Comparative analysis of clinical treatment outcomes: Breath-hold vs. free-breathing techniques in liver stereotactic body radiotherapy
K Morishima: MD; H Yamashita MD, PhD; T Noyama MD, PhD; A Katano MD, PhD.
Conflict of interest: None.
Abstract
Introduction
The aim of this study was to clarify the safety and efficacy of breath-hold irradiation in liver stereotactic body radiotherapy (SBRT).
Methods
A retrospective analysis was conducted on 57 consecutive patients who received SBRT for hepatocellular carcinoma or liver metastases between 2013 and 2021. Breath-hold irradiation was implemented for patients treated after April 2020.
Results
The median follow-up period for all patients was 16.4 months (IQR: 7.36–20.9). The 2-year overall survival rate was 64.4% (95% CI: 47.4–77.2), and the local control rate was 84.3% (95% CI: 69.7–92.3) for all patients. The 1-year overall survival was 80.0% (95% CI: 60.8–90.5) versus 82.0% (95% CI: 53.5–93.9) in the free-respiratory (FR) group versus the breath-hold (BH) group, respectively (P = 0.60). The 1-year local control rates were 78.1% (95% CI: 57.5–89.5) in the FR group and 95.7% (95% CI: 72.9–99.4) in the BH group, respectively (P = 0.16). Radiation-induced liver injury, defined by an escalation of ≥2 in Child–Pugh score, was observed in four patients within each group (FR 13% vs. BH 15%). There were no gastrointestinal adverse events of Grade 3 or higher.
Conclusion
Breath-hold irradiation can be safely administered and has demonstrated clinical potential in improving local control. Further research into dose escalation using breath-hold techniques is warranted.
Introduction
Hepatocellular carcinoma (HCC) accounted for 865,000 new cases and 757,000 deaths globally in 2022, making it the sixth most commonly diagnosed cancer and the third leading cause of cancer-related deaths.1 The primary curative modality for HCC is surgery, typically recommended for patients exhibiting preserved hepatic function and tolerance to the procedure.2 For inoperable patients, radiofrequency ablation (RFA) has been reported to demonstrate a local control rate comparable to that of surgery for HCC with small tumour size and limited lesions. RFA is a proven treatment approach for HCC cases with tumour sizes not exceeding 3 cm in diameter.3 Stereotactic body radiotherapy (SBRT) is an emerging technique and a viable treatment option when surgery or radiofrequency ablation (RFA) is not suitable.4 Previous clinical trials and retrospective analyses have demonstrated a 2-year local control rate ranging from 70% to 90%.5, 6 Rim et al. demonstrated that ablative radiotherapy can achieve oncologic outcomes comparable to RFA and may be more effective for treating tumours in challenging locations or larger tumours in their hybrid meta-analysis.7 Although there are no reports of prospective clinical trials comparing RAF and SBRT. Radiotherapy may be the first-line treatment for HCC cases with relatively preserved hepatic function in instances where surgery or RFA are contraindicated.8
Radiation-induced liver injury (RILD) is a known adverse event associated with radiotherapy, which leads to a temporary decline in Child–Pugh score after irradiation.9 The incidence of RILD correlates with hepatic function. In the case of Child–Pugh A, the irradiation to the focal liver could perform safely. In Child–Pugh B7, the dose was reduced or the number of fractions was increased than in Child–Pugh A.10
Image guidance is essential for the safe and effective delivery of high-dose treatments that account for inter- and intra-organ motion.11 Techniques for assessing and managing respiratory motion enable precise delineation of margins within the tumour volume and accurate beam delivery amidst intrafractional organ motion. Both prospective and retrospective trials exploring external beam radiotherapy (EBRT) for HCC have employed daily imaging guidance methods such as orthogonal kilovoltage imaging, computed tomography (CT) on rail imaging and cone-beam CT, alongside respiratory motion management techniques including breath holding, gating, tracking and abdominal compression. However, investigations specifically addressing the efficacy of daily imaging guidance and respiratory motion management remain scarce.12
In our previous practice, HCC was treated with free-breathing SBRT using image guidance. Concern arose regarding the necessity for additional margins due to respiratory migration during free breathing, resulting in an enlarged irradiation field.13 Treatment with breath-hold improves dose delivery accuracy and reduces PTV margins. The adoption of breath-hold techniques potentially improves the toxicity by reducing the dose of organ at risk (OAR). In liver SBRT, breath-hold irradiation has been associated with a reduced probability of normal liver complications.14 By eliminating the effects of respiratory movement, it is enable to increase the dose intensity of the target, thereby potentially enhancing tumour control.
Therefore, since April 2020, irradiation with breath-hold techniques has been integrated into liver SBRT protocols at our institution. In this retrospective study, we compared the toxicity and control rate of using breath-hold irradiation with those of previous free-breathing irradiation.
Methods
Patients who received SBRT for HCC or liver metastases at our institution from 2013 to 2022 were retrospectively surveyed. The current study was approved by the Institutional Review Board at the University of Tokyo Hospital (No. 3372-6). Cases in which hepatic veins, portal vein and inferior vena cava tumour thrombus were irradiated simultaneously in addition to intrahepatic masses were included. SBRT was performed using X-rays from a 6-MV or 10-MV linear accelerator. In all cases, CT registration, such as kilovoltage cone-beam CT (CBCT) was performed during each treatment. Radiotherapy was administered only after confirming that there were no significant deviations between the expiratory phase images from the planning CT and the CBCT. Breath-hold irradiation was implemented through volumetric-modulated arc therapy (VMAT), with a single arc being subdivided into six beams, each deliverable within 20 s or less. Our institution employs the repeated breath-hold method for breath-hold irradiation, with the breath-hold phase confirmed by kilovoltage projection imaging of the diaphragm. This technique requires patients to hold their breath multiple times during therapy to stabilize their liver position, enhancing the precision and effectiveness of the treatment. Detailed treatment protocols are outlined in previous publications.15-17 Figure 1 illustrates a schematic summary of the breath-hold method utilized at our institution. For free-respiratory irradiation, VMAT, multimodal irradiation or three-dimensional conformal radiation therapy was used.
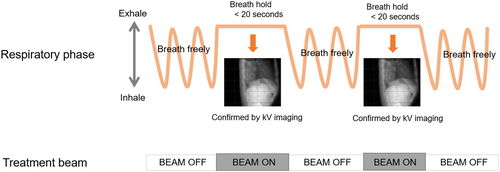
The gross tumour volume (GTV) was delineated by referencing gadolinium-ethoxybenzyl-diethylenetriamine penta-acetic acid-enhanced MRI or contrast-enhanced CT (CE-CT) images to accurately contour the tumour volume. In the breath-hold (BH) group, the clinical target volume (CTV) was created by adding a 0–5 mm margin to the GTV, and the internal target volume (ITV) was equivalent to the CTV. For the free-breathing (FB) group, four-dimensional computed tomography (4D-CT) with a time-synchronized external respiratory signal was used to quantify tumour motion. The CTV was contoured across all respiratory phases, and the ITV was generated by accumulating the CTVs from all respiratory phases, ensuring coverage of the entire tumour motion range. For the planning target volume (PTV), an individualized 5 mm margin was applied around the ITV in both the BH and FB groups to account for setup variations. Patients received either 48 Gy in 4 fractions or 50–60 Gy in 10 fractions, with the radiation oncologist determining the dose based on tumour size and normal tissue exposure during treatment planning. The prescription point was D50 (dose covering 50% volume within PTV), D95% or iso-center. The biologically effective dose (BED) (α/β = 10 Gy) was 75–105.6 Gy. The following formula for BED10 was used: BED (Gy10) = nd × (1 + d/10). Normal tissue dose constraints were below: liver mean dose <20 Gy, liver maximum dose <110% prescribed dose, stomach, bowel, oesophagus maximum dose <43.5 Gy, cord max dose <35 Gy, kidney mean dose <26 Gy.
Local control, recurrence-free survival, overall survival, and adverse events were compared between free-respiratory and breath-hold techniques. Local control, recurrence-free survival and overall survival were measured from the first day of irradiation to the event or of death. RILD was defined as a rise of ≥2 in Child–Pugh score at 3 or 6 months after treatment. Furthermore, comparisons were made regarding target volume, dosage and doses of organs at risk (OAR) during the planning phase between the two groups. The survival probability was estimated by Kaplan–Meier curves and the log-rank test was used to determine whether there was a statistically significant difference between the patient groups. To compare differences in patient characteristics, Pearson's chi-square or Fisher's exact test was used to analyse categorical variables, and Mann–Whitney U test (non-normally distributed data) or Student's t-test (normally distributed data) was used to analyse continuous variables. A two-tailed P-value of less than 0.05 was considered statistically significant. All statistical analyses were performed using R, version 4.1.1.
Result
Patient characteristics
We conducted a retrospective analysis involving 57 consecutive patients who received liver SBRT at our institution. Among them, 31 patients (54%) underwent free-respiratory irradiation and 26 (46%) underwent breath-hold irradiation. Patients eligible for breath-hold irradiation were able to hold their breath for at least 15 s. The majority of patients undergoing breath-hold techniques were treated after April 2020, with inclusion also extending to those treated before April 2020 if free-respiratory techniques failed to meet dose constraints. Patient background is shown in Table 1.
| Free respiratory | Breath-hold | |
|---|---|---|
| Gender, n (%) | ||
| Male | 23 (74%) | 18 (69%) |
| Female | 8 (26%) | 8 (31%) |
| Age (median) | 73.1 | 75.4 |
| KPS | ||
| KPS ≧ 80% n (%) | 28 (90%) | 25 (96%) |
| KPS < 80% n (%) | 3 (10%) | 1 (4%) |
| Total dose, n (%) | ||
| 48 Gy/4 fr | 20 (65%) | 15 (58%) |
| 50 Gy/10 fr | 10 (32%) | 10 (38%) |
| 60 Gy/10 fr | 1 (3%) | 1 (4%) |
| Tumour max diameter (median) | 18 mm (IQR: 13–25 mm) | 24 mm (IQR: 14–30 mm) |
| RT techniques, n (%) | ||
| VMAT | 6 (19%) | 17 (65%) |
| Conformal arc | 25 (81%) | 9 (35%) |
| Aetiology, n (%) | ||
| B-HCC | 6 (19%) | 5 (19%) |
| C-HCC | 13 (42%) | 13 (50%) |
| nBnC | 5 (16%) | 5 (19%) |
| Metastasis | 7 (23%) | 3 (12%) |
| Lesion, n (%) | ||
| 1 | 28 (90%) | 26 (100%) |
| 2 | 3 (10%) | 0 |
| Post-treatment n (%) | ||
| Surgery | 9 (29%) | 6 (23%) |
| RFA | 23 (74%) | 23 (88%) |
| TACE | 14 (45%) | 15 (58%) |
| Child–Pugh score, n (%) | ||
| A5-6 | 29 (90%) | 25 (96%) |
| B7 | 2 (10%) | 0 |
| >B8 | 0 | 1 (4%) |
- KPS, Karnofsky Performance Score; RFA, radiofrequency ablation; RT, radiotherapy; TACE, transarterial chemoembolization; VMAT, volumetric arc radiotherapy.
Breath-hold irradiation was notably more prevalent with VMAT (65% vs. 19%, P = 0.01). No significant differences were observed between the two groups, except for variations in irradiation techniques. The characteristics of target lesions and previous treatment histories were comparable across both groups. Most of the patients had a Child–Pugh score of A5-6 before treatment, and their liver function was preserved.
Survival outcome
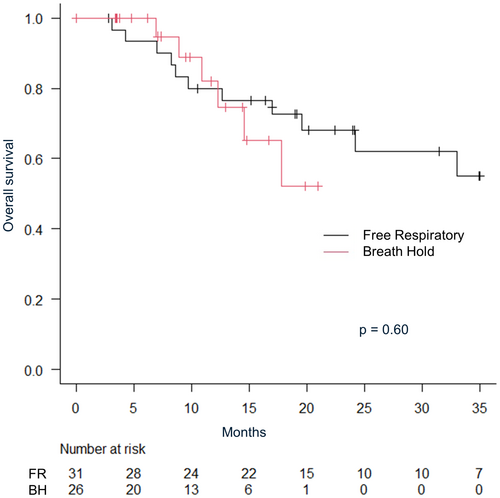
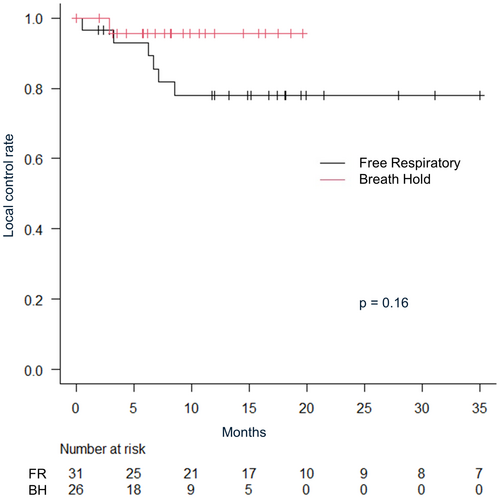
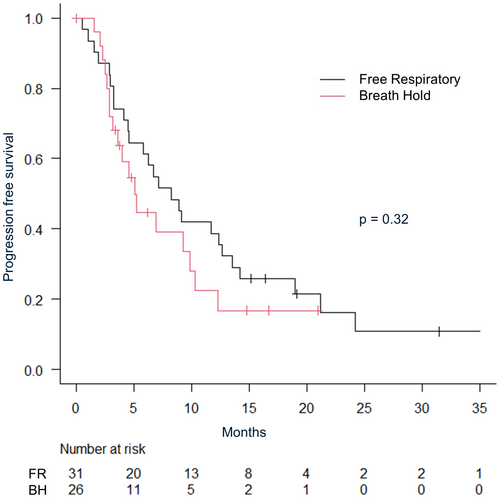
The median follow-up period for all patients was 16.4 months (IQR: 7.36–20.9). The median follow-up was shorter in the breath-hold irradiation group (breath-hold 9.7 months vs. free-respiratory 22.4 months). The 2-year overall survival rate was 64.4% (95% CI: 47.4–77.2), and local control rate was 84.3% (95% CI: 69.7–92.3) for all patients. The 1-year overall survival was 80.0% (95% CI: 60.8–90.5) versus 82.0% (95% CI: 53.5–93.9) in free-respiratory group versus breath-hold group, respectively (P = 0.60) (Fig. 2). Multivariate analysis revealed no significant differences in age, gender, Karnofsky Performance Status (KPS), tumour size, irradiation method or a number of irradiated sites. The 1-year local control rate were 78.1% (95% CI: 57.5–89.5) in free-respiratory group and 95.7% (95% CI: 72.9–99.4) in breath-hold group, respectively (P = 0.16) (Fig. 3). The 1-year progression-free survival were 38.7% in free-respiratory group (95% CI: 24.9–61.7) and 22.4% in breath-hold group (95% CI: 20.4–70.4), respectively (P = 0.32) (Fig. 4).
We administered BED10 75 Gy to 35 patients and BED10 105.6 Gy to 20 patients. The 2-year local control rate was 91.7% (95% CI: 70.6–97.8) for 48 Gy in the 4 fractions group and 75.4% (95% CI: 46.3–90.1) for 50 Gy in the 10 fractions group (P = 0.06). In terms of 2-year overall survival, the rate was 72.5% in the 48 Gy group (95% CI: 50.2–86.0) and 54.6% in the 50 Gy group (95% CI: 26.8–75.7, P = 0.29).
Toxicity
RILD, defined by an escalation of ≥2 in Child–Pugh score, was observed in four patients within each group (FR 13% vs. BH 15%) (Table 2). Among these patients, six patients returned to baseline after treatment. Two patients in the breath-hold group sustained elevated Child–Pugh scores. One patient developed ascites due to disease progression, and one patient had elevation of Child–Pugh score 5–7, which did not resolve. There were no gastrointestinal adverse events of Grade 3 or higher. Radiation-induced pneumonia was the only adverse event of Grade 3, which was observed in the BH group. Grade 2 bile duct stricture was observed in a case of FR irradiation, where the patient underwent SBRT subsequent to transarterial chemoembolization (TACE).
| Free respiratory | Breath hold | |
|---|---|---|
| GI ≧ Grade 3 | 0 | 0 |
| CP score increase of ≧2 | 4 (13%) | 4 (15%) |
| AST, ALT elevations (G2) | 0 | 1 (4%) |
| Liver dysfunction | 0 | 0 |
| Cholangitis G2 | 1 (3%) | 0 |
| Any AE ≧G2 | 3 (10%) | 1 (4%) |
| AE any ≧G3 | 0 | 1 (4%) |
- AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CP, Child–Pugh score; G2, grade 2; GI, gastrointestinal.
Dosimetry analysis
Dose and volume at treatment planning were assessed. Cases utilizing BH techniques had larger GTV volumes but smaller PTV volume (Table 3). The average dose to the normal liver was lower in BH, and the spared liver volume was less than 700 cc in three cases in FR and one case in BH. Spared liver is defined as below: normal liver volume with a dose lower than 21 Gy in four fractions or 32 Gy in 10 fractions. The maximum dose of the stomach, small intestine or oesophagus exceeded 43.5 Gy in 4 cases each.
| FR | BH | P value | |
|---|---|---|---|
| GTV median (cc) | 8.1 | 10.3 | 0.35 |
| Interquartile range | 4.7–17.4 | 4.9–23.4 | |
| PTV median (cc) | 40 | 31.3 | 0.91 |
| Interquartile range | 31.6–61.4 | 21.7–56.4 | |
| Liver mean dose (Gy) | 12.0 | 10.0 | 0.46 |
| >15 Gy (n) | n = 5 (16.1%) | n = 4 (15.4%) | |
| Liver-CTV median (cc) | 1063 | 1055 | 0.89 |
| Mean dose (Gy) | 11.7 | 9.7 | 0.42 |
| Spared liver < 700 cc | n = 3 (9.6%) | n = 1 (3.8%) | |
| GI max dose > 43.5 Gy (n) | n = 4 (12.9%) | n = 4 (15.4%) |
- GI, gastrointestinal; GTV, gross target volume; PTV, planning target volume.
Discussion
We compared retrospective cases of liver stereotactic radiation performed at our own institution. Breath-hold is routinely used as a control for respiratory motions, and we compared the clinical implications with and without breath-hold. The 2-year overall survival rate and local control rate were 64.4% (95% CI: 47.4–77.2) and 84.3% (95% CI: 69.7–92.3), respectively. The result was similar to previous reports, although overall survival varied depending on patient background and study design. Nalee Kim et al. conducted a cohort study involving 496 patients across seven hospitals, investigating SBRT for HCC. They reported 3-year cumulative local recurrence rates and 2-year cumulative mortality rates in the SBRT groups as 21.2% and 25.7%, respectively.18 Jun Won Kim et al. presented findings from a phase I/II study using helical IMRT-based SBRT for HCC, with dose escalation ranging from 36 Gy to 60 Gy. Patients were immobilized using an abdominal compression device. Their results indicated 1- and 2-year local control rates of 90.6% and 80.9%, respectively, with a significant difference observed in the local control rate between different dose levels.19
Although there was no statistically significant difference, the higher local control rate observed with the breath-hold method holds substantial clinical importance. The breath-hold technique may enhance treatment accuracy and improve tumour control, making it a valuable approach in clinical practice. Due to the small sample size in the study, statistical significance could not be confirmed. However, the observed higher local control rate remains clinically relevant and could influence treatment strategies. The findings of this study provide a foundation for larger-scale studies to further validate the safety and efficacy of the breath-hold technique. With advancements in radiotherapy technology, continued refinement of respiratory control could lead to improved long-term survival and reduced toxicity risks. The potential benefit of dose escalation in HCC has been inferred from published prospective and retrospective studies, although the optimal dose–response relationship has not been clearly defined in the available literature.20, 21 Several studies suggest that dose escalation (minimum BED10 65 to 79 Gy) improves local control. Nevertheless, concerns have been raised regarding the efficacy of doses exceeding BED10 of 100 Gy.22 Furthermore, a relationship between LC and tumour size has been shown. The LC decreases with increasing tumour size, suggesting that more intense dose escalation may be necessary when tumour size exceeds 3–5 cm.23 We treated BED10 75 Gy in 35 patients and BED10 105.6 Gy in 22 patients. The 2-year local control rate and the 2-year overall survival were both relatively higher in BED10 105.6 Gy group compared to the BED10 75 Gy group. This retrospective study had a bias of patient baseline characteristics. Specifically, the patients receiving 50 Gy in 10 fractions relatively had larger tumours or were difficult to treat due to the proximity of the OARs. Further studies are warranted to ascertain whether 50 Gy in 10 fractions is an inadequate dose for curative treatment. Increasing the tumour dose should take into account the risk of liver failure in cirrhosis, which is often irreversible and potentially fatal, as demonstrated in phase I/II trials.24 While careful observation is essential to monitor potential side effects of dose escalation, we aim to explore the feasibility of using breath-hold irradiation to facilitate dose escalation while ensuring adherence to dose constraints.
In this study based on practical clinical data, tumour diameters tended to be larger with breath-hold, although this disparity did not reach statistical significance. Furthermore, there was no significant difference in OAR dose between the two groups. This observation might be attributed to the fact that meeting dose constraints was potentially easier with breath-holding. Alternatively, it could be attributed to advancements in irradiation techniques, given that many cases treated with breath-holding were more recent.
Patients treated with or without breath-hold did not show a significant difference in adverse events, with only one case of Grade 3 pneumonitis reported in breath-hold group. No severe adverse events related to liver or gastrointestinal tract disorders were observed. The rate of adverse events was similar to that reported in previous years. We have experienced one case of bile duct injury, which was irradiation after TACE. Biliary complications associated with a hypofractionated RT were reported few, even in the perihilar region.25 TACE-related bile duct injury is often observed, the case we encountered might also be related to TACE.26
A limitation of our study is heterogeneity in patient background and the relatively short observation period. Cases with concurrent irradiation of IVC tumour plugs were included, which could introduce variability in treatment outcomes. Furthermore, it is important to acknowledge that most of the references utilized for establishing dose constraints are derived from retrospective analyses or small-scale prospective trials. These factors underscore the necessity for further research, potentially involving larger prospective cohorts, to validate our findings and establish more robust treatment guidelines.
In conclusion, this study did not demonstrate the efficacy of breath-hold, but it may have enabled the treatment of large tumours. Future prospective studies are warranted to examine the dose reduction of OAR with breath-holding and the dose increase to subjects using breath-hold.
Open Research
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




