Mechanism of action of adapalene for treating EGFR-TKI-induced skin disorder
Abstract
Background
Skin disorders are the most common side effect associated with epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) therapy. It is important to manage skin lesions. Adapalene has been used to treat skin lesions caused by EGFR-TKIs in some cases. The aim of this study was to investigate the functional mechanism of adapalene in erlotinib-induced skin disorder.
Methods
To analyze the effect of adapalene on skin rash, afatinib and adapalene were administered to mice. The relationship between the concentration of adapalene and skin disorders was also examined by analyzing AQP3 expression. A skin lesion model was experimentally established in human skin keratinocytes (HaCaT) by using erlotinib with TNF-α and IL-1β. We used qRT–PCR to analyze chemokine-induced inflammation and western blotting to analyze the effects of adapalene on the NF-κB signaling pathway. Antimicrobial peptides and adhesion factors were also examined using qRT–PCR.
Results
Mice administered 0.01% adapalene had less skin inflammation than mice treated with afatinib alone. The expression level of AQP3 decreased in an adapalene concentration-dependent manner. The mRNA levels of proinflammatory cytokines such as CCL2 and CCL27 in HaCaT cells were significantly reduced by adapalene. The expression of an antimicrobial peptide, hBD3, was upregulated after adapalene treatment. Adhesion factors, such as E-cadherin, were significantly downregulated by EGFR-TKI and significantly upregulated by adapalene treatment. Western blot analysis suggested that erlotinib-induced phosphorylation of p65 was decreased by adapalene.
Conclusion
We suggest that adapalene may be a possible treatment option for skin disorders induced by EGFR-TKIs.
INTRODUCTION
Lung cancer is the most common cause of cancer-related death. In non-small cell lung cancer (NSCLC), epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) have been effective in treating EGFR-mutated lung cancer.1, 2 However, the adverse events caused by EGFR-TKI therapy are unique from those caused by conventional cytotoxic chemotherapy. Among these adverse events, skin disorders occur frequently reduce patient quality of life.3 It is also known that the severity of EGFR-TKI-induced skin rashes are dose-dependent, with increased drug exposure leading to increased incidence and severity.4 It is important to control skin disorders since they can hinder further EGFR-TKI treatment.
EGFR-TKI-induced skin disorders include papulopustular rush, hair loss, eye/eyelash abnormalities, periungual/nail alterations and xerosis/pruritus.5 In particular, maculopapular rash has been reported to be present in 50%–100% of patients.6 Important skin tissues such as epidermal tissue, hair follicles, and tissues surrounding the nail express EGFR, and EGFR-TKI treatment inhibits the activity of EGFR in the skin, resulting in the abnormal keratinization of keratinocytes. In addition, chemokines such as C-C motif chemokine 2 (CCL2), CCL5, and C-X-C motif chemokine ligand 10 (CXCL10) are produced by epithelial cells, causing chemokine-induced inflammation and various skin disorders. In addition, secondary infections and barrier defects can exacerbate skin disorders.7 Inhibition of epidermal defense factors, such as antimicrobial peptides and intercellular adhesion factors, by EGFR-TKIs causes epidermal infection.8 Specifically, some of these factors include hBD3, an antimicrobial peptide, and E-cadherin, an adhesion factor.
The skin regulates water permeability through aquaporins (AQPs). In particular, aquaporin 3 is abundant in the epidermis, the outermost layer of the skin. Defects in AQP are known to reduce water content and delay wounding in the skin as a result of reduced water transport in keratinocytes and impaired keratinocyte migration.9, 10 Previously, it has been reported that retinoic acid can upregulate the expression of AQP3.11, 12
Adapalene is a naphthoic acid derivative similar to retinoids (vitamin A derivatives). The chemical structure of adapalene is 6-[4-Methoxy-3-(tricyclo[3.3.1.13,7]dec-1yl)phenyl]naphthalene-2-carboxylic acid, and its molecular formula is C28H28O3. Its structure is highly stable, and adapalene is virtually insoluble in water, acetonitrile, or ethanol. Adapalene activates gene transcription by binding to retinoic acid receptors in the cell nucleus and exerts effects on keratinocytes in follicular epithelial cells. It is used for the treatment of acne vulgaris and is recommended for patients with acne due to skin damage caused by EGFR-TKIs, since there have been reports that oral retinoic acid has been successful in treating skin disorders caused by EGFR-TKI therapy.13 Some studies report the use of adapalene for skin damage caused by EGFR-TKIs.14, 15 However, according to the Multinational Association for Supportive Care in Cancer (MASCC) Skin Toxicity Study Group, which is comprises international, interdisciplinary experts in dermatology, oral retinoic acid is recommended for the treatment of skin disorders caused by the use of EGFR-TKIs.16 Although adapalene plays crucial roles in antiproliferative and anti-inflammatory pathways,17 the therapeutic mechanism of action of adapalene in EGFR-TKI-induced acne-like eczema is not clear. Therefore, the aims of this study were to analyze the effect of adapalene on EGFR-TKI-induced skin disorders and to clarify the mechanism of action of adapalene.
METHODS
Reagents
Erotinib was purchased from Chugai Pharmaceutical. Afatinib was purchased from Boehringer Ingelheim Japan. Adapalene was purchased from Tokyo Chemical Industry.
Mice
Male Slc mice (5 weeks old) were purchased from SLC (Shizuoka, Japan). The mice were divided into five groups: control, afatinib, adapalene, afatinib and adapalene, and afatinib and low concentration 0.1% adapalene. Afatinib (0.1 mg/0.1 g) was applied to the skin of the mice. Adapalene (0.01% or 0.1%) was also administered to the mouse skin. All mice were observed for 7 days. This study was approved by the President of Kobe University after review by the Institutional Animal Care and Use Committee (permission no.: P211001) and carried out according to the Kobe University Animal Experimentation Regulations.
Cell culture and treatment
Human skin keratinocytes (HaCat) purchased from CLS Cell Lines Service GmbH were cultured in Dulbecco's modified Eagle medium (DMEM: Wako) with 10% fetal bovine serum (FBS) and 1% antibiotic solution. All cells were grown in an incubator with 5% CO2 at 37°C, and the medium was replaced every 2 days. To induce inflammation, 10 ng/mL TNF-α and 5 ng/mL IL-1β were added for 6 h or 24 h. Erlotinib (10 μM) and various concentrations of adapalene (25 nM, 250 nM, 2000 nM) were used.
Real-time quantitative PCR
After treatment for 24 h, total RNA was isolated using Sepasol as previously indicated.18 Total RNA was reverse transcribed from RNA to cDNA using 5X Primescript RT Master Mix (Takara). Real-time PCR was performed using TB Green Premix Ex Taq II (Takar). To stimulate cytokinesis, cells were treated with 100 ng/mL LPS. GAPDH was selected as the control. Primers were constructed for CCL2, CCL27, LL37, hBD3, and RNase-7.
Western blot analysis
Western blotting was performed using a previously reported protocol.18 In this study, the following primary antibodies were used: β-actin (#4967), NF-kB (#8242), and phospho-NF-kB (#3033). All were purchased from Cell Signaling Technology.
Statistical analysis
Data are presented as the mean ± standard deviation (SD). Statistical analysis was used to compare differences between the two groups using Student's unpaired t test. A p-value < 0.05 was considered statistically significant for all analyses.
RESULTS
Effect of adapalene on skin rash
Mice were observed for 7 days in the control, afatinib alone, and afatinib plus 0.01% adapalene groups, and skin rashes were observed on Day 3 in the afatinib group but not in the afatinib plus 0.01% adapalene group. Pathological findings showed less inflammation in the afatinib plus 0.01% adapalene group than in the afatinib group (Figure 1). Intriguingly, mice treated with 0.1% adapalene, with or without afatinib, showed more skin inflammation (Figure S1).

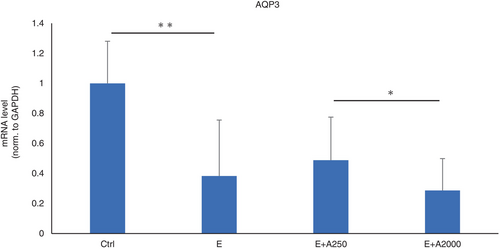
Effect of adapalene on the expression of AQP-3
To evaluate the relationship between adapalene concentrations and skin rashes, we investigated the expression of AQP3, which is present in the outer epithelial layer of the skin, by RT–PCR. HaCaT cells were treated with an EGFR-TKI only or an EGFR-TKI and adapalene. The results showed that the expression of AQP3 was downregulated by the EGFR-TKI. The expression of AQP3 further decreased with higher concentrations of adapalene (Figure 2). Taken together, these results suggest that adapalene can partially ameliorate EGFR-TKI-induced skin disorders by downregulating AQP3.
Role of adapalene in the inflammation of keratinocytes treated with EGFR-TKIs
The inhibitory effect of adapalene on the levels of inflammatory cytokines in the skin tissue of mice treated with EGFR-TKIs was analyzed by RT–PCR. Human inactivated epidermal keratinocytes (HaCaT cells) were treated with an EGFR-TKI only and an EGFR-TKI and adapalene. The results showed that the expression of chemokines CCL2 and CCL27 was upregulated by EGFR-TKI treatment and downregulated by adapalene treatment (Figure 3a, Figure S2).
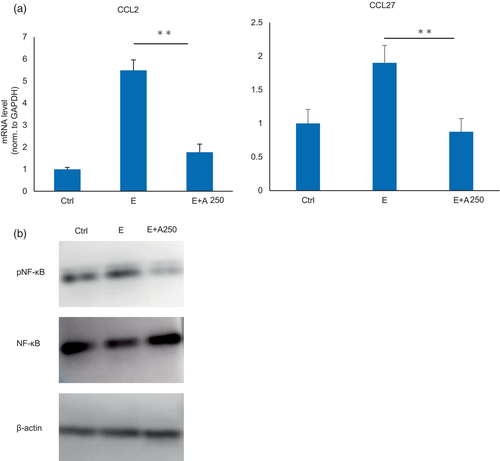
NF-κB, which regulates the transcription of cytokines, has been shown to be induced by EGFR-TKI stimulation in cancer cells. We used HaCaT cells to analyze the expression of NF-κB after EGFR-TKI and adapalene stimulation by western blotting and elucidate the mechanism by which adapalene reduces skin inflammation. pNF-κB was downregulated by adapalene treatment, suggesting that adapalene may ameliorate EGFR-TKI-induced skin damage via NF-κB signaling (Figure 3b).
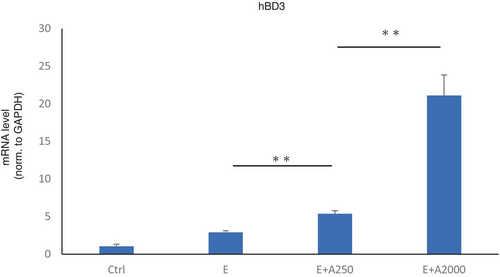
Role of adapalene in antimicrobial peptide production
HaCat cells were used to evaluate the antimicrobial role of adapalene using RT–PCR and HaCaT cells treated with an EGFR-TKI alone or an EGFR-TKI with adapalene. The expression of hBD3, an antimicrobial peptide, was upregulated by adapalene treatment in a dose-dependent manner (Figure 4). However, hBD3 was not downregulated by EGFR-TKI treatment, contrary to our expectation.
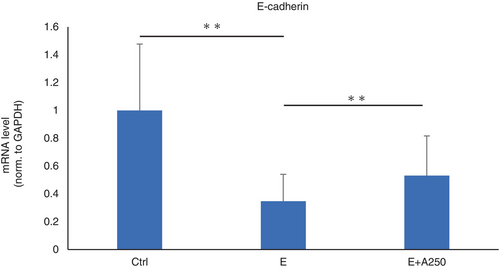
Role of adapalene in cell–cell adhesion
The expression of E-cadherin, an adhesion factor, was significantly downregulated by EGFR-TKI and significantly upregulated by adapalene treatment (Figure 5).
DISCUSSION
In this study, we investigated the effect of adapalene on EGFR-TKI-induced skin damage. We have experienced cases of acne-like skin rash during treatment with EGFR-TKI, in which the skin disorder improved with the application of adapalene.19 Previous reports have shown a correlation between skin rash severity and patient prognosis after treatment with erlotinib.20 Ameliorating skin disorders is very important for the continued administration of EGFR-TKIs.
EGFR-TKIs may cause skin damage by inducing aberrant keratinization and proinflammatory cytokine production in epithelial cells.21 Proinflammatory cytokines such as CCL2 have also been shown to be potentially involved in psoriatic skin inflammation and irritant contact dermatitis.22, 23 In this study, we demonstrated that adapalene may inhibit the production of inflammatory cytokines.
Adapalene binds to retinoic acid receptors (RAR). Adapalene treatment downregulated pNF-κB expression, suggesting that RARs may be involved in NF-κB expression after translocation into the nucleus. Retinoic acid has been reported to suppress NF-κB activation,24 and we speculate that adapalene may exert its effects through a similar mechanism. pNF-κB expression was downregulated, thereby reducing inflammatory cytokine expression and ameliorating the skin damage caused by EGFR-TKI. On the other hand, although NF-κB expression has been reported to be induced by EGFR-TKI stimulation,25 no upregulation was observed in the present study.
The skin protects against infection. EGFR-TKIs cause sterile acne, but bacterial infections, such as Staphylococcus aureus, may develop after prolonged periods of EGFR-TKI treatment.26 Topical antimicrobial agents are an option for acne-like skin eruptions,27 but long-term use of such agents may induce bacterial resistance. Adapalene upregulates the expression of antimicrobial peptides, which may reduce the risk of bacterial infections that contribute to the skin damage caused by EGFR-TKIs. It has also been reported that combination therapy with adapalene and clindamycin was more effective than monotherapy in treating acne vulgaris.28
The upregulated expression of adhesive factors with adapalene administration was demonstrated in this study. All-trans retinoic acid has been shown to activate E-cadherin expression via promoter hypomethylation.29 Previously, deletion of E-cadherin has been shown to cause overproliferation, differentiation defects and impaired barrier formation.30 Its upregulated may improve skin barrier function.
Mouse experiments suggested that diluted adapalene exerts a preventive effect on skin rashes. However, high doses of adapalene have been shown to induce skin inflammation. One of the main side effects of adapalene is irritation in the early stage of topical application. A previous report also showed that some concentrations of adapalene can cause severe inflammation and exacerbate skin damage caused by EGFR-TKIs.31 Previous reports have shown that in some cases, tazarotene administration was discontinued due to irritation caused by its application.32 Adapalene should be used once a day, but side effects may be reduced by decreasing the frequency of use or by diluting the dose.
Aquaporin expression has been implicated in the retention of epidermal water content in skin diseases.33 We showed that EGFR-TKI treatment downregulated AQP3 expression and that adapalene treatment upregulated AQP3 expression. Previously, it has been reported that EGFR-TKI decreased the expression of AQP3 and caused skin dryness in mice. One possible reason for this could be that EGFR-TKI administration inhibits EGFR in the skin, which in turn suppresses Ras/MAPK pathway activity and AQP3 transcription.34 On the other hand, AQP3 expression was downregulated by high concentrations of adapalene. In a previous report, the expression of AQP3 was downregulated when retinoic acid concentrations exceeded a certain value or the exposure time was prolonged.11, 35 The causes of these changes are not clear and require further study. High concentrations of retinoic acid might contribute to skin dryness, causing changes in osmotic pressure and decreasing the expression level of AQP3.
Topical steroids are the mainstay of treatment for acneiform eruptions caused by EGFR-TKIs, and strong topical agents are often used in Japan. Although topical steroids are an important treatment, long-term use can lead to rosacea-like dermatitis and skin desquamation, so concomitant use of adapalene may be expected to reduce the side effects of topical steroids. Another advantage of treatment with adapalene is that it can be used over a long period of time without adverse side effects, unlike topical steroids.
On the other hand, there are disadvantages of treatment with adapalene. Adapalene is currently recommended for the treatment of acne vulgaris only on the face, limiting its potential use. One important side effect is irritation in the early stages of use. If the skin disorder caused by EGFR-TKI is more severe, the side effects of adapalene may be more pronounced, and it is important to treat this inflammation with a moisturizer or other combination therapy.
In this study, we were not able to determine the effect of adapalene on skin wound healing. It has been reported that retinoic acid stimulates fibroblast growth and the production of extracellular matrix by fibroblasts, although the direct effects of adapalene and dermal fibroblasts are unknown.36 Adapalene is a retinoid-activated drug and may be involved in skin wound healing through similar effects. We believe this is an issue for future research.
In conclusion, adapalene suppressed inflammatory cytokine production and upregulated the expression of antimicrobial peptides and adhesion factors. An appropriate dose of adapalene may be beneficial for the treatment of skin disorders induced by EGFR-TKIs.
AUTHOR CONTRIBUTIONS
CM contributed to the project administration, methodology, data curation, statistical analysis, and writing the original draft. TN contributed to the conceptualization, methodology, investigation, writing and reviewing of the manuscript, and supervision. DT contributed to the conceptualization, methodology, investigation, writing and reviewing of the manuscript. NM, KM, JY, HS, and RS contributed to methodology, investigation, and writing and reviewing of the manuscript. DH, MY, MT, YN, and KK contributed to the writing and reviewing of the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
This work was supported by funding from J16KSPS KAKENHI 16K19452 to Daisuke Tamura. The authors thank Dr Kei Kunimasa (Department of Thoracic Oncology, Osaka International Cancer Institute, Osaka, Japan) and members of the Division of Respiratory Medicine of Kobe University Graduate School of Medicine for their helpful discussions. The authors created graphic abstract with BioRender.com Adapted from “Skin (Layout),” by BioRender.com (CurrentYear). Retrieved from https://app.biorender.com/biorender-templates.
CONFLICT OF INTEREST STATEMENT
The author reports no conflicts of interest in this work.




