhance T cell-mediated elimination of senescent cells in aging and cancer models. For example, anti-PD-1 therapy effectively increases the immune clearance of PD-L1+ senescent cells in aging models (Onorati et al. 2022), suggesting a strategy to exploit ICIs against senescent cell populations within tumors. Similarly, targeting senescent cell-specific markers alongside ICIs could restore anti-tumor immunity in preclinical models (Wang et al. 2022b), indicating a dual approach to address both exhaustion and senescence. Other strategies focus on disrupting DNA damage signaling pathways that contribute to T cell senescence. Blockade of proteins involved in these pathways, such as ATM, ATR, or MAPK, has shown potential to prevent T cell senescence and enhance ICI efficacy in multiple cancer types in mice (Liu et al. 2022). Inhibiting DNA damage response pathways in conjunction with anti-PD-L1 significantly delayed tumor progression and improved survival in preclinical models (Chaib et al. 2024). Additionally, cytokine therapies, including IL-2, IL-7, IL-12, and IL-15, are being explored to rejuvenate T cells and boost anti-tumor responses. For example, IL-7 and IL-15 have been shown to promote T cell survival, proliferation, and function, potentially reversing exhaustion and senescence (Kim et al. 2008). Recent clinical trials, such as a Phase I/II study combining pembrolizumab with IL-12, have shown promising early results in enhancing T cell activation and reducing tumor burden in NSCLC, supporting the rationale for combining cytokines with ICIs to overcome T cell dysfunction (Kim et al. 2022). However, designing clinical trials for cancer patients with prior chemotherapy and radiotherapy is challenging due to the development of treatment resistance and the confounding effects of previous therapies on the immune system, complicating the evaluation of new treatments, especially senolytic agents.
8.2 Modulating the TME
Modulating the TME is a crucial strategy to enhance the efficacy of immunotherapy. The TME is characterized by an abundance of Tregs, TAMs, and MDSCs, all of which cause senescence and exhaustion (Chen et al. 2005; Togashi et al. 2019; Lasser et al. 2024). Depleting these immunosuppressive cell populations is a promising strategy to prevent T cell senescence and enhance immunotherapy. For example, CSF-1R inhibitors have been shown to reduce TAMs, resulting in a less suppressive TME and enhanced T cell-mediated anti-tumor responses (Butowski et al. 2016). Additionally, anti-CD25 therapies targeting Tregs have demonstrated efficacy in preclinical GBM models by decreasing Treg populations and augmenting cytotoxic T cell function (Solomon et al. 2020; Nishikawa and Sakaguchi 2014). Inhibiting the cytokines released by these cells is another potential strategy to improve T cell function and therapeutic outcomes (Hambardzumyan et al. 2016; Quail and Joyce 2017). For instance, TGF-β inhibitors, such as galunisertib, have been demonstrated to reduce the immunosuppressive activity of the TME, thereby enhancing the effectiveness of ICIs and ACT (Holmgaard et al. 2018). Similarly, targeting IL-10, a cytokine that suppresses T cell activation, is being explored as a method to reverse immunosuppression (Widodo et al. 2021). However, it remains to be determined whether the benefits of depleting immunosuppressive cells and inhibiting their cytokine secretion are mediated by preventing T cell senescence and exhaustion.
Metabolic reprogramming of T cells in the TME is an emerging approach to counteract the suppressive influence of the TME. Targeting metabolic pathways, such as mTOR, which regulates T cell differentiation and function, can help reprogram T cells to resist exhaustion and senescence. Rapamycin and other mTOR inhibitors have been shown to enhance T cell function and reduce senescence markers in preclinical studies (Waickman and Powell 2012; Chen et al. 2023b; Sullivan and Pearce 2015). Additionally, inhibitors of DNA damage response pathways, such as PARP inhibitors, may prevent T cell senescence by limiting DNA damage accumulation, thereby enhancing anti-tumor activity (Li et al. 2023). Nutritional interventions, such as caloric restriction and antioxidant supplementation, are also being explored as non-pharmacological approaches to modulate the TME and attenuate T cell senescence (Golonko et al. 2024; Yan et al. 2021). Caloric restriction has been shown to reduce inflammation and improve immune function, including T cell activity, in various cancer models (Golonko et al. 2024). For example, an antioxidant N-acetylcysteine (NAC) mitigates oxidative stress, a key driver of senescence, and has shown potential in preclinical models to restore T cell function (Scheffel et al. 2018).
8.3 Enhancing T Cell Persistence and Function
Enhancing T cell persistence and functionality through genetic engineering and advanced cell modification techniques shows significant promise in overcoming T cell exhaustion and senescence in solid tumors. A key strategy involves engineering T cells with additional costimulatory domains, such as 4-1BB or CD28, which enhance their activation, persistence, and resistance to exhaustion and senescence when utilized in CAR-T cell therapies (López-Cantillo et al. 2022; Poorebrahim et al. 2021). These modifications help sustain T cell effector functions, maintain cytokine production, and improve overall T cell survival in the challenging TME. Incorporating cytokine receptors into T cells has also shown the potential to bolster T cell persistence and function by promoting cell survival and reducing senescence-associated markers (Bell and Gottschalk 2021). For example, IL-15 transduction in CAR-T cells enhances their expansion, persistence, and anti-tumor activity, particularly in the immunosuppressive environment of solid tumors (Giuffrida et al. 2020). These modifications not only improve T cell survival but also help sustain anti-tumor responses by mitigating the effects of immunosuppressive signals from the TME (Alizadeh et al. 2019). Additionally, engineering T cells to express anti-senescent factors, such as telomerase or inhibitors of p16 and p21, targets intrinsic aging pathways, reducing senescence and boosting anti-tumor activity (Janelle et al. 2021; Amor et al. 2020; Akincilar et al. 2016). Preclinical studies have demonstrated that T cells engineered to express telomerase maintain longer telomeres, exhibit better proliferative capacity, and show enhanced persistence in ACT settings (Rufer et al. 2001). Lastly, dual CAR-T cell therapies, which target both tumor antigens and senescent cell markers may offer a novel approach to simultaneously address cancer and senescent cells within the TME. For instance, dual CAR-T therapies targeting EGFRvIII on tumor cells alongside senescent cell markers like p16INK4a or Bcl-2 may enhance anti-tumor efficacy by reducing senescent cells that contribute to tumor growth and immunosuppression. This approach provides a comprehensive strategy by addressing both the direct elimination of cancer cells and the mitigation of the pro-tumorigenic effects of senescent cells, potentially improving immunotherapy outcomes.
8.4 Senolytic Therapy
Several studies show that the accumulation of senescent cells in tumors is associated with decreased CD8+ T cell activation and proliferation (Amor et al. 2020; Toso et al. 2014; Eggert et al. 2016). Further, eliminating senescent cells in older individuals or those with TIS can improve the effectiveness of cancer immunotherapies (Amor et al. 2020; Kang et al. 2011). Senolytic therapies selectively target and induce apoptosis in senescent cells by disrupting their pro-survival pathways, presenting a promising approach to mitigate their immunosuppressive effects in the TME. When combined with immunotherapies such as ICIs or ACT, senolytics have demonstrated synergistic effects in preclinical models of different tumors (Montero et al. 2011; Mustjoki et al. 2013; Salam et al. 2023). These combinations enhance cytotoxic T cell function, reduce tumor burden, and restore the immune landscape by reversing the suppressive profile of myeloid cells, thereby overcoming resistance to immunotherapy. For example, the senolytic agent ABT263 has been shown to improve the efficacy of anti-PD-L1 therapy by partially restoring the immunosuppressive phenotype of monocytes and enhancing CD8+ T cell activation and proliferation (Maggiorani et al. 2024; Saleh et al. 2020). Similarly, depletion of senescent cancer-associated fibroblasts (CAFs) using the senolytic drug ABT-199 (venetoclax) increases the proportion of activated CD8+ T cells, and its combination with immune checkpoint therapy significantly reduces tumor burden (Assouline et al. 2024). Furthermore, combining senolytic therapy to prevent tumor-specific T cell senescence with anti-PD-L1 checkpoint blockade has also been found to synergistically enhance anti-tumor immunity and improve immunotherapy outcomes in vivo (Liu et al. 2022).
Senolytics also show potential when combined with standard treatments such as TMZ and radiation therapy in preclinical models, suggesting their role in enhancing overall treatment efficacy (Fletcher-Sananikone et al. 2021; Beltzig et al. 2022). By modulating the immune landscape, senolytics reverse the immunosuppressive profile of myeloid cells and restore cytotoxic T cell functions, ultimately reducing resistance to immunotherapy. Overall, targeting T cell exhaustion and senescence through a combination of immunomodulation, TME modulation, advanced cell engineering, and senolytic interventions provides a multifaceted approach to improving immunotherapy outcomes. However, further research is required to refine these strategies and determine their efficacy in clinical settings, paving the way for more effective and personalized cancer treatments.
9 Conclusions and Perspectives
The landscape of cancer immunotherapy has evolved significantly, yet challenges related to T cell exhaustion and senescence continue to impede progress. The persistent dysfunction of T cells within the TME underscores the need for novel strategies to enhance therapeutic efficacy. The evaluation of senolytics specifically for solid tumors remains in its nascent stages. Additionally, overcoming barriers to effective drug delivery, such as the dense extracellular matrix and abnormal vasculature typical of solid tumors, remains a significant hurdle for the administration of senolytics and other therapeutic agents. Advances in drug delivery technologies, including nanoparticle-based systems and direct intratumoral administration, are being explored to enhance drug penetration and efficacy (Di Filippo et al. 2021). Further, recent findings suggest that cellular senescence, traditionally seen as a permanent state, might be more dynamic than previously believed and hence senescent cells may possess the potential for reversal and proliferation (Aguirre-Ghiso 2007; Roberson et al. 2005). This insight opens exciting possibilities for developing therapies aimed at rejuvenating senescent T cells. Future research should prioritize the clinical trial of senolytics and T cell rejuvenation in combination with immunotherapies to address T cell senescence more effectively.
Key challenges include identifying reliable biomarkers for detecting senescent cells within the TME and distinguishing them from exhausted T cells. Identification of reliable biomarkers and innovative strategies to modulate the TME will be crucial. A multidisciplinary approach is essential to dissect senescence and exhaustion. Understanding the complex interactions between these two cell types and their interaction with other immune cells, such as B cells—which influence TCR diversity and senescence (Khan et al. 2023)—will be critical for developing next-generation immunotherapies.
Moreover, reengineering the TME to overcome physical and biochemical barriers remains a critical challenge. Strategies such as metabolic reprogramming of immune cells (Pauken and Wherry 2015; Cerezo and Rocchi 2020) to enhance their resilience in hypoxic and nutrient-depleted environments, remodeling stromal and extracellular matrix components (Mai et al. 2024; de Visser and Joyce 2023) to facilitate immune cell infiltration, and targeting suppressive cytokines secreted by senescent cells (Lelarge et al. 2024) hold significant promise. Further, Advanced CAR-T designs to target cancer and senescent cells, resist exhaustion, or secrete immunomodulatory cytokines represent the next frontier in cellular therapies for solid tumors (Deng et al. 2024; Feucht and Abou-El-Enein 2020).
In summary, the future of cancer immunotherapy hinges on continued exploration and innovation. Addressing the challenges of T cells senescence and exhaustion, immune evasion, TME immunosuppression, and senescence-immune cells interactions will be key for developing effective, transformative therapies. The path forward involves leveraging emerging insights and technologies to enhance treatment efficacy and improve patient outcomes. Future research must focus on understanding and overcoming barriers in the TME to unlock new potential treatments. Harnessing the mechanisms of senescence in the TME could lead to breakthroughs in enhancing the effectiveness of current treatments and discovering new therapeutic avenues.
Author Contributions
T.D.A. and J.S.Y. drafted and edited the manuscript. T.D.A. drafted all the images.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




