Intraosseous and intravenous administration of antibiotics yields comparable plasma concentrations during experimental septic shock
Conflicts of interest:
Dr Eriksson has received a travel grant from Vidacare Corporation, and Dr Michalek received payment for statistical assistance from the same company.
Funding:
This study was financially supported by the Uppsala University Hospital Research Fund and by an unrestricted grant from Vidacare Corporation.
Abstract
Background
We aimed to investigate whether comparable antibiotic concentrations could be reached with intraosseous and intravenous administration during septic shock.
Methods
In this randomized, prospective experimental study conducted at an animal research laboratory at the University Hospital of Uppsala, eight anesthetized pigs, weighing 21.2 to 29.1 kg (mean: 25.2 ± 2.3 kg), received endotoxin infusion at 4 μg/kg/h for 6 h. At the onset of clinical shock, alternatively after 3 h of endotoxemia, they received 75 mg/kg of cefotaxime and 7 mg/kg of gentamicin either in a proximal tibial intraosseous catheter or in a peripheral intravenous catheter. Mixed venous samples were taken after 5, 15, 30, 60, 120 and 180 min and analyzed for antibiotic concentrations.
Results
For both antibiotics, plasma concentrations after intraosseous and intravenous administration followed similar curves throughout the observation period, and peak concentrations were comparable. Mean concentration area under the curve (AUC mg × h/l) for cefotaxime was 108.1 ± 19.5 after intraosseous and 116.5 ± 11.1 after intravenous administration; ratio 0.93, (95% CI 0.71–1.19). Mean AUC for gentamicin was 28.1 ± 6.8 for intraosseous and 32.2 ± 3.5 for intravenous administration; ratio 0.87 (95% CI 0.62–1.19).
Conclusions
In this porcine septic shock model, intraosseous and intravenous administration of gentamicin and cefotaxime yielded comparable concentrations. In an emergency, intraosseous administration of these antibiotics may be considered in severe infections when venous access is difficult.
In patients with an acute life-threatening infection such as septic shock or meningitis, timely administration of parenteral antibiotics is paramount in order to increase the likelihood of survival. If no intravenous access is immediately available in such patients, intraosseous administration should be considered.
In the patient with an acute life-threatening infection such as septic shock or meningitis, timely administration of parenteral antibiotics is paramount in order to increase the likelihood of survival.1-3 However, gaining access to the circulation could be challenging in hemodynamically unstable patients. A special concern is the pediatric patient, where venous access is often difficult even under stable conditions.4 Today, the intraosseous route of access is widely recommended when there is an urgent need for vascular access and the peripheral intravenous route proves difficult.5-7 Equipment for intraosseous cannulation is often available in emergency departments and pre-hospital care,8-10 and the method is reported to have a high success rate in both of these settings.11, 12 It is documented that many drugs administered intraosseously show similar pharmacokinetics as when administered intravenously.13, 14 One recent study showed that tibial intraosseous drug administration delivered a lower dose compared with sternal intraosseous administration or administration in a central venous catheter during cardiopulmonary resuscitation.15 In antibiotic treatment, peak concentrations may be of importance for antimicrobial effect, as is the case with the aminoglycosides.16 For beta-lactam antibiotics, commonly used for treatment of severe infections, the time above minimal inhibitory concentration (MIC) seems to be of greater importance.17 Previous studies have compared serum concentrations of antibiotics after intraosseous vs. intravenous administration in anesthetized animals. Jaimovich et al. found lower peak concentrations of cefotaxime, chloramphenicol, vancomycin, and tobramycin after intraosseous compared with intravenous administration, while Pollack et al. found comparable levels of gentamicin, cefotaxime, and ampicillin but lower concentrations of ceftriaxone after intraosseous administration.18, 19 However, the animals in these studies were hemodynamically stable.
The aim of this study was to compare, in a septic shock model, the central plasma concentrations after intraosseous vs. peripheral intravenous administration of cefotaxime and gentamicin, two drugs commonly used for empirical treatment of severe infections. The rationale was to simulate a clinical situation with deranged physiology in which intraosseous antibiotic administration might be considered.
Methods
Animals and concession
Eight apparently healthy domestic-breed pigs weighing between 21.2 kg and 29.1 kg (mean 25.2 ± 2.3 kg) were included in the study. All animals were handled according to the guidelines of the Swedish Board of Agriculture and the European Directive on Animal Care. The Animal Ethics Committee of Uppsala University, Sweden, approved the experiment (C140/10, date of approval 28 May 2010).
Anesthesia and preparatory procedures
The animals received pre-medication with 50 mg of xylazin intramuscularly before transport from the breeder.
Anesthesia was induced by an injection of 6 mg/kg of tilétamin-zolezepam mixed with 2.2 mg/kg of xylazin in the neck muscles. General anesthesia was deepened and maintained by a continuous infusion of 8 mg/kg/h of pentobarbital mixed with 1.6 mg/kg/h of rocuronium bromide and 0.48 mg/kg/h of morphine.
After receiving a bolus dose of 20 mg of morphine and 100 mg of ketamine, all animals were tracheotomized in order to secure a free airway and permit mechanical ventilation. Ventilation was maintained by a Servo I ventilator (Maquet Critical Care, Solna, Sweden). During the experiment, the animals received 8 ml/kg/h of a balanced solution containing 25 mg/ml of glucose and 7 ml/kg/h of Ringer's Acetate. After the induction of anesthesia, they also received a 10 ml/kg bolus of a 4% gelatin solution to promote initial circulatory stability. An arterial catheter was placed into a right cervical artery, and a central venous catheter was introduced through the right external jugular vein into the superior caval vein. A Swan-Ganz catheter was introduced through the right external jugular vein into the pulmonary artery for monitoring purposes. A 15G intraosseous cannula (EZ-IO, Vidacare Corp., Shavano Park, TX) was inserted into the proximal tibia. Correct placement was verified by the needle standing without support, aspiration of blood and by an incision to the bone after finishing the experiment. A minor vesicotomy was performed, and a urinary catheter was inserted into the urinary bladder. After preparation procedures, a stabilization period of 30 min in supine position preceded the experiment.
Experimental protocol
An infusion of endotoxin, 4 μg/kg/h, was started to induce experimental septic shock. After induction of endotoxemia, sequential bolus injections of cefotaxime, 75 mg/kg, and gentamicin, 7 mg/kg, each in 10 ml of saline, were given when any of two pre-defined criteria were met. These were: (1) A reduction in mean arterial pressure (MAP) of ≥ 30% of baseline (after induction of anesthesia) level or (2) 3 h of endotoxin infusion, if the first criterion was not met during this time. The antibiotics were randomly administered in the tibial intraosseous cannula in half of the animals and in a peripheral intravenous cannula (ear vein) in the other half in a non-blinded manner. Injections were given over 2 min after which the intraosseous/intravenous cannula was flushed with 10 ml of saline. After the injection, mixed venous samples were taken at 5, 15, 30, 60, 120, and 180 min from the pulmonary artery catheter. During this time, norepinephrine infusion was used when needed, aiming to keep MAP > 60 mmHg. Also, epinephrine in bolus doses of 0.1 mg was used as rescue treatment for circulatory collapse with MAP ≤ mean pulmonary artery pressure (MPAP).
Bioanalytical method
The laboratories analyzing the samples were blinded to modes of administration for the individual samples. Cefotaxime in pig plasma was quantified with a protein precipitation-based method using reversed-phase high-pressure liquid chromatography separation coupled to mass spectrometry at the Therapeutic Drug Monitoring Laboratory, Dept. of Pharmacology, Karolinska University Hospital, Stockholm, Sweden. Plasma samples were mixed with internal standard solution (ceforanide) and precipitated with acetonitrile. An aliquot of 0.8 μl of the supernatant was injected on a Kinetex column (C18. 2.6 μm, 50 × 2.1 mm, Phenomenex, Torrance, CA, USA) using gradient condition. Mobile phases consisted of aqueous 25 mM formic acid and 100% acetonitrile. Detection was performed by a Agilent 1100 MSD (Santa Clara, CA) in SIM mode using electrospray ionization monitoring the transition of the pseudomolecular ion for cefotaxime at m/z 457 and internal standard, at m/z 520. The process efficiency for the bioanalytical method was 123%. Limit of detection was approximately 0.03 μg/ml using a sample volume of 50 μl plasma, and the method was validated for the concentration range 0.39–50 μg/ml. There were no significant differences in validation parameters between human and pig plasma. Gentamicin was analyzed on an Architect Ci8200 analyzer (Abbott Laboratories, Abbott Park, IL, USA) using reagent from the same manufacturer (1P31). The total coefficients of variation for the gentamicin assay were 1.7% at 3.0 mg/l and 2.2% at 5.5 mg/l.
Statistical methods
Based on previous findings, given a standard deviation of 0.1 and with an alpha error of 0.05, a sample size of four animals per group was calculated to give an 80% power of detecting a 25% difference in mean concentration, which was considered clinically relevant.19 The total sample size was eight; four randomly selected subjects were measured with regard to plasma concentrations of cefotaxime and gentamicin after intraosseous administration, and the remaining four subjects were measured with regard to plasma concentrations of cefotaxime and gentamicin after intravenous administration at 5, 15, 30, 60, 120, and 180 min. The data were reduced to two concentration by time curves, one for each method of administration (intraosseous, intravenous) for each drug (cefotaxime, gentamicin). The area under the curve (AUC; mg × h/l) was calculated for each of these curves. Methods were contrasted with regard to the mean AUC with a linear model of AUC in terms of method. Data were summarized with the sample size, mean, standard deviation, median, minimum, and maximum. The P-value for testing Ho: μIO = μIV vs. H1: μIO ≠ μIV, where μIO and μIV are the mean AUC under the intraosseous and intravenous concentration by time curves, and the 95% confidence intervals for the difference μIO − μIV and for the ratio μIO/μIV of AUC means were tabulated. For each subject, the AUC was determined with the trapezoidal rule, and the confidence interval for the ratio of means was computed by analyzing in log units then applying the antilog to the confidence interval for the difference of means. All statistical testing was two sided with a significance level of 5% and SAS Version 9.3 for Windows (SAS Institute, Cary, North Carolina) was used throughout.
Results
After starting the endotoxin infusion, most of the animals showed signs of hemodynamic instability with reduced MAP and cardiac index and elevated MPAP. All animals except animal 3 needed norepinephrine to keep MAP > 60 mmHg. Animal 5 had two short episodes of pulseless electric activity but recovered after resuscitation with a total of 0.3 mg of epinephrine, and animal 6 died after 4 h from circulatory collapse despite attempted resuscitation with 0.3 mg of epinephrine. Physiologic data over the course of the experiment are presented in Fig. 1. All animals except animal 3 received antibiotics based on the criterion of a ≥ 30% reduction of baseline MAP.
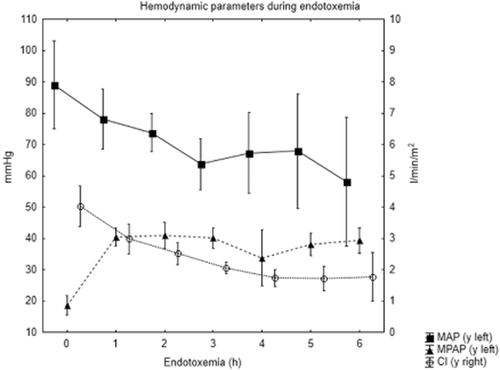
Hemodynamic parameters during endotoxemia (mean ± SD). MAP, mean arterial pressure, MPAP, mean pulmonary artery pressure, CI, cardiac index.
Antibiotic concentrations are shown in Figs 2 and 3. As is seen here, intraosseous and peripheral intravenous administration both resulted in high central plasma concentrations of the two antibiotics. Median (range) concentration of cefotaxime at 5 min after intraosseous administration was 200 mg/l (135–260) compared with 183 mg/l (161–201) after intravenous administration. For gentamicin, median concentrations at 5 min were 34 mg/l (28.8–38.2) after intraosseous and 29.2 mg/l (27.5–34.8) after intravenous administration. Plasma concentrations of both antibiotics followed similar elimination curves for both modes of administration. Due to the death of one animal and one missing sample, intraosseous data from 120 and 180 min are from two and three subjects, respectively. As shown in Tables 1 and 2, mean concentration area under the curve (AUC mg × h/l) for cefotaxime was 108.1 ± 19.5 after intraosseous and 116.5 ± 11.1 after intravenous administration; P 0.48, ratio 0. 93 (95% CI 0.71–1. 19). Mean AUC for gentamicin was 28.1 ± 6.8 for intraosseous and 32.2 ± 3.5 for intravenous administration; P 0.32, ratio 0.87 (95% CI 0.62–1.19).
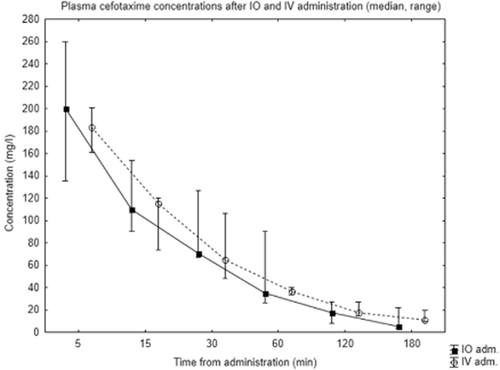
Plasma concentrations of cefotaxime after intraosseous (IO) and intravenous (IV) administration.
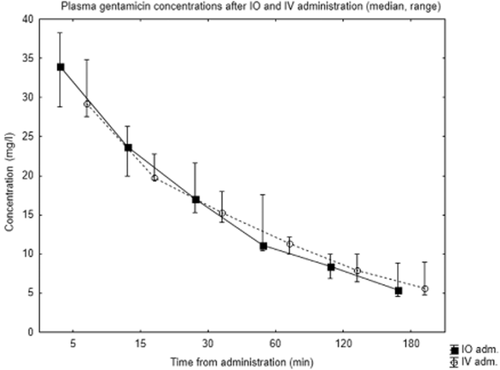
Plasma concentrations of gentamicin after intraosseous (IO) and intravenous (IV) administration.
| Method | |||||
|---|---|---|---|---|---|
| IO | IV | P-value* | 95% CI† | 95% CI‡ | |
| N | 4 | 4 | |||
| Mean (SD) | 108.1 (19.5) | 116.5 (11.1) | |||
| Median | 113.6 | 111.8 | |||
| Range | 81.7, 123.4 | 109.5, 133 | |||
| ● | ● | 0.48 | (−35.95–19.07) | (0.71–1.19) | |
- *For the inequality of means based on analysis of variance. †95% confidence interval CI) for the difference of means IO minus IV). ‡ 95% confidence interval for the ratio of means IO divided by IV). AUC, area under the curve; IO, intraosseous; IV, intravenous.
| Method | |||||
|---|---|---|---|---|---|
| IO | IV | P-value* | 95% CI† | 95% CI‡ | |
| n | 4 | 4 | |||
| Mean (SD) | 28.1 (6.8) | 32.2 (3.5) | |||
| Median | 28.3 | 32.4 | |||
| Range | 21.1, 34.9 | 28.5, 35.7 | |||
| ● | ● | 0.32 | (−13.47–5.26) | (0.62–1.19) | |
- *For the inequality of means based on analysis of variance. †95% confidence interval C) for the difference of means IO minus IV). ‡95% confidence interval for the ratio of means IO divided by IV). AUC, area under the curve; IO, intraosseous; IV, intravenous.
Discussion
Administration of antibiotics using an intraosseous access may be considered when facing a critically ill patient with a suspected severe infection such as septicemia or meningitis where venous access is difficult to establish. This could be of particular importance in remote or pre-hospital situations and with pediatric patients, where intravenous access is often challenging. It is important to know if effective antibiotic concentrations can be achieved in the circulatory unstable septic patient. This animal study indicates that high peak concentrations of the commonly used broad-spectrum drugs cefotaxime and gentamicin can be reached with intraosseous administration under such conditions. Average levels with intraosseous administration were generally similar to those seen with intravenous administration, although the dispersion of the concentrations was greater for the intraosseous route. Cefotaxime has a higher, although still only around 35%, degree of protein binding than gentamicin, which might lead to a somewhat less predictable uptake from the bone marrow.19 The findings indicate that intraosseous administration may be a better alternative than intramuscular injection, where peak concentrations are more slowly reached compared with intravenous administration, in the setting of difficult venous access.20 In this animal model, serum half-lives of the drugs were shorter than what is described in humans,20, 21 this applied to both methods of administration.
The study has some obvious limitations. It is an animal model, and the results are likely to be, but not necessarily applicable in humans. However, randomizing septic shock patients to intraosseous or intravenous antibiotic administration would not be ethically acceptable. Further, the study size does not allow detection of smaller differences and, because of missing data, the 120 and 180 min intraosseous concentrations should be viewed with special caution. Ideally, in order to evaluate bioequivalence, a cross-over design including washout periods should be used. This was not considered feasible from an animal ethical perspective. However, considering commonly observed MIC values for community-acquired pathogens, both modes of administration clearly yielded therapeutic drug concentrations.22 Another potential concern is the reported toxic effect on marrow-derived mesenchymal stem cells from high local concentrations of gentamicin,23 although in a medical emergency one may be willing to accept this risk. Results from this study should not be extrapolated to antibiotics with other pharmacokinetic properties than the ones investigated.
Conclusions
In summary, this animal model indicates that intraosseous access may be an effective way to administer cefotaxime and gentamicin in septic shock. Intraosseous antibiotic administration should be considered when time is important and there is a difficulty establishing venous access.
Acknowledgements
We thank Monika Hall for the excellent assistance in the animal laboratory, and Tommy Pettersson for performing the cefotaxime analyses.




