Activation of insulin-like growth factor-1 receptor confers acquired resistance to osimertinib in non-small cell lung cancer with EGFR T790M mutation
Abstract
Background
Osimertinib (AZD9291) is a third-generation EGFR-tyrosine kinase inhibitor (TKI) that selectively inhibits the activating EGFR mutation and T790M mutation, and is currently used globally to treat EGFR-mutant non-small cell lung cancer (NSCLC). However, acquired resistance to osimertinib is inevitable.
Methods
We established osimertinib-resistant cells (PC9/T790M/AZDR and H1975/AZDR) derived from EGFR-mutant NSCLC cells harboring T790M mutation, and investigated the mechanism of acquired resistance to osimertinib by whole-exome sequencing and multiple phospho-receptor tyrosine kinase (RTK) array. A tumor specimen from an EGFR-mutant NSCLC patient with acquired resistance to osimertinib was also subjected to immunohistochemical analysis.
Results
Whole-exome sequencing analysis demonstrated that genetic alterations, such as acquisition of EGFR C797S, loss of T790M mutation, MET amplification, or mutated KRAS, MEK, BRAF, PIK3CA, were not detected. Analysis of phospho-RTK array revealed that insulin-like growth factor-1 receptor (IGF1R) was activated in PC9/T790M/AZDR and H1975/AZDR cells. Knockdown of IGF1R by siRNA as well as inhibition of IGF1R activation by linstinib (IGF1R inhibitor) significantly restored the sensitivity to osimertinib. Immunohistochemical analysis revealed that the expression level of phosphorylated IGF1R was higher in the tumor specimen from the EGFR-mutant NSCLC patient with acquired resistance to osimertinib than in the specimen collected prior to the treatment.
Conclusions
IGF1R activation could occur following treatment with osimertinib in EGFR-mutant NSCLC with T790M mutation, and might be one of the mechanisms underlying osimertinib resistance. Combined treatment of osimertinib and IGF1R inhibitor might be effective in overcoming the acquired resistance to osimertinib induced by IGF1R activation.
Key points
Significant findings of the study: Using osimertinib-resistant cells, we found that IGF1R activation induced by osimertinib treatment in EGFR-mutant NSCLC with T790M mutation is involved in resistance. Increased phosphorylation of IGF1R was observed in the tumor specimen from an EGFR-mutant NSCLC patient with acquired osimertinib resistance.
What this study adds: IGF1R activation might be one of the mechanisms of osimertinib resistance. A combination therapy with osimertinib and an IGF1R inhibitor might be an optimal approach for overcoming the acquired resistance to osimertinib induced by IGF1R activation.
Introduction
Advanced non-small cell lung cancer (NSCLC) is the leading cause of cancer-related deaths worldwide.1 Somatic mutations in the epidermal growth factor receptor (EGFR) gene, such as an in-frame deletion in exon 19 and the point mutation L858R, are associated with favorable response to the first- and second-generation EGFR tyrosine kinase inhibitors (EGFR-TKIs), gefitinib, erlotinib, and afatinib.2-4 However, the progression-free survival of patients is reported to be nine to 13 months.2-8 The EGFR T790M mutation is the most common cause of acquired resistance to first- and second-generation EGFR-TKIs and is detectable in 50% or more patients with such acquired resistance.9
Osimertinib (AZD9291) is a third-generation EGFR-TKI that selectively suppresses both EGFR “activating” mutations and the T790M “resistance” mutation.10 Osimertinib is approved globally for the treatment of T790M-positive NSCLC patients who have disease progression after therapy with first- or second-generation EGFR-TKIs.11, 12 A recent phase-3 clinical trial (the FLAURA study) revealed that osimertinib, used as a first-line treatment for EGFR mutation-positive NSCLC patients, was associated with a longer progression-free survival and promising overall survival than the first-generation EGFR-TKIs.13 Based on the positive results of the FLAURA trial, first-line treatment with osimertinib has become one of the standard of care therapies for treatment-naive NSCLC patients with mutated EGFR.12, 13 However, acquired resistance to osimertinib is also inevitable. On the basis of preclinical and clinical studies, various mechanisms of resistance to third-generation EGFR-TKIs, including osimertinib, have been reported; these mechanisms include acquisition of EGFR C797S or L798I mutation, amplification of wild-type EGFR, activation of MAPK pathway by KRAS or MEK mutation, activation of a bypass pathway through MET or HER2, and small-cell lung cancer transformation.14-21 With the widespread use of osimertinib, it is extremely important to address the resistance to osimertinib for improving the prognosis of EGFR-mutant NSCLC patients. However, the mechanisms of acquired resistance to osimertinib are not fully understood.
In this study, we established osimertinib-resistant EGFR-mutant NSCLC cells with T790M mutation. We found that activation of insulin-like growth factor-1 receptor (IGF1R) occurred by the treatment with osimertinib and conferred acquired resistance to osimertinib in EGFR-mutant NSCLC cells with T790M mutation. IGF1R phosphorylation was also detectable by immunohistochemistry in tumor specimen from a patient of EGFR-mutant NSCLC with acquired resistance to osimertinib as compared with the specimen collected before the EGFR-TKI treatment. The biological significance of IGF1R activation in the resistance to osimertinib was investigated.
Methods
Cell culture and reagents
NSCLC cell lines, PC9 (EGFR E746-A750 del) and H1975 (EGFR L858R + T790M), were used in this study. PC9 cells were established at the Tokyo Medical University (Tokyo, Japan), as previously described,22 and were kindly provided by Dr Kazuto Nishio (Department of Genome Biology, School of Medicine, Kinki University, Osaka). H1975 cells were obtained from the American Type Culture Collection (Manassas, VA, USA). Osimertinib was provided by AstraZeneca UK Ltd., UK. The culture conditions and other reagents are described in Supplementary Materials and Methods.
Cell proliferation assay
The WST-8 cell proliferation assay was performed as previously described.22 Briefly, the cells were seeded in 96-well plates at 1 × 103 cells/well in quadruplicate. The cells were grown in the absence or presence of osimertinib at various concentrations for 72 hours. The cell viability was assessed by using a Cell Counting Kit-8 (Wako, Japan).
Western blotting
The cells were lysed with a buffer containing protease and phosphatase inhibitors (Roche, Basel, Schweiz). Immunoblot analysis was performed as previously described.22 The details of the method and antibodies are provided in Supplementary Materials and Methods.
Quantitative real-time PCR
The quantitative polymerase chain reaction (qPCR) conditions and sequences of the primers used for transcript detection are described in Supplementary Materials and Methods.
Phospho-receptor tyrosine kinase array
Phospho-receptor tyrosine kinase array was performed according to manufacturer's instructions (R&D Systems, Minneapolis, MN). The procedure is explained in detail in Supplementary Materials and Methods.
Whole-exome sequencing
Genomic DNA of each cell line was extracted using a QIAamp DNA Mini Kit (QIAGEN, Hilden, Germany). Fragment libraries were created from sheared samples by sonication and target enrichment was performed using the SureSelectXT Human All Exon V6 kit (Agilent, Santa Clara, CA). Captured DNA was amplified followed by solid-phase bridge amplification and paired-end sequencing on an Illumina NovaSeq 6000 System (Illumina, San Diego, CA). Details of the method are provided in Supplementary Materials and Methods.
RNA interference
Short interfering RNAs (siRNAs) targeting IGF1R (Stealth Select RNAi siRNA), the negative control, and lipofectamine RNAiMAX were purchased from Invitrogen (Carlsbad, CA, USA). The procedure is described in detail in Supplementary Materials and Methods.
Immunohistochemistry
We used tumor tissues from an EGFR-mutant NSCLC patient at Juntendo University Hospital, with the patient's consent, following a protocol that was approved by the Institutional Review Board. Immunohistochemistry for phosphorylated IGF1R and IGFBP3 was carried out as described in Supplementary Materials and Methods.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 7.0 (GraphPad Software, La Jolla, CA). The two-tailed Student's t-test and ANOVA were used to compare the values. Significant differences between the means were considered statistically significant at P < 0.05.
Results
Establishment of osimertinib-resistant cells
To investigate the mechanisms of acquired resistance to the third generation EGFR-TKI, osimertinib (AZD9291), we established osimertinib-resistant cells using EGFR-mutant NSCLC cells with T790M mutation. To generate T790M mutation-positive cells, NSCLC cell line PC9 cells, which express EGFR exon 19 deletion mutation (ΔE746-A750), were continuously exposed to a first-generation EGFR-TKI, gefitinib, over six months, and clonal-resistant cells were isolated. These resistant cells exhibited the presence of T790M mutation, and were, therefore, named as PC9/T790M. The PC9/T790M cells were cultured with stepwise escalation of the osimertinib concentration from 150 nmol/L to 1 μmol/L over six months (following the dose-escalation method), and the osimertinib-resistant cells harboring the T790M mutation, thus obtained, were referred to as PC9/T790M/AZDR. We also established osimertinib-resistant cells from the NSCLC cell line, H1975, which expresses the EGFR L858R/T790M mutation, using the high-concentration method. The H1975 cells were cultured with 1 μmol/L of osimertinib over three months, and the resistant cells that were obtained were named as H1975/AZDR.
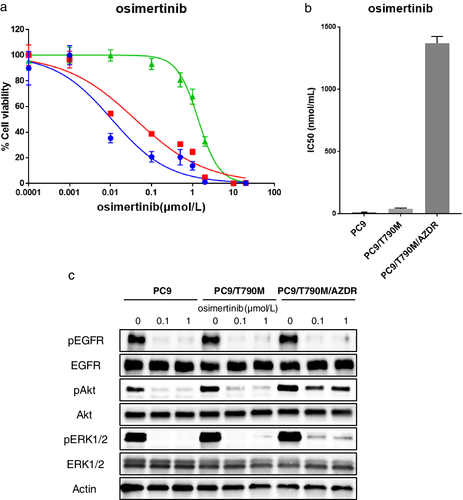
As shown in Fig 1a,b, the PC9/T790M cells were sensitive to osimertinib. However, the PC9/T790M/AZDR cells were highly resistant to osimertinib, and acquired an approximately 30-fold higher 50% inhibitory dose (IC50) to osimertinib than the PC9/T790M cells. The PC9/T790M/AZDR cells showed cross-resistance to gefitinib (data not shown). The H1975/AZDR cells were also highly resistant to osimertinib (Fig S1a,b).
 ) PC9, (
) PC9, ( ) PC9/T790M, and (
) PC9/T790M, and ( ) PC9/T790M/AZDR.
) PC9/T790M/AZDR.We next examined the status of EGFR-related molecules in the absence or presence of osimertinib. The cells were either left untreated or were treated with increasing concentrations of osimertinib (0.1–1 μmol/L) for 3 hours. After the treatment, the cells were lysed, and western blot analysis was performed. As shown in Fig 1c, treatment with osimertinib suppressed the level of phosphorylated EGFR in the PC9 parent, PC9/T790M, and in PC9/T790M/AZDR cells, but the level of phosphorylated AKT was not inhibited in PC9/T790M/AZDR cells. The ERK1/2 phosphorylation was partially suppressed in the PC9/T790M/AZDR cells by osimertinib treatment.
Whole-exome sequencing of osimertinib-resistant PC9 cells
To identify the potential mechanism of osimertinib resistance, DNA isolated from the PC9 parent, PC9/T790M, PC9/T790M/AZDR, and the H1975 parent, H1975/AZDR cells was subjected to whole-exome sequencing (Table S1). Several genetic mutations not observed in the PC9 parent, PC9/T790M, and H1975 parent were observed in PC9/T790M/AZDR (Table S2) and H1975/AZDR (Table S3) cells. However, genetic alterations potentially relevant to third-generation EGFR-TKI resistance, such as acquisition of EGFR C797S or L798I mutation, loss of T790M mutation, amplification of wild-type EGFR or MET, or mutated KRAS, MEK, BRAF, PIK3CA, were not detected in the PC9/T790M/AZDR and H1975/AZDR cells. The EGFR E746-A750 del + T790M mutation was preserved in PC9/T790M/AZDR cells. The EGFR L858R + T790M mutation was also preserved in H1975/AZDR cells.
Activation of IGF1R and loss of IGFBP3 expression in PC9/T790M/AZDR cells
The activation of bypass signaling pathway contributes to acquired resistance to EGFR-TKIs.16 We analyzed the phosphorylation level of multiple receptor tyrosine kinases (RTKs) in the osimertinib-resistant PC9 cells by using a human phospho-RTK array kit. This phospho-RTK array kit is designed to simultaneously detect the relative phosphorylation of 49 different RTKs as described in Supplementary Materials and Methods. As shown in Fig 2a,b, IGF1R was markedly activated in the PC9/T790M/AZDR cells, whereas this was not found in the PC9 parent and PC9/T790M cells. Any activation of other reported bypass pathways, such as HER2, MET, and AXL signaling, resulting in the resistance to EGFR-TKIs was not detected in the PC9/T790M/AZDR cells. The IGF1R activation was also observed in the H1975/AZDR cells (Fig S2).
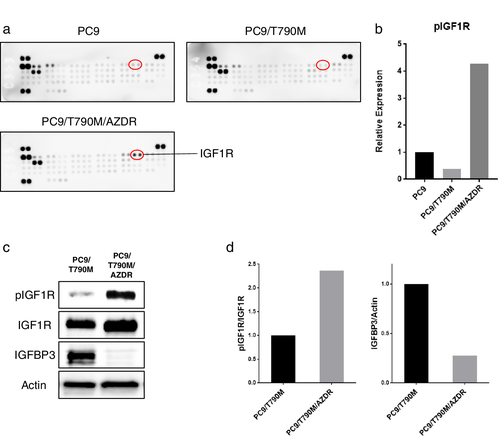
Previous reports have demonstrated that downregulation of the expression of insulin-like growth factor binding protein 3 (IGFBP3) is involved in the activation of the IGF1R pathway leading to EGFR-TKI resistance.23 To investigate the mechanism of IGF1R activation in PC9/T790M/AZDR cells, we analyzed the expression of IGFBP3 by western blotting. Consistent with the findings of the RTK array, IGF1R was phosphorylated in the PC9/T790M/AZDR cells compared with that in the PC9/T790M cells (Fig 2c,d). Furthermore, the expression of IGFBP3 was downregulated in the PC9/T790M/AZDR cells (Fig 2c,d).
Activation of IGF1R is involved in osimertinib resistance in PC9/T790M/AZDR cells
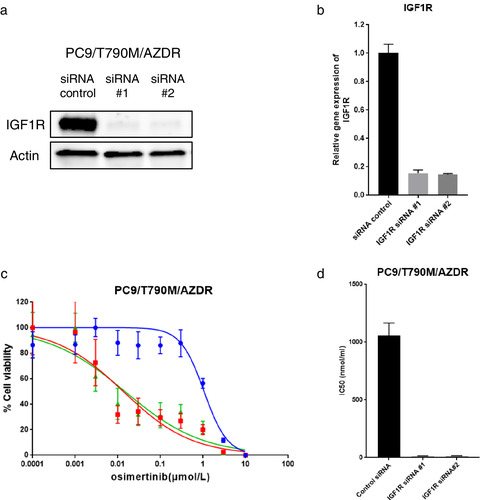
To examine whether IGF1R activation could contribute to the osimertinib resistance in PC9/T790M/AZDR cells, the IGF1R expression in these cells was silenced by using two different siRNAs (siRNA#1 and #2). The expression of IGF1R was significantly suppressed by both these siRNAs, as detected by western blotting and qPCR analysis (Fig 3a,b). The sensitivity of cells to osimertinib was significantly restored in PC9/T790M/AZDR cells through the silencing of IGF1R (Fig 3c,d). Similar results were obtained in H1975/AZDR cells (Fig S3a-c).
 ) control siRNA, (
) control siRNA, ( ) IGF1R siRNA #1, and (
) IGF1R siRNA #1, and ( ) IGF1R siRNA #2.
) IGF1R siRNA #2.Inhibition of IGF1R activation could overcome osimertinib resistance in PC9/T790M/AZDR cells
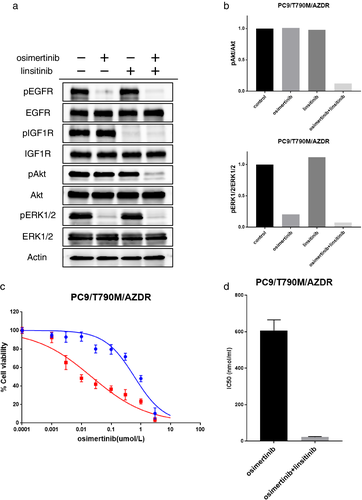
To investigate whether IGF1R inhibition could overcome the osimertinib resistance in PC9/T790M/AZDR cells, we used linstinib, a small-molecule inhibitor of IGF1R. The osimertinib treatment suppressed the phosphorylation of EGFR, and partially inhibited the phosphorylation of ERK1/2 in PC9/T790M/AZDR cells (Fig 4a,b). However, AKT phosphorylation was not inhibited by osimertinib alone. Treatment with linstinib suppressed the phosphorylation of IGF1R, but the phosphorylation of AKT was not suppressed by linstinib alone. Notably, the combined treatment with osimertinib and linstinib effectively suppressed AKT phosphorylation in PC9/T790M/AZDR cells (Fig 4a,b). Furthermore, treatment with linstinib significantly restored the sensitivity to osimertinib in PC9/T790M/AZDR cells, when used in combination with osimertinib (Fig 4c,d). These findings suggest that IGF1R activation is involved in the acquired resistance to osimertinib in PC9/T790M/AZDR cells. Similar results were obtained in H1975/AZDR cells (Fig S4a-c).
 ) osimeritnib and (
) osimeritnib and ( ) osimeritnib+linsitinib.
) osimeritnib+linsitinib.Detection of IGF1R phosphorylation in tumor specimen from an EGFR-mutant NSCLC patient with acquired resistance to osimertinib
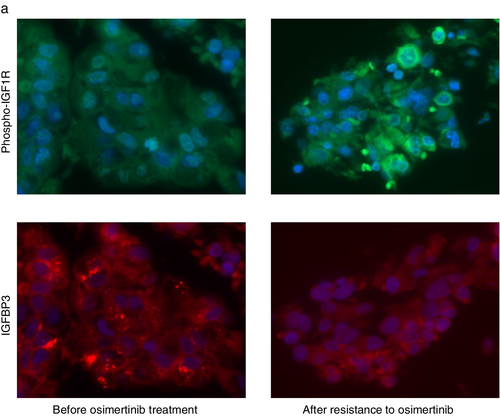
Finally, we examined the IGF1R phosphorylation in a pair of tumor specimens obtained before EGFR-TKI treatment and after relapse during the osimertinib treatment of an EGFR-mutant NSCLC patient. A 78-year-old female patient was diagnosed with stage IV lung adenocarcinoma harboring the EGFR L858R mutation. Treatment with gefitinib was initiated as the first-line therapy. At six months after initiation of the gefitinib treatment, the axillary lymph node was enlarged. Biopsy of the axillary lymph node tissue was performed, and EGFR T790M mutation detected. Osimertinib treatment was started, but the primary lung tumor relapsed after eight months. We performed computed tomography-guided lung biopsy, and obtained the relapsed tumor tissue after osimertinib treatment. Although axillary lymph node tissue after gefitinib treatment was not available, we tried to examine the IGF1R phosphorylation in tumor specimens obtained before the TKI treatment and after relapse during the osimertinib treatment by immunohistochemistry. The level of phosphorylated IGF1R was higher in the tumor specimen with acquired resistance to osimertinib as compared to that in the specimen collected prior to the treatment (Fig 5). In contrast, the expression of IGFBP3 was decreased after the development of resistance to osimertinib (Fig 5). These findings were consistent with our in vitro results in osimertinib-resistant PC9/T790M/AZDR cells.
Discussion
IGF1R is a transmembrane receptor tyrosine kinase that is responsible for cellular survival and proliferation, and is expressed in various types of cancer cells, including NSCLC.24 IGFBP3 is a carrier protein for insulin-like growth factor, and loss of its expression has been shown to induce the activation of IGF1R.23 It has been reported that increased activation of IGF1R through the loss of IGFBP3 expression is implicated in resistance to first- and second-generation EGFR-TKIs.23, 25
In this study, we established PC9/T790M/AZDR cells resistant to a third-generation EGFR-TKI, osimertinib, and found the activation of IGF1R and loss of IGFBP3 expression in PC9/T790M/AZDR cells. Genetic alterations, such as acquisition of EGFR C797S or L798I mutation, amplification of wild-type EGFR or MET, or mutated KRAS, MEK, BRAF, and PIK3CA, were not detected by whole-exome sequencing. The IGF1R activation was also found in osimertinib-resistant H1975/AZDR cells with T790M mutation. The siRNA-mediated silencing of IGF1R, as well as inhibition of IGF1R activation by linstinib, significantly restored the sensitivity to osimertinib in both PC9/T790M/AZDR and H1975/AZDR cells. Our findings suggest that IGF1R activation could be induced by the treatment with osimertinib in EGFR-mutant NSCLC with T790M mutation, and is involved in osimertinib resistance.
Recently, Park et al. reported that IGF1R activation was associated with acquired resistance to another third-generation EGFR-TKI, WZ4002, which was accompanied by downregulation of IGFBP3 expression.26 The combined treatment of WZ4002, either with a chemical IGF1R inhibitor or with a ligand-neutralizing monoclonal antibody, led to the restoration of drug sensitivity to WZ4002 in WZ4002-resistant NSCLC cells.26 Cortot et al. also demonstrated that activation of IGF1R with IGFBP3 loss was involved in the resistance to WZ4002.25 However, IGF1R activation as a mechanism of resistance to osimertinib has not previously been reported. Various third-generation EGFR-TKIs, including WZ4002, osimertinib, rociletinib, olmutinib, and others, have been developed.12, 16 However, WZ4002 is not allowed for use in clinical practice, and osimertinib is the only third-generation inhibitor that is currently approved by the FDA.12 Therefore, our data for IGF1R activation in osimertinib-resistant cells are important for the clinical practice in cases of EGFR-mutant NSCLC patients, although that in WZ4002-resistant cells has been previously reported for the two above-mentioned studies.
IGF1R activation and loss of IGFBP3 expression have not yet been validated in clinical specimens from NSCLC patients with acquired resistance to the first-, second-, or third-generation EGFR-TKIs. In our study, we selected the EGFR-mutant NSCLC patient who received the tumor biopsy before EGFR-TKI treatment and after relapse to osimertinib therapy. Increased phosphorylation of IGF1R and decreased expression of IGFBP3 were observed by immunohistochemistry in the tumor specimen after acquired resistance to osimertinib as compared to that in the specimen collected prior to the EGFR-TKI treatment. This study is the first to provide the evidence that IGF1R is activated and the IGFBP3 expression is downregulated, using a clinical specimen from an EGFR-mutant NSCLC patient with acquired resistance to osimertinib, although the evidence is based on the analysis of a single patient. The analysis of DNA from tumor tissues and cell-free plasma collected from NSCLC patients by next-generation sequencing identified various mechanisms of acquired resistance to osimertinib. However, the activation of IGFIR is not a genetic event, unlike EGFR C797S mutation or MET amplification, and it will not be easily detected in clinical specimens by NGS analysis. Tissue-based analyses, such as immunohistochemistry, would be necessary to fully characterize the resistance to osimertinib in NSCLC patients.
There are several limitations of this study. First, we did not perform an in vivo animal experiment using a xenograft tumor model of PC9/T790M/AZDR to evaluate the efficacy of the combined therapy of osimertinib and IGF1R inhibitor. Second, a greater number of tumor specimens from NSCLC patients are required to investigate the significance of IGF1R activation in the acquired resistance to osimertinib. Third, osimertinib is also currently used for the first-line treatment of EGFR-mutant NSCLC patients without T790M. To address the biological significance of IGF1R activation in acquired resistance to osimertinib in this clinical setting, further studies to establish osimertinib-resistant cells from EGFR-mutant NSCLC cells without T790M, such as parental PC9 or HCC827 cells, are necessary. In addition, relapsed tumor tissues from EGFR-mutant NSCLC patients without T790M after first-line treatment with osimertinib are required.
In conclusion, our study suggests that IGF1R activation through the loss of IGFBP3 expression might be one of the mechanisms of osimertinib resistance in EGFR-mutant NSCLC. This study is the first to reveal that the activation of IGF1R conferred acquired resistance to osimertinib in EGFR-mutant NSCLC cells with T790M mutation in a preclinical model, and increased the phosphorylation of IGF1R and decreased the expression of IGFBP3 in the tumor specimen from a patient of EGFR-mutant NSCLC with acquired resistance to osimertinib. Although the small-molecule IGF1R inhibitor is not allowed for use in clinical practice, a combination of osimertinib and IGF1R inhibitor could be an optimal approach for overcoming the acquired resistance to osimertinib in EGFR-mutant NSCLC patients as we observed in this study in osimertinib-resistant cells. However, further studies are necessary in this regard.
Acknowledgments
This work was supported by JSPS KAKENHI Grant Number 15K09230 (Kazuhisa Takahashi), a Grant for Cross-disciplinary Collaboration, and a Grant from the Institute for Environmental & Gender-specific Medicine, Juntendo University. This study was also financially supported in part through grants from the Practical Research for Innovative Cancer Control under Grant Number JP18ck0106252 from the Japan Agency for Medical Research and Development, AMED.
Disclosure
Kazuhisa Takahashi received research funding from Chugai Pharm, Ono Pharma, Taiho Pharm, Nippon Boehringer Ingelheim, AstraZeneca, Pfizer, MSD, and Lilly Japan, outside the submitted work. Fumiyuki Takahashi received research funding from AstraZeneca, Nippon Boehringer Ingelheim, MSD, Novartis, and Lilly Japan, outside the submitted work. Ryo Ko received grants and personal fees from Nippon Boehringer Ingelheim; personal fees from AstraZeneca, Taiho Pharmaceutical, Ono Pharmaceutical, Chugai Pharmaceutical, Novartis, and MSD, outside the submitted work. Takehito Shukuya received grants and personal fees from MSD, personal fees from AstraZeneca, Chugai, Eli Lilly, and Ono Pharmaceutical, outside the submitted work. Hiroyuki Mano serves as a board member for CureGene and a scientific advisor for Chugai, and received research funds from Sysmex and Konica-Minolta, outside the submitted work. The remaining authors declare that they have no conflict of interest.




