High levels of uric acid in systemic lupus erythematosus is associated with pulmonary hypertension
Abstract
Aim
To estimate the point prevalence of pulmonary hypertension (PH) and determine the associated factors for PH in patients with systemic lupus erythematosus (SLE).
Methods
A prospective cross-sectional study of 114 patients with SLE was conducted in a single tertiary center. Transthoracic echocardiography was performed to estimate the pulmonary arterial pressures. PH was defined as resting systolic pulmonary artery pressure (sPAP) ≥ 40 mmHg, in the absence of left heart disease.
Results
PH was identified in nine patients (7.9%) who had few cardiopulmonary symptoms. SLE patients with PH had higher SLE disease activity index score. In particular, serum uric acid (UA) was significantly higher in patients with PH than in those without PH. In multivariate analysis, UA remained significant for the presence of PH. Moreover, serum UA level correlated significantly with plasma NT-pro-B-type natriuretic peptide level as well as sPAP. At the cutoff level of 6.5 mg/dL, serum UA had reasonable accuracy for predicting the presence of PH in SLE patients (sensitivity 66.7% and specificity 96.2%).
Conclusion
A significant number of SLE patients in rheumatology practice have undiagnosed PH with few discernible symptoms. Serum UA level may be useful as a surrogate marker for screening of PH in patients with SLE.
Introduction
Pulmonary hypertension (PH) is characterized by remodeling of the small pulmonary arteries, leading to a progressive increase in pulmonary vascular resistance and right ventricular failure.1 PH is one of the unusual cardiopulmonary manifestations in systemic lupus erythematosus (SLE), but a serious complication that carries a high mortality.2 Right heart catheterization studies have not been launched in patients with SLE and the prevalence of PH on echocardiography-based cohorts ranges between 0.5 and 14%.3-8 This variation in the reported frequency of PH is probably due to the differences in the study design, methods and criteria used to make a diagnosis, as well as to the differences in the patient populations. Given that significant number of patients with systemic sclerosis (SSc) or mixed connective tissue disease in a community rheumatology practice have undiagnosed PH and the majority of them exhibit no or nonspecific symptoms,9 it is supposed that more SLE patients with unidentified or missed PH would also exist in rheumatology clinics.
The reasons for the underestimation of PH are that the awareness of physicians of the early asymptomatic phase is low and that symptoms associated with PH, such as dyspnea, non-productive cough, impaired exercise tolerance and fatigue, are frequently considered to be attributable to the underlying connective tissue disease and its comorbidities.6, 9 Accumulating evidences demonstrated that currently available therapeutic agents when administered timely in the early phase of PH, significantly improved pulmonary vascular resistance, functional capacity and survival.10, 11 Thus, early disease detection in the preclinical asymptomatic or mildly symptomatic phase is a critical step in the therapeutic strategy to improve the outcome.10, 12-14
Previous reports documented that several factors were associated with the presence of PH in SLE but yielded conflicting results. Positive rates of anti-cardiolipin antibody were significantly higher in SLE patients with PH than those without in retrospective studies.15, 16 In a recent prospective study, lupus anticoagulant was the only significant risk factor for PH in SLE.6 However, these associations were not confirmed by other reports.3 Raynaud's phenomenon was suggested to be associated with the presence of PH in SLE patients in some studies15, 17 but not in the others.3, 16 These inconsistent results give us a lesson that these factors are not the determining factors and they are insufficient to predict the development of PH in SLE patients. Both the plasma brain natriuretic peptide (BNP) and the N-terminal pro-BNP (NT-proBNP), have been shown to detect PH with reasonable sensitivity and specificity in high-risk populations.18, 19 However, elevation in BNPs is a relatively late event related to right ventricle dysfunction, and lacks sensitivity to be used as a stand-alone test to detect early PH.14 Therefore, a further in-depth study to establish the clinical or serologic factors for predicting PH in SLE patients is required.
Using Doppler echocardiography, which has been shown to be the most practical and reliable non-invasive tool to screen for PH,9, 20 we sought to determine the point prevalence of undiagnosed PH in SLE patients, that was not detected during a routine clinical practice. We also assessed the clinical characteristics and laboratory features of all included SLE patients and investigated the useful factors for screening PH in SLE patients.
Patients and Methods
Patients
This prospective cross-sectional study was conducted in the Department of Rheumatology at Yeouido St. Mary's Hospital, The Catholic University of Korea between 2008 and 2010. Of the 381 SLE patients who are followed up regularly in this hospital, patients were excluded if they had previous history of symptomatic PH, congenital heart disease, valvular heart disease, left-sided heart failure, chronic thromboembolic disease or chronic obstructive pulmonary disease. Patients who had suspected active infections were also excluded. Finally, 114 patients with SLE gave informed consent to participate in this study. All these patients fulfilled at least four of the 1982 revised criteria of the American College of Rheumatology for SLE.21 As disease controls, nine patients with interstitial lung disease (ILD) and 22 patients with chronic obstructive pulmonary disease (COPD) who were diagnosed as having PH, and 12 patients with idiopathic pulmonary arterial hypertension (IPAH) were compared. All ILD patients were afflicted with underlying SSc. This study was carried out in accordance with the Declaration of Helsinki and was approved by the Yeouido St. Mary's Hospital Institutional Review Board (IRB), Clinical Research Coordinating Center of the Catholic Medical Center.
Clinical and laboratory profiles
The clinical and laboratory data of SLE patients were obtained at the time of transthoracic Doppler echocardiography. A clinical evaluation, including the review of their medical records, physical examination, blood tests and assessment of SLE disease activity was undertaken. SLE Disease Activity Index (SLEDAI) was used to estimate general disease activity. Laboratory parameters included complete blood count, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), creatinine, electrolytes, uric acid, and NT-proBNP. Antibodies against double-stranded DNA (dsDNA), Sm, ribonucleoprotein (RNP), ribosomal P, histone, Ro/SS-A (Sjögren's sydrome antigen A), La/SS-B, cardiolipin and β2-glycoprotein I were measured. Lupus anticoagulant was measured by the dilute Russell Viper Venom Test. The glomerular filtration rate (GFR) was estimated by the Modification of Diet in Renal Disease (MDRD) GFR (170 × PCr−0.999 × Age−0.176 × blood urea nigtrogen [BUN] −0.170 × albumin0.318 × 0.762 [for women]).
Doppler echocardiography
Transthoracic echocardiography (TTE) was performed in all patients as a screening test for PH, by experienced cardiologists. Two-dimensional, M-mode and color Doppler echocardiography were used to evaluate the cardiac morphology, flow abnormalities and cardiac functional status, including ejection fraction. Calculation of the left ventricular ejection fraction (LVEF) was performed using the modified Simpson's method of disc calculation from an apical four-chamber view at end systole and end diastole and patients with LVEF < 50% were excluded. Continuous wave Doppler sampling of the peak regurgitant jet velocity was used to estimate the right ventricular to the right atrial systolic pressure gradient using the modified Bernoulli equation (4× [tricuspid regurgitant jet velocity]2). sPAP was calculated by adding the Bernoulli-derived pressure gradient to the estimated mean right atrial pressure.
The current hemodynamic definition of PH is a mean pulmonary artery pressure > 25 mmHg; a pulmonary capillary wedge pressure, left atrial pressure, or left ventricular end-diastolic pressure of ≤ 15 mmHg.1 In this study, PH was defined as sPAP above 40 mmHg at rest, estimated by TTE, as described previously.3, 7, 9, 22
Statistical analysis
The results are presented as mean ± SE for continuous data and as percentages for categorical data. Comparisons of the numerical data between the groups were performed by Student's t-test or Mann–Whitney U-test. Qualitative differences between the groups were analyzed by the Chi-square test or Fisher's exact test. Correlation analysis between the two variables was performed using Pearson's correlation coefficient. The independence of the association with PH was assessed using a multivariate logistic regression procedure in which all variables that had a significant bivariate relation with the outcome in the previous reports were evaluated for inclusion in the model; results are reported as odds ratios with 95% confidence intervals. Two-sided P-values < 0.05 were considered to indicate statistical significance.
Results
Prevalence of PH in SLE patients
For 114 study subjects, the mean LVEF was 62.4 ± 0.7 mmHg with a range 50.5–84.6. Eighty-four patients (73.7%) had sPAP < 30 mmHg and 21 patients (18.4%) had sPAP of 30–39 mmHg. Nine of them (7.9%) were identified as having PH, as defined by a sPAP of more than 40 mmHg. All of the nine were female and showed no evidence of coexisting ILD. Among nine patients with PH, five individuals had sPAP above 50 mmHg and all of them were symptomatically corresponding to New York Heart Association (NYHA) functional class I or II. They conveyed an experience of mild dry cough or chest discomfort on exertion when asked specifically but no definite chest pain or dyspnea. Most of them have been essentially keeping low physical and social activity due to long-term suffering and sequelae of previous debilitating complications involving central nervous, hematologic or vascular systems (Table 1).
| Patient | Sex | Onset age | Disease duration | SLEDAI | Major clinical findings | aPL serology | Cardiopulmonary symptoms | sPAP (mmHg) |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 26 | 21 | 26 | Refractory thrombocytopenia (post-splenectomy), lupus nephritis | Negative | Unremarkable | 46 |
| 2 | F | 24 | 14 | 6 | Lupus nephritis | Negative | Chest discomfort | 47 |
| 3 | F | 25 | 8 | 4 | CNS lupus (seizure), antiphospholipid syndrome | aβ2GP1+ aCL+ | Dry cough | 90 |
| 4 | F | 16 | 9 | 12 | Retinal vasculitis, hemophagocytic lymphohistiocytosis, thrombotic thrombocytopenic purpura, CNS lupus (seizure), antiphospholipid syndrome | aβ2GP1+ aCL+ | Unremarkable | 87 |
| 5 | F | 39 | 1 | 10 | Neuropsychiatric lupus (hallucination) | Negative | Unremarkable | 40 |
| 6 | F | 24 | 10 | 8 | Serositis | Negative | Minimal chest tightness | 43 |
| 7 | F | 29 | 2 | 26 | Lupus nephritis, deep vein thrombosis | Negative | Chest discomfort on exertion | 61 |
| 8 | F | 19 | 1 | 7 | Lupus nephritis, serositis, cerebral infarction, retinal vasculitis, antiphospholipid syndrome | LAC+ | Unremarkable | 60 |
| 9 | F | 15 | 2 | 6 | Meningoencephalitis, Raynaud's phenomenon | Negative | Mild shortness of breath on exertion | 69 |
- SLE, systemic lupus erythematosus; PH, pulmonary hypertension; SLEDAI, SLE Disease Activity Index; aPL, antiphospholipid antibody; sPAP, systolic pulmonary artery pressure; CNS, central nervous system; LAC, lupus anticoagulant; αCL, anti-cardiolipin antibody; αβ2GPI, anti-β2 glycoprotein I antibody.
Clinical and laboratory characteristics of SLE patients with PAH
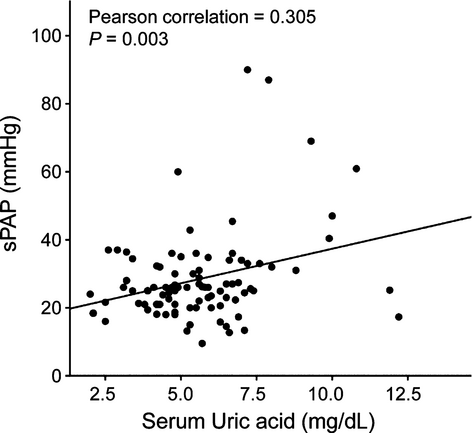
Clinical features of SLE patients with or without PH are summarized in Table 2. There were no statistically significant differences in age, disease duration, previous history of cardiovascular disease (coronary artery disease, stroke), and risk factors for cardiovascular disease (diabetes, hypertension), between the two groups. Frequency of Raynaud's phenomenon, ILD, and lupus nephritis did not differ between patients with and without PH. However, patients with PH exhibited significantly higher disease activity measured by SLEDAI than those without (11.7 ± 2.8 vs. 6.2 ± 0.5, P = 0.005). Comparison of laboratory variables is also shown in Table 2. Anti-Ro/SS-A positivity was less frequent in patients with PH than in those without PH (P = 0.014). However, the positivity of lupus anticoagulant, anti-cardiolipin, and anti-β2 glycoprotein I antibody did not differ between the two groups. Interestingly, serum UA levels were significantly higher in patients with PH than in those without PH (8.00 ± 0.71 vs. 5.29 ± 0.20 mg/dL, P < 0.001) and showed a positive correlation with sPAP (γ = 0.305, P = 0.003) (Fig. 1). None of the patients with PH had a history of gout or were being treated with diuretics. The association between serum UA levels and the presence of PH in SLE patients was further assessed using multivariate logistic regression, adjusting for variables affecting serum UA levels (sex, presence of hypertension and lupus nephritis, use of aspirin and cyclosporine, and renal insufficiency) and covariates with P < 0.2 in a univariate analysis (Table 3). As a result, only UA remained independently correlated with PH in patients with SLE.
| sPAP (mmHg) | P-value | ||
|---|---|---|---|
| < 40 (n = 105) | ≥ 40 (n = 9) | ||
| Sex, male : female | 13:92 | 0:9 | |
| Age, years | 36.9 ± 1.0 | 31.7 ± 3.2 | 0.150 |
| Echocardiography | |||
| LVEF,% | 62.6 ± 0.7 | 60.2 ± 4.6 | 0.610 |
| sPAP, mmHg | 23.9 ± 0.6 | 60.3 ± 6.2 | < 0.001 |
| Disease duration, years | 9.4 ± 0.9 | 7.4 ± 2.3 | 0.524 |
| Diabetes, n (%) | 1 (0.9) | 0 (0.0) | 0.769 |
| Hypertension, n (%) | 18 (17.1) | 1 (11.1) | 0.641 |
| CAD, n (%) | 1 (0.9) | 0 (0.0) | 0.769 |
| Stroke, n (%) | 11 (10.5) | 1 (11.1) | 0.953 |
| Raynaud's phenomenon, n (%) | 14 (13.3) | 1 (11.1) | 0.842 |
| Interstitial lung disease, n (%) | 8 (7.6) | 0 (0.0) | 0.390 |
| Lupus nephritis, n (%) | 50 (47.6) | 4 (44.4) | 0.855 |
| Renal insufficiency, n (%)† | 4 (3.8) | 1 (11.1) | 0.309 |
| SLEDAI | 6.2 ± 0.5 | 11.7 ± 2.8 | 0.005 |
| Medications | |||
| Steroid, n (%) | 103 (98.1) | 8 (88.9) | 0.220 |
| Prednisolone, mg | 8.4 ± 0.8 | 11.8 ± 2.6 | 0.259 |
| Hydroxychloroquine, n (%) | 92 (87.6) | 7 (77.8) | 0.337 |
| Immunosuppressants, n (%) | 64 (60.9) | 5 (55.6) | 0.738 |
| Aspirin, n (%) | 22 (20.9) | 2 (22.2) | 1.000 |
| Anti-Ro/SS-A, n (%) | 67 (63.8) | 2 (22.2) | 0.014 |
| Anti-La/SS-B, n (%) | 20 (19.1) | 2 (22.2) | 0.817 |
| Anti-Sm, n (%) | 36 (34.3) | 2 (22.2) | 0.461 |
| Anti-histone, n (%) | 51 (48.6) | 5 (55.5) | 0.688 |
| Anti-RNP, n (%) | 51 (48.6) | 5 (55.5) | 0.688 |
| Anti-Ribosomal P, n (%) | 36 (34.3) | 3 (33.3) | 0.954 |
| LAC, n (%) | 24 (22.8) | 1 (11.1) | 0.414 |
| αCL, n (%) | 25 (23.8) | 2 (22.2) | 0.914 |
| αβ2GPI, n (%) | 9 (8.6) | 2 (22.2) | 0.183 |
| NT-proBNP, pg/mL | 593.4 ± 372.2 | 3797.6 ± 1981.8 | 0.024 |
| Creatinine, mg/dL | 0.96 ± 0.05 | 1.15 ± 0.38 | 0.476 |
| GFR, mL/min | 77.5 ± 2.2 | 90.8 ± 14.9 | 0.404 |
| LDH, IU/L | 534.9 ± 64.3 | 632.0 ± 88.6 | 0.062 |
| Uric acid, mg/dL | 5.3 ± 0.2 | 8.0 ± 0.7 | < 0.001 |
- LVEF, left ventricular ejection fraction; sPAP, systolic pulmonary artery pressure; αβ2GPI, anti-β2 glycoprotein I antibody; RNP, ribonucleoprotein; CAD, coronary artery disease; LAC, lupus anticoagulant; αCL, anti-cardiolipin antibody; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index; NT-proBNP, N-terminal pro-brain natriuretic peptide GFR, glomerular filtration rate; LDH, lactic dehydrogenase. Values are expressed as mean ± SEM unless otherwise stated. †Renal insufficiency was defined by serum creatinine level ≥ 2 mg/dL.
| Variables | Beta coefficient | Odds ratio (95% CI)a | P-value |
|---|---|---|---|
| Uric acid | 0.685 | 1.983 (1.301–3.022) | 0.001 |
| SLEDAI | 0.118 | 1.125 (0.982–1.290) | 0.089 |
| Anti-Ro/SS-A | −1.782 | 0.168 (0.019–1.499) | 0.110 |
| Age | −0.050 | 0.951 (0.865–1.047) | 0.306 |
| αβ2GPI | 1.015 | 2.760 (0.255–29.823) | 0.403 |
- SLEDAI, Systemic Lupus Erythematosus Disease Activity Index; αβ2GPI, anti-β2 glycoprotein I antibody. aCI denotes confidence interval.
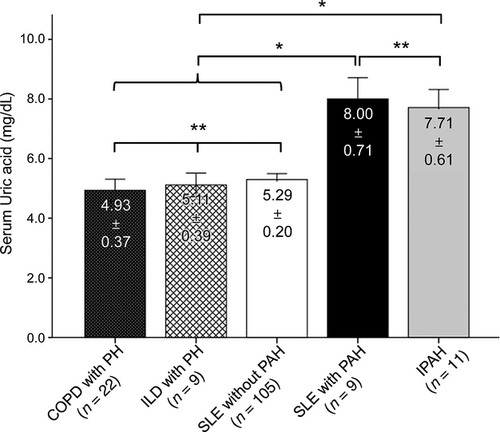
Serum UA levels in patients with pulmonary hypertension according to underlying causes
Despite a similar hemodynamic profile, the pathobiology and clinical course of PH differs considerably according to the underlying or associated diseases.1, 23, 24 To determine whether association of serum UA levels with PH observed in SLE patients is also applied to different forms of PH, we compared serum UA levels in patients with different underlying diseases at the time of diagnosis of PH. Nine patients with ILD, 22 patients with COPD who were diagnosed with PH and 11 patients with IPAH were compared. All of them were symptomatic in cardiopulmonary systems. Serum UA levels in patients with IPAH were highly comparable to those of SLE patients with PH. However, serum UA levels in the patients with underlying ILD or COPD were as low as those of SLE patients without PH (Fig. 2). Taken together, SLE patients with PH had significantly higher serum UA levels than those in the ILD and COPD patients with PH (P < 0.01) (Fig. 2).
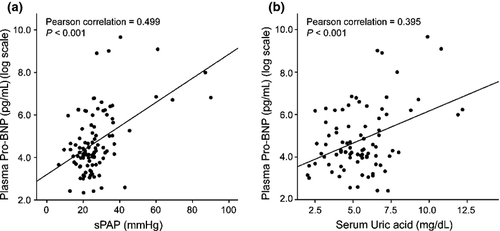
Clinical usefulness of serum UA measurement for screening of PH in SLE patients
In recent studies, plasma NT-proBNP level has been presented as a potential screening tool for early PH in high-risk populations.18, 19 In the present study, plasma NT-proBNP showed significant correlation with sPAP (γ = 0.499, P < 0.001) (Fig. 3). Serum UA levels correlated positively with plasma NT-proBNP levels (γ = 0.395, P < 0.001), as well as sPAP (Fig. 3). This correlation was even significant after adjusting for the known confounding factors, age and GFR (γ = 0.353, P < 0.001).25, 26 Optimal cutoff values to differentiate patients with PH from those without PH were deduced from receiver operating characteristics (ROC) curves. These were 6.5 mg/dL for UA and 800 pg/mL for NT-proBNP with specificities of 82.8% and 96.2%, and sensitivities of 77.7% and 66.7%, respectively. The positive predictive value (PPV) was 28.0% for UA and 60.6% for NT-proBNP. Combination of UA with NT-proBNP raised the specificity and PPV up to 98.1% and 71.4%, respectively. Using a logistic regression model, we determined the diagnostic accuracy for PH with respect to serum UA and plasma NT-proBNP adjusted for the confounding factors, age and GFR. As a result, serum UA became less sensitive (11.1%) and plasma NT-proBNP was more specific (97.1%) for the prediction of PH. However, the combination of two variables exhibited the diagnostic accuracy equivalent to the unadjusted model.
Discussion
Based on national registry data, PH is still considered as an orphan disease with prevalence of about 15 cases per million adult inhabitants.27, 28 However, previous studies indicated that the incidence of PH in connective tissue diseases (CTD), such as SLE and SSc is not unusual.6, 9 In our study, the prevalence of PH, as defined by a sPAP of ≥ 40 mmHg, was 7.9%. By cutting off the value of sPAP ≥ 50 mmHg, 4.4% was estimated. This rate is a bit higher when compared to those of the previous prospective study, in which the prevalence of PH, defined by sPAP ≥ 30 mmHg on TTE, was estimated as 4.2%.6 In our study, SLE patients with PH appeared to have higher disease activity compared to those without PH, which is consistent with the previous result studied in the same ethnic background.29 SLE patients included in this study were from a tertiary center, to which the patients with high disease activity are referred from regional clinics.
It is intriguing that the patients had little discernable symptoms in spite of high sPAP measured up to 90 mmHg. This result is consistent with the previous reports that the majority of PH cases in SLE were mild and asymptomatic.6, 29 This was probably because most of the patients have been sedimentary, with low physical activity to manifest PH-associated symptoms and because gradually increasing pulmonary vascular resistance and pulmonary arterial pressure are met with right ventricular hypertrophy and resultant preservation of cardiac output.30 Nonetheless, pathologic change of pulmonary vessels has already initiated in this compensated phase.31 Although the clinical significance and natural history of asymptomatic or mild PH in this phase is unclear, patients with identification of subclinical PH are closely monitored, thus providing at least an opportunity for early detection and treatment of future symptomatic PH since the several data available from PH trials have supported the notion that earlier intervention may improve the efficacy of current therapies as well as prognosis.10, 14
Of notable interest, serum UA levels were significantly higher in SLE patients with PH than in those without PH. The significance and independence of serum UA levels were confirmed by a multivariate analysis, involving variables affecting serum UA levels and probable PH-associated factors. This association is compelling since hyperuricemia is not usually observed in SLE patients except in the case of renal insufficiency and in use of drugs, such as diuretics.32 Then, what are the the mechanism(s) by which an increased UA is observed in SLE patients with PH? A previous study reported that serum levels of interferon-γ are correlated with disease activity in SLE patients.33 Interestingly, interferon-γ was shown to potently induce the xanthine oxidase (XO) enzyme in pulmonary endothelial cells.34 Moreover, tissue hypoperfusion and ischemia from reduced pulmonary circulation could deplete adenosine triphosphate and further stimulate the expression of the XO enzyme.35-37 Therefore, PH in active SLE might set up a favorable environment for generating UA. However, such a metabolic change in association with reduced pulmonary circulation would be modulated by the distinct pathobiology of the underlying diseases, giving rise to PH. For instance, high levels of oxidative stress was detected in the lungs of patients with COPD,38 and peroxynitrite, a major and powerful oxidative product, has been known to decrease plasma UA levels by rapid oxidation of UA.39, 40 Moreover, XO was not detected in the lung tissue of interstitial pneumonia, while inducible nitric oxide synthase (iNOS) was intensively expressed.41 The former finding is explained partly by high expression of transforming growth factor-β (TGF-β) in the lung tissue of patients with ILD, since the induction of XO was suppressed by treatment of TGF-β in rat cardiac cells.42 Thus, it is speculated that serum UA levels are not elevated in patients with COPD and ILD, even coexisting with pulmonary hypertension. Consequently, serum UA level can be a useful indicator distinguishing SLE-associated PH from lung disease-associated pulmonary hypertension.
Serum UA levels closely correlated with functional severity and have independent prognostic implications in IPAH.36 In SSc patients, uric acid levels have been correlated with sPAP and functional capacity and were independent predictors of the presence of PH in asymptomatic patients with SSc.43, 44 However, its levels have not been evaluated as a screening biomarker or predictor in SLE-associated PH. In this study, serum UA levels were not only independently increased in SLE patients with PH but also had a strong correlation with NT-proBNP, which is the validated marker of right ventricular dysfunction in PH.45 Not only do NT-proBNP values have a significant association with functional status as well as sPAP,46 they were qualified for a screening tool with the sensitivity of 56–69% and specificity of 95–100% in detecting PH in SSc patients.18, 19 NT-proBNP had comparable diagnostic values for detecting PH in this SLE patient group as well. With the use of a cutoff point of 6.5 mg/dL, the serum UA had reasonable accuracy for predicting the presence of PH in SLE patients as much as NT-proBNP. Therefore, if a variety of common causes of hyperuricemia such as decreased renal function, hypertension, use of drugs like diureitcs, salicylate and cyclosporin are ruled out, and then unexplained increase of UA would carry clinical relevance for detecting PH in SLE patients with better specificity.
This study has several limitations. First, echocardiography is an excellent non-invasive screening tool for PH but the diagnostic accuracy for PH with a sPAP threshold of 40 mmHg is relatively modest.47 sPAP can be over- or under-estimated when the tricuspid regurgitant jet is not of good quality. As a result, there is significant discrepancy between sPAP estimated from echocardiography and measured by right heart catheterization. Second, echocardiography is unable to differentiate between pre- and post-capillary PH for its inability to measure pulmonary capillary wedge pressure. Therefore, invasive hemodynamic confirmation by right heart catheterization is mandatory for confirming the diagnosis and establishing the cause of elevated sPAP. However, this invasive test was not offered except in the case of having sPAP > 40 mmHg with symptoms corresponding to WHO functional class 3 or 4, at the request of the Institutional Review Board committee given that the risk outweighed the benefits.6 For the same reason, right heart catheterization was not performed routinely on patients with elevated sPAP in our study. Third, due to the cross-sectional nature of this study, it is susceptible to bias, including selection bias and information bias.
Given a long asymptomatic period before clinically overt disease and the nonspecific symptoms and subtle physical signs, particularly in the early stages, a high clinical index of suspicion is necessary to detect the disease before irreversible pathophysiologic changes occur. In this regard, serum UA may be useful as a surrogate marker for screening of PH in SLE patients since its level has a significant correlation with NT-proBNP and sPAP and offers reasonable diagnostic values. Moreover, compared with NT-proBNP, UA measurement is more useful because it is inexpensive and it can be repeatedly and easily performed in a variety of clinical settings ahead of NT-proBNP. Therefore, it is worth consideration that NT-proBNP measurement and TTE would be performed on SLE patients with unexplained high UA levels.
Conflicts of Interest
None.




