Value of Routine Pelvic Examination in the Follow-Up of Patients Receiving Adjuvant Radiation Therapy for Endometrial Cancer: An Australian Tertiary-Centre Experience
ABSTRACT
Introduction
Pelvic examination is a routine component of post-treatment surveillance for endometrial cancer, supported by global guidelines. However, there is no evidence of oncological or quality-of-life benefit, with data suggesting associated discomfort and potential psychological harm. We evaluate the value of routine pelvic examination in follow-up protocols after adjuvant radiation therapy.
Methods
We retrospectively reviewed all patients receiving adjuvant radiation therapy for endometrial carcinoma across two combined cancer services between January 2017 and December 2022. All stages and histological subtypes were eligible. At least 12 months of documented follow-up was required. Patients were stratified by FIGO stage (2009 and 2023) and ESGO/ESTRO risk group.
Results
Two hundred and sixty-four of 395 patients met inclusion criteria, with a median follow-up of 34 months. Whilst demographics were widely distributed, the most common features included endometrioid histology (76.5%), FIGO 2023 stage II (48.5%) and ESGO/ESTRO high-risk (36.7%).
Disease recurrence was identified in 41 patients (15.5%). Only four patients had isolated local recurrence, with most also having distant disease at detection. Only three patients had asymptomatic recurrence found on examination (1.1% of cohort), with only one proceeding to salvage therapy (0.4% of cohort). As expected, higher-risk groups were associated with worse overall survival (p < 0.001).
Conclusions
We found routine pelvic examination following adjuvant radiation therapy for endometrial cancer results in low recurrence detection rates, with salvageable local recurrences being rare. We advocate for the omission of routine pelvic examination from follow-up protocols for patients receiving adjuvant radiation therapy, with either clinic-based or telephone-based follow-up being offered on a risk-stratified basis.
1 Introduction
- To allow early identification of disease recurrence such that disease-trajectory-modifying salvage treatments can be offered.
- To identify, monitor and manage toxicities that are consequential from treatment.
- To provide psychosocial support.
Follow-up protocols vary between tumour streams, with further patient-specific individualisation based on cancer risk stratification and patient-specific factors (e.g., risk of toxicity). Follow-up must be sufficiently frequent to serve its purpose, but must also be rational enough to consider the logistical and economic burdens on clinicians and services [1-3].
Rationalisation of follow-up is especially important in gynaecological malignancies. A major component of every visit is a pelvic examination to assess for local recurrence, which is invasive and uncomfortable [4]. Noninvasive imaging is unable to replace or substitute the role of a pelvic examination with respect to sensitivity of assessment [5]. As such, it is important that there is a clear and reasonable justification for clinicians to request consent for these procedures.
Currently, the NSW Agency for Clinical Innovation Gynaecological Oncology Network guidelines suggest patients should be followed up every 3 months for year 1, every 4 months for year 2, every 6 months for year 3 and then annually for years 4 and 5 [6]. At each of these visits, a routine pelvic examination is recommended—a total of 11 examinations during follow-up. The Cancer Council's Optimal Care Pathway provides similar but less specific guidance, recommending less intensive follow-up for those with low-risk disease (defined as a risk of recurrence of 5% of less) [7].
However, there is limited evidence to support these recommendations with respect to routine examination, with no evidence for improved survival associated with early identification of recurrence [3, 8]. This is of particular concern in patients who have received adjuvant radiation therapy, for whom local salvage options are far more limited should recurrence be identified.
This has led to the introduction of patient-initiated follow-up (PIFU) programmes within the National Health Service (NHS) in the United Kingdom, with the backing of the British Gynaecological Cancer Society [9]. This provides for risk-stratified timing for transition to PIFU, with both phone and clinic consultations being appropriate whilst routine follow-up continues.
Despite this lack of evidence and changing patterns globally, Australian practice and guidelines continue to require routine pelvic examinations for all patients [6].
We aimed to evaluate the value of routine pelvic examination within our gynaecological oncology service in the Australian setting, to identify the frequency of asymptomatic disease recurrence and resulting rates of salvage therapy being offered.
2 Methods
2.1 Setting & Study Population
This study was conducted within the two Radiation Oncology Network sites within the Western Sydney Local Health District (WSLHD). This combined practice is the major tertiary referral oncology centre within the largest catchment in NSW, estimated at 1,114,280 residents in 2021. In 2023, this service managed 804 new gynae-oncology patients, including 213 patients with uterine malignancies [10].
All patients with a diagnosis of primary endometrial carcinoma who received adjuvant radiation therapy following primary surgical management within WSLHD during the study period were eligible for inclusion. Exclusion criteria included noncarcinoma histology (e.g., primary endometrial sarcoma), patients receiving definitive radiation therapy without primary surgical management and patients receiving salvage radiation therapy for disease recurrence.
The study period was defined as the course of adjuvant radiation therapy being completed between January 2017 and December 2022. For meaningful analysis, patients required at least 12 months of follow-up after radiation therapy to be included in the analysis (unless censored by death).
Cases were identified via search of the oncology information system by appropriate ICD-10 diagnosis codes (C54 & C55).
2.2 Data Collection
Data were retrospectively accessed from the electronic clinical records. The oncology information system was used to access most of the clinicopathological data, whilst histopathological and staging data were sourced from the local pathology database.
- –
Patient demographic and tumour features (age, histological diagnosis including subtypes, lymphovascular invasion, 2009 & 2023 FIGO stages)
- –
Treatment features (treatment modality, prescribed dose, treatment dates)
- –
Follow-up (most recent attendance, disease status, date of death)
- –
Disease recurrence (location of recurrence, nature of presentation, subsequent treatments recommended and then received)
Description of lymphovascular invasion was inconsistent between pathology reports. Where a detailed description was available, extensive lymphovascular invasion was defined as ≥ 5 vessels being involved (as per FIGO guidelines) [11]. Otherwise, the presence of lymphovascular invasion was reported in a binary manner.
Risk stratification was defined as per the ESGO/ESTRO/ESP consensus guideline [12], incorporating the nonmolecular low, intermediate, high-intermediate and high-risk groups.
Data were stored in a Microsoft Excel database (Microsoft, Washington, USA) within the secured WSLHD local network.
2.3 Statistics
Much of the data is presented in a descriptive manner to allow observation of trends amongst our patient cohort.
When comparing categorical data, statistical comparison was conducted via Fisher's exact test for 2 × 2 contingencies (Freeman–Halton extension used for larger contingency tables). Kaplan–Meier graphs with log-rank testing were used for survival analysis. A p-value of < 0.05 was considered statistically significant.
Statistical analysis was performed within SPSS Statistics v29 (IBM, New York, USA). Graphical representations of data were performed within SPSS Statistics v29 and SankeyMATIC.
2.4 Ethics
Ethical approval for the study was granted by the Western Sydney Local Health District Human Research Ethics Committee (approval number = 2405–03 QA). This study was also reviewed and approved by the local Radiation Oncology Network research committee.
3 Results
Three hundred and ninety-five patients were identified in the initial database search. Ultimately, 288 cases were eligible for analysis following the exclusion of duplicate cases, patients receiving salvage radiation therapy, patients receiving definitive/palliative radiation therapy for intact endometrial cancers and patients with noncarcinoma histologies.
Of these, 264 patients met the criteria for at least 12 months of documented follow-up (the database was locked for collection of follow-up data on 12 May 2024). The median follow-up of the resulting cohort was 34.37 months.
The demographic, tumour and treatment features for included patients are displayed in Table 1. Notably, because of the updated FIGO 2023 staging system incorporating extensive lymphovascular invasion and high-grade histologies into grade 2 disease, considerable stage migration between stages 1 and 2 is demonstrated between the 2009 and 2023 staging systems.
| Patients (n = 264) | ||
|---|---|---|
| Median Age—years (range) | 66.81 (32.01–90.94) | |
| Histology | ||
|
247 (93.6%) | |
|
202 (76.5%) | |
|
38 (14.4%) | |
|
6 (2.3%) | |
|
1 (0.4%) | |
|
15 (5.7%) | |
|
2 (0.8%) | |
| Histological Grade | ||
|
101 (38.3%) | |
|
55 (20.8%) | |
|
93 (35.2%) | |
|
15 (5.7%) | |
| Lymphovascular Invasion | ||
|
49 (18.6%) | |
|
57 (21.6%) | |
|
42 (15.9%) | |
|
116 (43.9%) | |
| FIGO Stage | FIGO (2009) | FIGO (2023) |
|
168 (63.6%) | 79 (29.9%) |
|
56 (21.2%) | 9 (3.4%) |
|
112 (42.4%) | 60 (22.7%) |
|
— | 10 (3.8%) |
|
39 (14.8%) | 128 (48.5%) |
|
— | 20 (7.6%) |
|
— | 35 (13.3%) |
|
— | 73 (27.7%) |
|
51 (19.3%) | 53 (20.1%) |
|
10 (3.8%) | 10 (3.9%) |
|
9 (3.4%) | 11 (3.9%) |
|
32 (12.2%) | 32 (10.9%) |
|
6 (2.3%) | 4 (1.6%) |
|
2 (0.8%) | 2 (0.8%) |
|
4 (1.5%) | 2 (0.8%) |
| ESGO/ESTRO/ESP Risk Group | ||
|
9 (3.4%) | |
|
79 (29.9%) | |
|
79 (29.9%) | |
|
97 (36.7%) | |
| Adjuvant Treatment Received | ||
|
256 (100%) | |
|
160 (60.6%) | |
|
84 (31.8%) | |
|
20 (7.6%) | |
|
62 (23.5%) | |
- Abbreviations: EBRT, external-beam radiation therapy; VBT, vaginal brachytherapy.
Based on collaborative guidelines and local institutional practice [12], patients with low-risk disease are typically provided a recommendation for observation in lieu of adjuvant radiotherapy. However, a small number of patients in our cohort did receive adjuvant radiotherapy despite having low-risk disease, following multidisciplinary consensus. Indications for this included morcellated specimen limiting assessment of depth of invasion (n = 3), large macroscopic tumour size (n = 3), lack of nodal staging (n = 2) and discordance/uncertainty in histopathology between curette and hysterectomy specimens (n = 1).
3.1 Patients With Disease Recurrence
Forty-one patients (15.5% of cohort) developed disease recurrence during the follow-up period, with a median time to recurrence of 14.33 months. Most patients with recurrence had high-risk disease at diagnosis (n = 31; 75.6%; p < 0.001). Carcinosarcoma (6/15; 40%) and serous adenocarcinomas (13/28; 34.2%) were more likely to develop recurrence than any other histological subtype (p < 0.001).
Summary and visual representation of the recurrence characteristics is provided in Table 2 and Figure 1. Of note, whilst surveillance imaging is not a component of our practice locally, seven patients (17.1% of recurrences) had recurrent malignancy identified based on imaging arranged by external practitioners for either surveillance or unrelated indications.
| Patients with recurrence (n = 41) | |
|---|---|
| Duration of follow-up—months (median + range) | 34.73 (2.40–77.90) |
| Time to recurrence—months (median + range) | 14.33 (1.00–60.20) |
| Risk group at initial diagnosis | |
|
0 (0%) |
|
4 (9.8%) |
|
6 (14.6%) |
|
31 (75.6%) |
| Mode of recurrence detection | |
|
31 (75.6%) |
|
7 (17.1%) |
|
3 (7.3%) |
| Anatomic distribution of recurrence | |
|
4 (9.8%) |
|
7 (17.1%) |
|
22 (53.7%) |
|
2 (4.9%) |
|
3 (7.3%) |
|
3 (7.3%) |
| Received salvage therapy | 11 (26.8%) |
|
6 (14.6%) |
|
1 (2.4%) |
|
2 (4.9%) |
|
2 (4.9%) |
- Abbreviation: EBRT, external beam radiation therapy.
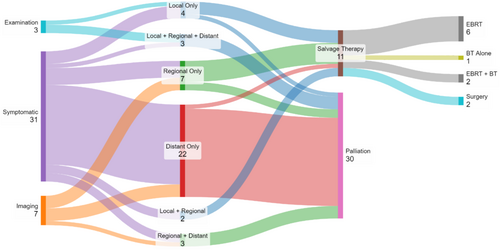
Isolated local recurrences occurred in four patients, and of these, only one was asymptomatic and identified on routine examination. This patient proceeded to salvage therapy with brachytherapy (EQD2 was limited to 38Gy due to previous dose), developing short-interval progressive disease in-field after 14 months.
The two other patients with an asymptomatic diagnosis of recurrence on examination were found to have a biopsy-proven vaginal vault recurrence and a palpable abdominal mass respectively. Further staging for both patients demonstrated both regional and distant metastatic disease, with palliative management then initiated.
A total of 11 patients underwent salvage treatments for disease recurrence with either EBRT (n = 6), brachytherapy (n = 1), EBRT + brachytherapy (n = 2) or surgical resection (n = 2). Each case of salvage brachytherapy involved intracavitary treatment using the Venezia applicator set (0 mm tandem and vaginal caps). Radiation therapy doses varied depending on target site, previous irradiation and time interval. One patient underwent hepatic resection of oligometastasis, and another underwent resection of an abdominal wall metastasis.
3.2 Overall Survival
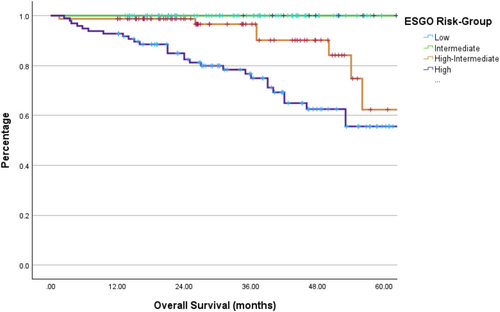
The median overall survival for the entire cohort was 71 months, with stratification by risk group shown in Figure 2. As expected, patients who are in higher ESGO risk groups at diagnosis were at a higher risk of death (p < 0.001).
4 Discussion
Our study presents the patterns of follow-up for all endometrial cancer patients receiving adjuvant radiation therapy within our centre over a 6-year period, with all having at least 12 months of routine follow-up.
Of the 41 patients (15.5% of cohort) who developed disease recurrence, only three patients (1.1% of cohort) were asymptomatic, with recurrence identified on examination. Of these, only one patient (0.4% of cohort) had isolated local recurrence and proceeded to salvage brachytherapy. Ultimately, due to previous radiation therapy, a truly curative salvage dose was not feasible, and this patient died of progressive disease.
Our experience is very similar to that published by Sarwar, et al. regarding their experience at University College London [13]. This retrospective study from a large single centre reviewed 900 patients who were managed surgically for endometrial cancer (all stages and histological subtypes included). 16% of all patients relapsed and of the 126 recurrences identified, only 18 (2% of cohort) were salvageable and only 12 (1.3% of cohort) were both asymptomatic and salvageable. ‘Asymptomatic’ in this study did not distinguish between clinical or radiological identification of recurrence.
It should be noted that only 53% of patients received adjuvant therapy in the Sarwar study, the majority of whom had some form of radiation therapy [13]. Salvage radiation therapy is often quite feasible for isolated local recurrence following hysterectomy alone, whilst both salvage surgery and radiation therapy are more challenging following a course of adjuvant radiation therapy. Therefore, the Sarwar study presents a cohort with potentially higher salvage potential than those included in our study.
However, there is no high-level evidence to suggest that any form of post-treatment surveillance will improve overall survival or patient quality of life [3, 14, 15]. For guidance, in addition to the data from Sarwar, et al., a retrospective analysis of 234 cases of recurrent endometrial cancer in a single centre found that 8.5% of all recurrences were identified by pelvic examination in an asymptomatic patient [16]. However, this does not evaluate the overall detection rate of routine pelvic examination, nor does it discuss the suitability for salvage therapy following detection.
Whilst an imperfect comparison, the only randomised guidance is provided by the TOTEM trial, which compared an intensive follow-up schedule with a more minimalist approach for women after curative management of endometrial cancer [8]. The differences in follow-up schedules pertained to routine serology, vaginal cytology and CT surveillance, as well as a small difference in the number of clinic visits (however all patients received gynaecological examination). Only 33.3% of included patients had any form of adjuvant therapy. The intensive follow-up programme was not associated with an improvement in overall survival and a small nonsignificant trend towards an increased likelihood of detecting early recurrence which did not translate to a survival benefit. Whilst this study represents the only randomised data on the topic, this study is not very applicable to Australian practice as the routine local practice employs a minimalist approach.
It is important to also note that whilst some women are greatly reassured by an unremarkable routine pelvic examination, they can also be associated with harm to patients [7]. At the most basic level, vaginal examinations are associated with some degree of physical discomfort, particularly when associated with vaginal stenosis following radiation therapy [17, 18]. This can be compounded by psychological discomfort associated with existing risk factors such as previous sexual trauma [18, 19]. However, with some women placing greater significance on the results of the examination than clinicians, there is also the risk of anticipatory anxiety in the lead-up to scheduled appointments [4].
As a result of the issues discussed above, the NHS in the United Kingdom has broadly implemented a system of patient-initiated follow-up (PIFU), which has been supported by the British Gynaecological Cancer Society (BGCS) [9]. The protocol is risk-stratified to guide appropriate follow-up protocols for individual patients. For patients with high-intermediate or high-risk disease (who are most likely to receive adjuvant radiation therapy), it is recommended that patients are transitioned to PIFU after 2 years of routine follow-up. Notably, both clinic-based and telephone follow-up are recommended as reasonable approaches whilst routine follow-up is offered, though vaginal examination is still recommended when clinic-based review is employed.
However, guidelines from other co-operative groups have not yet undergone similar modifications. As summarised in Table 3, guidelines from Europe, Asia, Oceania and the United States demonstrate significant variation in the recommendations for follow-up following definitive management of gynaecological cancers. Incorporation of risk adaptation, follow-up intervals and the role of routine imaging in high-risk groups varied quite widely between guidelines. However, all guidelines uniformly recommend routine pelvic examination during clinic appointments, with only the Australian Cancer Council, BCGS and European Society for Medical Oncology (ESMO) guidelines providing telephone consultation as a reasonable alternative. It should be noted that most guidelines do expressly acknowledge the absence of evidence to support a benefit to routine examination.
| Sponsoring organisation(s) | Risk adapted protocols | Frequency of clinic-based follow-up | Telephone-based alternative | Routine pelvic examination recommendation | Routine imaging recommendation |
|---|---|---|---|---|---|
| NCCN (v2024.2) [20] | No |
- 3–6 monthly for 2–3 years—6–12 monthly until 5 years—then annually |
No | Yes | Not recommended |
|
ESMO [21] |
Yes |
LR = 6 monthly for 2 years & then 12 monthly until 5 years HR = 3 monthly for 3 years & then 6 monthly until 5 years |
Yes (for LR group only) | Yes | Can be considered in HR groups |
|
ESTRO/ESGO/ESP [12] |
— |
No specific guidance |
— | — | — |
| Cancer Council (Australia) [7] | Yes |
LR = 3–6 monthly for 2 years, then 6–12 monthly for 1 year, then annually until 5 years HR = more frequent than LR (no specific guidance) |
Yes | — | — |
|
NSW ACI Gynaecological Oncology Network [6] |
No |
- 3 monthly for 1 year—4 monthly for 1 year—6 monthly for 1 year—then annually until 5 years |
No | Yes | Not recommended |
| NZGCG [22] | Yes |
LR = annually for 2 years IR = 6 monthly for 3 years HR = 3 monthly for 1 year & then 6 monthly until 3 years |
No | Yes | Not recommended |
| SEOM-GEICO [23] | Yes |
LR = 6–12 monthly for 2 years & then annually HR = 3–6 monthly for 2 years & then 6–12 monthly |
No | Yes | Can be considered in HR groups |
| BGCS [24] | Yes | No specific guidance (protocols should be individualised) |
Yes (for all risk groups) |
Yes |
Not recommended |
|
Pan-Asian adapted ESMO [25] |
Yes |
LR = 6 monthly for 2 years & then 12 monthly until 5 years HR = 3 monthly for 3 years & then 6 monthly until 5 years |
Yes (for LR group only) | Yes | Can be considered in HR groups |
|
JSGO [26] |
No |
- 1-4 monthly for 3 years—6 monthly for 2 years—annually thereafter |
No | Yes | Can be considered in HR groups |
- Note: Note that risk groupings are not consistently defined between guidelines.
- Abbreviations: HR, high risk; IR, intermediate risk; LR, low risk.
Current clinical trials are also following a more intensive follow-up schedule, which is to be expected given the need to closely monitor patients when introducing an experimental treatment approach. The PORTEC-4 trial requires 3-monthly reviews for the first 3 years, with 6-monthly reviews until 5 years of total follow-up [27]. These reviews require clinical evaluation for the early detection of local recurrence, which implies (but does not explicitly state) routine pelvic examination is required. Similarly, after an initial post-treatment review, the EN.10 trial requires 6-monthly reviews for the first 3 years, with annual reviews until 5 years of total follow-up, with a routine pelvic examination at each visit [28]. The potential role of pelvic examination remains unclear should molecular risk features dictate routine management, with higher rates of de-escalation with radiation therapy omission being likely.
Based on the data presented and the literature published to date, we would advocate for routine pelvic examination to be excluded from the routine follow-up of women who receive surgical management and adjuvant radiation therapy for endometrial cancer. Not only is there no evidence to support its benefit with considerable potential for harm to patients, but the identification of vaginal vault recurrence is unlikely to be actionable—isolated vaginal vault recurrence is rare, and salvage management options following previous radiation therapy are limited. As a caveat, we do fully support intensive follow-up including pelvic examination when treatment protocols change from a well-established standard, such as when introducing trial protocols as detailed above.
It is also very important to distinguish this recommendation for the exclusion of routine pelvic examination from the omission of follow-up altogether. As previously mentioned, follow-up serves not only to detect disease recurrence, but to identify and manage toxicities that may arise [1, 2]. However, in the absence of symptoms, we believe pelvic examination has a limited role in the appropriate management of post-radiotherapy toxicities. Frequently identified findings such as atrophy and telangiectasia do not require intervention in the absence of bleeding or recurrent urinary infections, and so examination can safely be triggered when indicated by symptoms.
One possible exception is surveillance for vaginal stenosis. All patients should be encouraged to regularly use vaginal dilators as prophylaxis for vaginal stenosis (protocols vary between institutions), with few options for intervention available if stenosis does occur. As vaginal stenosis typically occurs following the complete omission of vaginal dilators or poor dilator technique, serial examinations may provide a valuable educational opportunity for patients to ensure correct dilator use.
A rational approach may be to advocate for a UK-style approach, with routine clinic or telephone follow-up for the first 2 years, with a PIFU protocol thereafter [9]. In this approach, pelvic examination would only be conducted if symptoms or signs indicate the need.
Finally, an essential component to the success of any PIFU approach is to ensure easy and rapid referral pathways back to the service should an issue arise. An important qualitative study was performed by Amirthanayagam et al. regarding clinician perspectives of the PIFU programme [29]. Several barriers to re-presentation were raised, including patient stoicism, poor communication on flags for re-presentation, and non-English speaking background resulting in communication difficulties. Further, there was an emphasis on individualising follow-up such that PIFU should be offered, but not forced upon all patients as there are some who may gain psychological benefit from an ongoing clinical review.
Careful planning, co-ordination and clinician education are essential to ensure safe local introduction of such a programme, with guidance available from multiple previously implemented models shown to be safe and acceptable to patients [30-32]. It is critical to ensure a smooth return to care when required, and encouraging involvement of general practitioners and/or a gynaecological specialist nurse co-ordinator in a shared-care approach may assist. In this case, clearly delineated roles between professionals will ensure efficiency and avoid missing flags for review. Further, the provision of a detailed end-of-treatment summary within a survivorship framework can help provide the patient with clear and concise guidance on triggers for re-presentation, and the appropriate pathways to follow.
4.1 Study Limitations
Whilst our study is naturally limited by the retrospective nature of data collection, we do present a comprehensive cohort of patients who live within our catchment area. Patients excluded because they were lost to follow-up were typically those who were referred from regional centres for treatment, with follow-up conducted by the local services thereafter. Similarly, our study suffers the limitations of a single-centre experience; however, our centre is a large tertiary service that provides the full range of gynaecological oncology services and so would be representative of Australian practice.
Notwithstanding these limitations, we feel that this study provides an important insight into the value of follow-up for patients receiving radiation therapy after endometrial cancer surgery, which is an often assumed conclusion.
An important caveat is that given the low rate of vaginal recurrence expected after adjuvant radiation therapy (and limited salvage options available thereafter), these data and conclusions should not be applied to patients who do not receive adjuvant radiation therapy.
5 Conclusions
We present data which adds to existing literature suggesting the limited value associated with routine pelvic examination in the follow-up of women who receive adjuvant radiation therapy following surgical management of endometrial cancer. We found that recurrence detection rates are low and salvageable local recurrences are rare. We advocate for the omission of routine pelvic examination from follow-up protocols, with either clinic-based or telephone-based follow-up being offered on a risk-stratified basis. However, there is insufficient data to recommend the omission of follow-up altogether, with management of toxicity and psychological benefits remaining likely.
Acknowledgements
Open access publishing facilitated by The University of Sydney, as part of the Wiley - The University of Sydney agreement via the Council of Australian University Librarians.
Disclosure
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




