Aggressive spinal cord involvement in granulomatosis with polyangiitis
Dear Editor,
Spinal cord involvement represents a rare complication of granulomatosis with polyangiitis (GPA).1-3 Hypertrophic pachymeningitis, as a possible manifestation, can arise in proteinase-3 antibody (anti-PR3) positive cases as well as in myeloperoxidase antibody (anti-MPO) associated GPA, whereas primary spinal angiitis seems to be a unique complication of anti-PR3 positive GPA.4, 5
Here, we report a case of long-term immunosuppressive treatment in a patient with anti-PR3 positive GPA, who developed an acute paraplegia caused by extensive pachymeningitis which despite treatment escalation only partially responded.
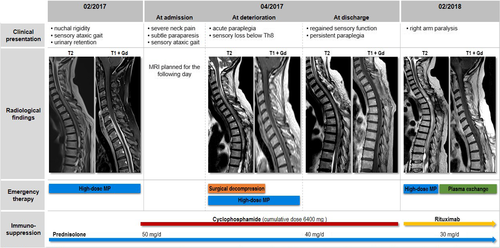
A 54-year-old Caucasian woman was diagnosed with anti-PR3 positive GPA in 2014 after presenting with bilateral conductive hearing loss and lower respiratory tract involvement (asymptomatic pulmonary nodules). After initiating an immunosuppressive treatment with prednisolone (10 mg/day) and methotrexate (15 mg/week), disease activity was sufficiently controlled for 2 years. In December 2016, the patient noticed slowly progressive neck and upper back pain, slight gait instability and intermittent numbness in both legs. In February 2017, she was admitted to our neurological ward due to acute urinary retention. Clinical examination showed nuchal rigidity, positive Kernig's and Brudzinski's signs and sensory ataxic gait disturbance in absence of leg paresis. Magnetic resonance imaging (MRI) showed a long contrast-enhanced swelling of the spinal meninges (C5-T8) combined with extensive spinal edema (Figure 1). Cerebrospinal fluid analysis showed elevated protein levels and an increased leukocyte count consistent with a spinal block. Further microbiological analyses did not show any pathological findings (Figure S1), but anti-PR3 antibodies were elevated. Histological examination of a meningeal biopsy (T3) revealed chronic granulating inflammation (Figure 2). We diagnosed a chronic hypertrophic spinal cord pachymeningitis and started intravenous steroid pulse therapy (1000 mg/day for 5 days) followed by initiation of intravenous cyclophosphamide treatment (800 mg) and an increase of oral prednisolone (50 mg/day). As a result, neurological deficits partly receded. At discharge the patient was able to walk small stretches without help. Urinary dysfunction as well as neck and upper back pain fully recovered. In April 2017, the patient was readmitted due to reoccurrence of gait instability and urinary dysfunction and severe neck and upper back pain. At hospitalization she presented with subtle paraparesis, which showed a significant deterioration within 1 day with painless leg paraplegia and sensory loss below T8. MRI showed a slightly progressive spinal edema (Figure 1) but did not reveal a definite cause for the acute deterioration. Immediate steroid pulse therapy (1000 mg/day for 5 days) and surgical spinal decompression were attempted without clinical improvement. Simultaneously, the patient developed sudden hemoptysis. A computed tomography scan revealed pulmonary granulomas with one large cavern, and the anti-PR3 antibodies were consistently elevated (Figure S1). Cyclophosphamide pulses with a cumulative dose of 6400 mg, (the patient received 8 pulses with 15 mg/kg every 4 weeks, the second pulse was delayed by 10 weeks because of a severe wound healing disorder following the decompression surgery) and oral steroid therapy (40 mg/day) were continued and hindered disease progression until January 2018. Oral cyclophosphamide was not attempted. During the next clinical visit in February 2018, a moderately severe paresis of the right arm was observed. MRI showed an increasing edema of the spinal cord extending up to C2 (Figure 1). Following steroid pulse therapy (1000 mg/day for 5 days), 5 cycles of plasma exchange and initiation of rituximab, short-term follow-up (August 2018) showed a significant regression of the arm paresis. For long-term control of GPA, a continuation of rituximab combined with oral steroid therapy (30 mg/day) was initiated.
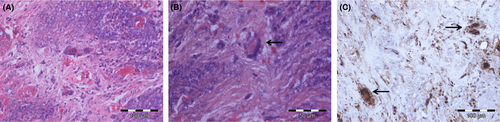
So far, only a few cases of spinal cord involvement as a manifestation of GPA have been reported.1, 6 In some of these patients, a subacute clinical aggravation was observed when being left untreated or under baseline therapy. Although cyclophosphamide-resistant disease courses are more common in anti-PR3 positive than in anti-MPO positive GPA,4, 6 no comparable case of acute and dramatic deterioration, despite escalated immune therapy, has been reported yet. Hence, we here combined highly dosed cortisone, plasma exchange and rituximab, while continuing oral steroid therapy. This aggressive approach had previously only been tried in GPA-associated pulmonary hemorrhage or progressive renal failure.7
As acute worsening of GPA rarely occurs in patients under escalated therapy, differential diagnoses were considered.7 Aside from a sole pachymeningitis with spinal edema, a concurrent vasculitis in addition to the granulomatous phenotype was taken into account.4, 8 Extensive spinal edema and extensive white matter lesions caused by vasculitis would result in comparable MRI findings. Concurrent vasculitis would furthermore explain the missing success of surgical intervention. Spinal cord vessel abnormalities should be discussed as an additional disease-related manifestation. In the event of uncertainty, spinal angiography could be considered. Although a long cord lesion is the hallmark of neuromyelitis optica spectrum disorders, the isolated contrast-enhancement of the spinal meninges in the absence of contrast-enhancement of the cord itself makes this differential diagnosis improbable.9, 10
In summary, physicians should keep in mind the possibility of rapid and aggressive spinal involvement in anti-PR3 positive GPA patients, which could occur as standalone in the absence of other typical life-threatening manifestations (eg renal or pulmonary failure).11, 12 Therefore, neurological symptoms as well as non-specific clinical signs, such as headaches and backaches, should be taken seriously and should result in a thorough clinical examination and, if necessary, in swift further diagnostics.
ETHICAL STATEMENT
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
CONFLICTS OF INTEREST
MP received speaker honoraria from Roche, Genzyme and Novartis and travel/accommodation/meeting expenses from Novartis, Biogen Idec, Genzyme and MERCK Serono. MK received speaker honoraria from the DMSG and travel/accommodation/meeting expenses from MERCK Serono. The other authors declare that there is no conflict of interest.
AUTHOR CONTRIBUTION
TH and MP have access to all the data and take responsibility for the data, accuracy of the data analysis and interpretation, and drafting the manuscript for intellectual content. VP, MK, CM, CB: Design and conceptualization of the case; revising the manuscript for intellectual content.




