Cytopathological findings of primary pulmonary Ewing family of tumors with EWSR1 translocation: A case report
Abstract
Primary pulmonary neoplasms of the Ewing family of tumors (EFT) are extremely rare and usually occur in adolescents or young adults. Only about 40 cases of pulmonary EFT have been reported in English literature, and no cytological studies have been documented. In this report, we describe the cytopathological findings of a primary pulmonary EFT in an elderly patient. A 70-year-old man sought care because of a progressing cough and dyspnea. Chest computed tomography revealed a circumscribed mass of 6 cm in the left upper lobe. Fine needle aspiration cytology and core needle biopsy revealed uniform round cell proliferation. The predominant population consisted of cells with thickened nuclear membranes, finely dispersed chromatin, single distinct nucleoli, and indistinct cytoplasm. The other population consisted of smaller cells with darker chromatin. The cytoplasm stained positive for periodic acid–Schiff stain and was digested by diastase. Immunohistochemistry showed positivity for MIC2 (CD99), and focal positivity for neuron specific enolase, synaptophysin, and chromogranin A. Fluorescence in situ hybridization (FISH) revealed EWSR1 translocation. Although rare, pulmonary EFT cannot be disregarded, regardless of age. When two populations of uniform, round cells are observed, immunohistochemistry with MIC2 (CD99) and cytogenetic analysis by reverse transcription polymerase chain reaction or FISH should be considered. Cytological diagnosis may play an important role in the early diagnosis and treatment of pulmonary EFT.
Introduction
The Ewing family of tumors (EFT) is a group of undifferentiated round cell sarcomas, including Ewing sarcoma (ES) of the bone, Askin's tumor of the chest wall, extraskeletal Ewing sarcoma (EES), and primitive neuroectodermal tumor (PNET). The tumors are characterized by chromosomal translocation involving the EWSR1 gene.1 It generally develops at a young age and has an aggressive course.2 Primary pulmonary EFT is extremely rare, with only 40 cases reported in English literature. None of these reports has focused on cytopathological findings. Here, we describe the cytopathological findings of a primary pulmonary EFT in a 70-year-old man.
Case report
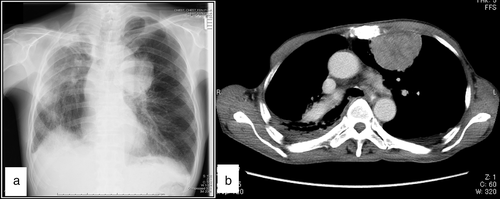
A 70-year-old man sought care because of a progressing cough and dyspnea. Chest radiograph revealed a mass in the left lung (Fig 1a). Computed tomography revealed a circumscribed 6 cm tumor in the left upper lung (Fig 1b). The tumor did not appear to invade the thoracic wall. Systemic magnetic resonance imaging and bone scintigraphy were negative for other lesions. Laboratory tests showed anemia (hemoglobin, 11.0 g/dL), a slight increase of squamous cell carcinoma antigen (3.8 ng/mL; reference range < 1.5 ng/mL), neuron specific enolase (NSE; 19.1 ng/mL; reference range < 16.3 ng/mL), and pulmonary surfactant-associated protein D (234 ng/mL; reference range < 110 ng/mL). The patient underwent percutaneous fine needle aspiration cytology (FNAC) and core needle biopsy of the tumor, and a diagnosis of EFT was established by pathological examination. After the diagnosis, he was treated with chemotherapy, but died four months after the diagnosis.
The Papanicolaou-stained FNAC specimen showed a uniform population of small round cells (Fig 2a,b). The cells predominantly exhibited thickened nuclear membranes with finely dispersed chromatin and single nucleoli. The cytoplasms were indistinct. Smaller and more hyperchromatic cells that apparently referred to so-called dark cells were mixed.3 Rosette formation was not observed. Malignant small round cell sarcoma was suspected by cytology, but a definitive diagnosis was not made.
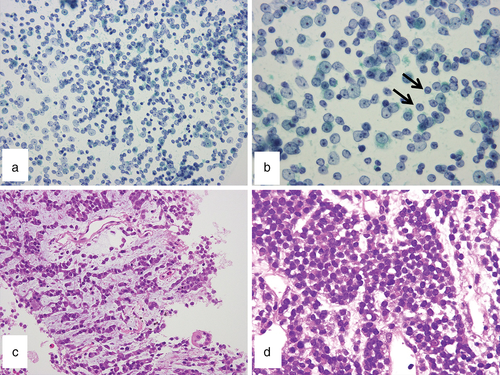
Histology of the needle biopsy specimen showed solid, trabecular, cord-like, or singular proliferation of discohesive small round cells in a myxofibrous stroma (Fig 2c,d). Mitotic figures were rare. No necrosis was present. The cytoplasm stained positive for periodic acid–Schiff stain and was digested by diastase (Fig 3a).
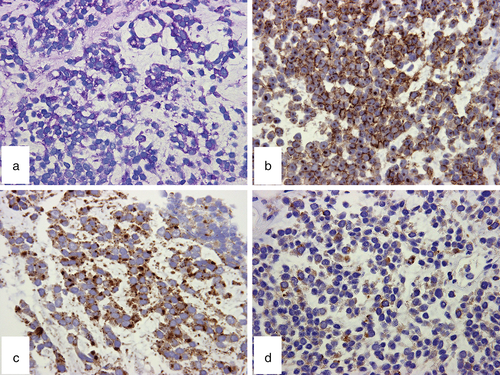
Immunohistochemical study showed diffuse membranous positivity for MIC2 (CD99) and focal cytoplasmic positivity for NSE, chromogranin A, and synaptophysin (Fig 3b–d). Cytokeratins (AE1/AE3, CAM5.2), leukocyte common antigen (CD45RB), S-100 protein, muscle specific actin (HHF35), desmin, thyroid transcription factor 1 (TTF-1), calretinin, and WT-1 were negative.
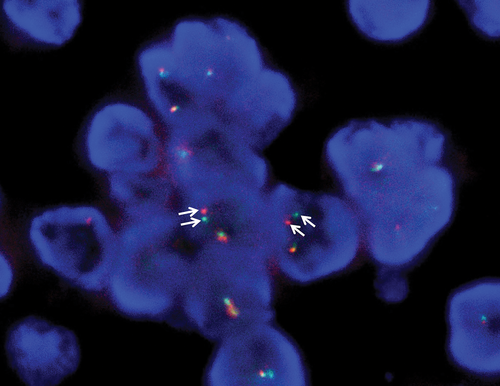
Based on these pathological findings, EFT was strongly suspected. Thus, fluorescence in situ hybridization (FISH) was performed on a section from the paraffin-embedded biopsy specimen, using an EWSR1 (22q12) dual-color, break-apart rearrangement probe. The analysis revealed break-apart signals of EWSR1 in more than 70 of the 200 tumor cells (Fig 4). The result, in combination with the cytopathological findings, yielded the diagnosis of pulmonary EFT.
Discussion
We report the first detailed cytological findings of pulmonary EFT in the oldest pulmonary EFT patient recorded. FNAC findings were basically similar to those of EFTs in other organs.3, 4 A uniform population of discohesive round cells with larger and smaller cells may be a characteristic feature.
Since the first case of possible pulmonary PNET was reported in 1989, 42 cases of primary pulmonary EFTs have been reported in English literature.5-12 The mean patient age is 28 years (8–67 years), slightly older than in the EFT of bone and soft tissue (mean 20 years), but most patients are adolescents or young adults. Our patient was 70 years old, making him the oldest pulmonary EFT patient reported so far.
Because MIC2 is expressed in many small round cell tumors, cytogenetic analysis of EWSR1 translocation is recommended to confirm diagnosis.1 Only 10 cases of pulmonary EFT have been confirmed via translocation by reverse transcription polymerase chain reaction or by FISH, including the present case.8, 10, 12-16
Differential diagnosis of pulmonary EFT comprises many other small round cell malignancies, such as small cell carcinoma, carcinoid, small cell type of squamous cell carcinoma, nuclear protein in testis midline carcinoma, malignant lymphoma, desmoplastic small round cell tumor (DSRCT), and poorly differentiated synovial sarcoma. Differentiation from these diseases is important because of different prognostic/therapeutic implications. In addition to an epithelial morphology, carcinomas are generally immunoreactive for cytokeratins and non-reactive for MIC2. Immunohistochemical study with TTF1, cytokeratin 5/6, p63, and/or p40 is also helpful. Malignant lymphomas exhibit more irregular nuclear contour, chromatins, and lymphoglandular bodies, and exhibit lymphoid markers. Cytological findings of DSRCT are limited and differentiation from EFT is challenging. DSCRT may present a rosette-like appearance and sometimes exhibits immunoreactivity for MIC2. However, DSCRT usually shows more smudged chromatin and nuclear moldings, and immunoreactivity of both cytokeratin and desmin. Synovial sarcomas rarely occur in the lung or in older patients, and cytopathological differentiation of the poorly differentiated variant of EFT may also be challenging. Synovial sarcomas tend to show more spindle morphology than EFTs and are immunoreactive for transducer-like enhancer of split-1 protein (TLE1) and more for epithelial markers.
Pulmonary EFT prognosis is poor. In reported studies, about half of the patients died of their disease 1–54 months (median, 11 months; n = 18) after diagnosis.7, 12-15, 17-20 Radical surgical resection at early stages with multiagent chemotherapy would more likely obtain a better outcome.12 Important prognostic factors of EFT include the stage, anatomical location, and size of the tumor.1 Although primary visceral EFTs may be associated with more aggressive biological behavior than those of the soft tissue, larger series with concurrent statistical analysis are needed to validate this finding.12 Previous reports have indicated that young age at the time of the diagnosis was associated with favorable prognosis; however, others have reported that event-free and overall survival were essentially comparable in the younger and older groups.21, 22
Our study reports the cytopathological findings of pulmonary EFT for the first time. When two populations of uniform round cells are observed with thickened nuclear membranes, dispersed chromatin, and scanty cytoplasm, EFT must be considered, regardless of age, to allow early surgical resection and aggressive chemotherapy.
Acknowledgments
This study was supported by Grants-in-Aid for Scientific Research by the Japan Society for the Promotion of Science (Grant Number 26460660).
Disclosure
No authors report any conflict of interest.




