Hip dysplasia hiding in plain sight: A retrospective analysis of radiology reports
RM Woodward: BSc, BHB, MBChB, FRANZCR; NJ Lightfoot MBChB, FANZCA; RM Vesey BSc; SA van Dijck BHB, MBChB, FRACS; JT Munro PhD, MBChB, FRACS; MJ Boyle BSc, MBChB, FRACS.
Abstract
Introduction
Timely recognition of dysplastic hip morphology is critical to facilitate appropriate management before significant joint damage has developed. It is likely that radiologist under reporting contributes to delays in diagnosis. This study aimed to assess how often adult hip dysplasia goes undetected in radiological reports and to identify clinical and radiological variables that impact the likelihood of detection of dysplasia by radiologists.
Methods
Referral details and radiology reports of patients who underwent periacetabular osteotomy by a single surgeon for symptomatic hip dysplasia between 1 January 2016 and 30 June 2020 were reviewed. Four assessors measured the lateral centre edge angle from the pelvic radiograph performed at time of referral. Film quality and other radiographic parameters were also assessed.
Results
Sixty-eight patients were included, 84% were female and the median age was 28.1 years. Dysplasia was not documented in the radiology report in 49% of cases. Dysplasia was more likely to be reported with no history of injury, an aspherical femoral head, lower lateral centre edge angle, higher acetabular index, increased femoral head shaft angle, higher femoro-epiphyseal acetabular roof index, or if there was disruption of Shenton's line, with the first three variables being independent predictors of radiologist detection.
Conclusion
Hip dysplasia should be considered in all adolescents/young adults presenting with hip pain. Causes of radiologist under reporting are likely multifactorial. Clinical information can cause cognitive biases and result in selective looking. A systematic approach to pelvis radiographs should include assessment of acetabular coverage and active search for evidence of femoral head migration.
Introduction
Dysplasia predisposes the hip to acetabular rim overload, dynamic instability, pain and premature development of osteoarthritis (OA).1, 2 Acetabular reorientation surgery is the mainstay of dysplasia management in skeletally mature patients.1, 3 Periacetabular osteotomy (PAO) alters the natural history of hip dysplasia, slowing hip degeneration, however, outcomes are better if surgery is performed early, before irreversible cartilage damage has developed.4 Once patients have high-grade chondral damage over the load-bearing surface of the acetabulum, they may no longer be considered suitable candidates for PAO.3 Unfortunately, often multiple healthcare providers are seen before diagnosis5 and frequently the duration of symptoms is several years before treatment.6
Mild hip dysplasia is not uncommon in the general population2, 7 with reported prevalence varying depending on the radiographic criteria applied and method of measurement.2, 8 The primary feature of hip dysplasia is a three-dimensional deficiency of the acetabulum which has been traditionally characterized by the lateral centre edge angle (LCEA).9 However, dysplastic hip morphology is complex, involving the femur as well as the acetabulum, and cannot be characterized by the LCEA alone.10, 11 Multiple imaging parameters should be assessed, in combination with evaluation of symptoms and clinical findings, when considering a clinical diagnosis of symptomatic hip dysplasia.
It is likely that radiologist under reporting contributes to delays in diagnosis,7 yet there is no research assessing the reasons for radiologist under reporting. The purpose of this study was to assess how often adult hip dysplasia goes undetected in radiological reports and to identify clinical and radiological variables that impact the likelihood of radiologists detecting adult hip dysplasia.
Methods
This study was a retrospective cohort analysis evaluating patients with symptomatic hip dysplasia who underwent unilateral PAO performed by a single surgeon (MJB) between the 1 January 2016 and the 30 June 2020. After obtaining an institutional ethics exemption, the operating surgeon's patient database, referral details and radiology reports were reviewed. Patients were separated into two groups—those where dysplasia was mentioned in the radiology report (‘reported’ group) and those where the radiology report made no mention of hip dysplasia or undercoverage (the ‘unreported’ group). Patients who had prior diagnosis of developmental dysplasia, history of surgical procedures on their ipsilateral hip or incomplete radiology reports were excluded.
Baseline demographics and clinical details were collected including patient age, gender, body mass index, place of residence, side of hip affected, history of injury, referrer specialty and place of surgery. The time from the referral radiograph to clinical review and diagnosis of hip dysplasia by the operating surgeon and the time from referral to PAO were also measured. The referral radiograph reports were reviewed and identification of hip dysplasia or under coverage and documentation of LCEA noted. The adequacy of the radiographs was assessed by evaluating rotation of the film and degree of pelvic tilt, with >1 cm rotation and >5 cm of posterior tilt deemed excessive respectively.
Radiographs of the hip and pelvis were reviewed by three experienced orthopaedic surgeons (MJB, SvD and JTM) and a musculoskeletal radiologist (RW) to assess the LCEA; all assessors measured the angle between a line perpendicular to the transverse axis of the pelvis and a line drawn from the femoral head centre (determined by a best fit circle) to the lateral edge of the weight-bearing surface (sourcil). The radiologist measured additional radiographic parameters which included femoral head sphericity, posterior wall sign, Shenton's line continuity, lateralization of the femoral head as well as measurement of the acetabular index (AI), the femoral neck-shaft angle and the femoro-epiphyseal acetabular roof index, see Appendix I. Assessors were blinded to the clinical details associated with a particular patient's film as well as others' radiological measurements.
Analyses were performed with SPSS Version 27 (IBM Corporation, Armonk, NY, USA) and NCSS Version 21.0.3 (NCSS LLC, Kaysville, UT, USA). All statistical tests were two-tailed with the level of significance to reject the null hypothesis set at P ≤ 0.05. Statistical comparisons between groups were made with the Mann–Whitney U-test for continuous variables and the Fisher exact test or Chi-square test with an appropriate continuity correction for binomial variables. Continuous parameters were reported as median (interquartile range, IQR) and discrete or binomial parameters as number (per cent) due to the anticipated sample size. Multivariate regression models were generated using logistic regression when appropriate to determine predictors of radiologist detection and reference LCEA. Correlation between the LCEA and the AI, femoral neck-shaft angle and the femoro-epiphyseal acetabular roof index was performed using the Spearman rho. Interobserver agreement of the LCEA was assessed using the interclass correlation coefficient (ICC, 3, 1 model), with the operating surgeon's measurements used as the reference LCEA to compare against other assessors as well as the radiologist report measures. ICC measures were reported as an average (95% confidence interval) and were interpreted as follows: values <0.5 were considered poor agreement, between 0.5 and 0.75 for moderate agreement, between 0.75 and 0.9 for good agreement and values >0.9 were considered excellent agreement.12
Results
Of the 68 patients included in this study, 57 (84%) were females and the median age was 28.1 (IQR: 20.7–36.0) years. The radiology report made mention of hip dysplasia or undercoverage in 35 (51%) patients (‘reported’ group), while no mention of dysplasia was made in the remaining 33 (49%) patients (‘unreported’ group). There was no difference between the groups in terms of sex, age, body mass index, side of surgery, place of residence, radiograph adequacy as assessed by rotation or pelvic tilt, specialty of the referring practitioner, time from referral to surgery or place of surgery (P > 0.05 for all), see Table 1. Those in the ‘reported’ group were more likely to have the LCEA value included in their radiology report compared with the ‘unreported’ group (26 vs. 3%, P = 0.02). Additionally, the ‘reported’ group were less likely to have a history of injury to the hip (31 vs. 63%, P = 0.01) and underwent PAO less rapidly following their diagnosis of hip dysplasia (293 vs. 189 days, P = 0.02).
| Overall cohort (n = 68) | Reported (n = 35) | Unreported (n = 33) | P-value | |
|---|---|---|---|---|
| Female gender | 57 (84) | 29 (83) | 28 (85) | 1.00 |
| Age at surgery (years) | 28.1 (20.7–36.0) | 24.5 (20.2–32.7) | 30.3 (23.8–37.6) | 0.10 |
| Body mass index (kg/m2) | 24.6 (22.2–27.6) | 24.5 (23.3–27.9) | 24.8 (21.6–26.9) | 0.35 |
| Left side | 26 (38) | 14 (40) | 12 (36) | 0.81 |
| History of injury | 32 (47) | 11 (31) | 21 (64) | 0.008 |
| Residence outside Auckland | 27 (40) | 17 (49) | 10 (30) | 0.14 |
| LCEA included in report | 10 (15) | 9 (26) | 1 (3) | 0.007 |
| Excessive film rotation | 5 (7) | 4 (11) | 1 (3) | 0.36 |
| Excessive pelvic tilt | 21 (31) | 9 (26) | 12 (36) | 0.43 |
| Time from dysplasia diagnosis to surgery (days) | 240 (150–368) | 293 (170–483) | 189 (119–260) | 0.021 |
| Referrer | ||||
| Orthopaedic surgeon | 42 (62) | 22 (63) | 20 (61) | 0.48 |
| Sports physician | 13 (19) | 7 (20) | 6 (18) | |
| General practitioner | 10 (15) | 6 (17) | 4 (12) | |
| Physiotherapist | 2 (3) | 0 (0) | 2 (6) | |
| Self-referral | 1 (1) | 0 (0) | 1 (3) | |
| Time from referral to surgery (days) | 185 (118–282) | 234 (104–311) | 162 (118–242) | 0.27 |
| Surgery at private hospital | 42 (62) | 18 (51) | 24 (73) | 0.07 |
- Data represents number (per cent) or median (interquartile range) as appropriate. Two-tailed P < 0.05 defines statistical significance.
- LCEA, lateral centre edge angle. Bold indicates significance level at P < 0.05.
Both the operating surgeon and radiologist reviewed all 68 radiographs. The other orthopaedic surgeons reviewed a subset (72% and 52%). Considering the LCEA values reported by each assessor, overall, the average ICC measure 0.933 (P < 0.001) indicates excellent reliability, see Table 2. The average ICC measures between the reference LCEA and each of the remaining assessors was between 0.831 and 0.949, ranging from good to excellent. Only 10 (15%) radiology reports documented the LCEA, their agreement with the reference LCEA was poor (ICC 0.435, P = 0.18).
| ICC | 95% confidence interval | P-value | |
|---|---|---|---|
| Reference LCEA vs. referral radiologists | 0.435 | −0.992–0.846 | 0.18 |
| Reference LCEA vs. MSK radiologist | 0.949 | 0.915–0.969 | <0.001 |
| Reference LCEA vs. Paeds Ortho surgeon | 0.927 | 0.870–0.959 | <0.001 |
| Reference LCEA vs. arthroplasty surgeon | 0.831 | 0.662–0.915 | <0.001 |
- Two-tailed P < 0.05 defines statistical significance. Bold indicates significance level at P < 0.05
- ICC, Interclass correlation coefficient (3, 1 model); LCEA, Lateral centre edge angle.
There were significant negative relationships between the reference LCEA and the AI and the femoro-epiphyseal acetabular roof index (ρ = −0.73 and −0.49, respectively; P < 0.001 for both). No relationship was found between the reference LCEA and the femoral neck-shaft angle between the groups (ρ = −0.09, P = 0.49). On linear regression, AI and a break in Shenton's line were the only predictive factors of reference LCEA (coefficient − 0.56 [odds ratio −0.72 to −0.39], P < 0.001 and −2.72 [odds ratio −5.20 to −0.23], P = 0.03, respectively).
Overall, 64 (94%) patients were classed as dysplastic, while the remaining 4 (6%) were borderline dysplastic when using the reference LCEA and cut-off values of <20° and 20–25°, respectively. Interestingly, in the ‘unreported’ group, 25% had a reference LCEA of <15° and 36% had an AI >15. Nevertheless, those in the ‘reported’ group had a lower reference LCEA than those in the ‘unreported’ group (14° vs. 18°, P < 0.001), this relationship also held true for all assessors, see Table 3. Additionally, the ‘reported’ group had a higher AI (17.2° vs. 13.6°, P = 0.013), femoral neck-shaft angle (139.6 vs. 135.9, P = 0.006) and femoro-epiphyseal acetabular roof index. The ‘reported’ group were also more likely to have an aspherical femoral head (66 vs. 36%, P = 0.007) and display a break in continuity in Shenton's line (29 vs. 6%, P = 0.015). There was no difference in posterior wall sign or lateral migration of the femur (P = 0.08 and 0.11 respectively). On multivariate analysis, aspherical femoral head, a lower reference LCEA and no history of injury were independent predictors of radiologist detection (P = 0.039, P = 0.041 and P = 0.022 respectively), see Table 4.
| Overall cohort (n = 68) | Reported (n = 35) | Unreported (n = 33) | P-value | |
|---|---|---|---|---|
| LCEA (degrees) | ||||
| Reference surgeon (n = 68) | 16.0 (14.0–18.0) | 14.0 (8.0–17.0) | 18.0 (15.0–19.0) | <0.001 |
| MSK radiologist (n = 68) | 14.9 (11.3–18.3) | 12.0 (7.6–16.0) | 17.7 (14.8–18.9) | <0.001 |
| Paeds Ortho surgeon (n = 49) | 16.5 (13.0–20.0) | 14.0 (6.7–17.5) | 18.5 (14.5–22.0) | 0.007 |
| Arthroplasty surgeon (n = 35) | 17.5 (14.5–21.3) | 15.0 (10.6–19.1) | 17.7 (14.8–18.9) | 0.01 |
| Referral radiologists (n = 10) | 17.0 (15.0–20.0) | 16.0 (15.0–20.0) | 23.0 (23.0–23.0) | 0.40 |
| Ancillary signs | ||||
| Acetabular index (degrees) | 15.7 (11.0–18.9) | 17.2 (11.7–21.7) | 13.6 (10.5–16.2) | 0.013 |
| Neck-shaft angle (degrees) | 138.4 (134.2–142.4) | 139.6 (136.0–143.3) | 135.9 (132.9–139.4) | 0.006 |
| FEAR index (degrees) | 1.8 (−0.5 to 8.1) | 6.3 (1.4–12.0) | 0.2 (−4.3 to 5.5) | 0.001 |
| Aspherical femoral head | 35 (52) | 23 (66) | 12 (36) | 0.007 |
| Posterior wall sign | 41 (60) | 23 (66) | 18 (55) | 0.08 |
| Break in Shenton's line | 12 (18) | 10 (29) | 2 (6) | 0.015 |
| Lateral migration of the femur | 19 (28) | 13 (37) | 6 (18) | 0.11 |
- Data represents number (per cent) or median (interquartile range) as appropriate. Two-tailed P < 0.05 defines statistical significance.
- FEAR, Femoro-Epiphyseal Acetabular Roof; LCEA, Lateral centre edge angle; MSK, musculoskeletal; Ortho, orthopaedic; Paeds, paediatric. Bold indicates significance level at P < 0.05.
| Univariate odds ratio | Final model odds ratio | P-value | |
|---|---|---|---|
| History of injury | 3.82 (1.49–10.44) | 0.270 (0.077–0.950) | 0.041 |
| Reference LCEA | 0.78 (0.67–0.92) | 0.824 (0.698–0.972) | 0.022 |
| Acetabular index | 1.13 (1.03–1.25) | – | – |
| Neck-shaft angle | 1.12 (1.03–1.23) | – | – |
| FEAR index | 1.13 (1.05–1.23) | – | – |
| Aspherical femoral head | 4.03 (1.44–11.24) | 3.79 (1.07–13.41) | 0.039 |
| Posterior wall sign | 1.41 (0.84–2.37) | – | – |
| Break in Shenton's line | 5.80 (1.16–29.01) | – | – |
| Lateral migration of the femur | 2.46 (0.80–7.58) | – | – |
- R2 0.208, Hosmer and Lemeshow goodness of fit test P = 0.27. Two-tailed P < 0.05 defines statistical significance. Bold indicates significance level at P < 0.05.
Discussion
In this group with symptomatic dysplasia, the referral radiograph report failed to document dysplastic morphology in about half of the cases. In contrast, the operating surgeon classified >90% with dysplasia and the remainder with borderline dysplasia. Although the dysplasia was more severe in the ‘reported’ group and there is ongoing discussion about the appropriate cut-off values for LCEA, this cannot account for the failure to report dysplasia. A quarter of those in the ‘unreported’ group had an LCEA <15 which is universally agreed to be dysplastic.9, 13-15 The LCEA was infrequently included in reports and the agreement between these values and the operating surgeons was poor, while assessors' interobserver reliability was excellent overall and is comparable with that reported in the literature.16, 17
There are many potential pitfalls in the measurement of the LCEA. Errors in measurement such as inclusion of the lateral bony margin in the LCEA calculation and failure to correct for pelvic orientation contributed to the under reporting of dysplasia in our cohort, see Figure 1. LCEA values vary widely depending on whether the angle is measured to the lateral acetabular margin or the lateral edge of the weight-bearing surface (sourcil)18, 19; in dysplasia, it is the weight-bearing surface that is relevant.9 The landmark to use for the lateral margin of the sourcil can be difficult to identify reliably when there is a cranial acetabular retroversion and a crossover sign. Anderson et al.20 suggest that the appropriate landmark is where the sourcil meets the posterior wall rather than the more laterally positioned anterior wall. Use of landmarks such as the inter teardrop line are necessary to establish the transverse axis of the pelvis.10 Other considerations include accurate identification of the femoral head centre as even a slight shift in the estimated centre can alter the LCEA, in one study a shift of 3 mm resulted in a difference of six degrees.20 Matching a best fit circle with the superior weight-bearing surface of the femoral head contained by the acetabulum has been suggested to improve calculation of LCEA.20
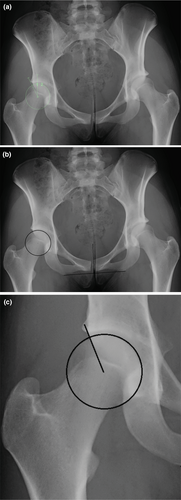
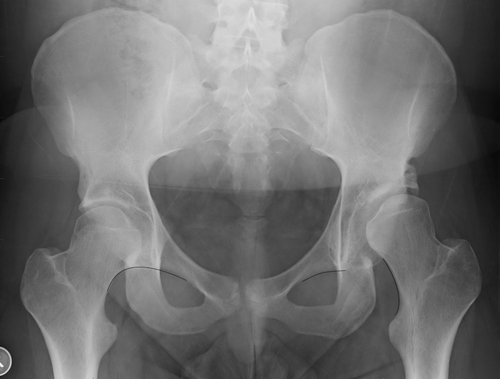
While LCEA is the primary parameter used to define dysplasia, there are multiple indicators of dysplastic change. This is demonstrated by our findings that all radiological variables assessed, except for the posterior wall sign and lateral migration of the femur, were more severe in the reported group. Many of these parameters are interrelated.11 Although a broken Shenton's line was closely related to a lower reference LCEA, two of the 12 cases with this feature were in mild/borderline dysplasia which was not reported by the radiologist, see Figure 2. In mild/borderline cases, where determining the appropriate management is challenging, it is particularly important that the radiologist documents any findings suggesting instability. A broken Shenton's line is a predictor of a poor outcome after isolated hip arthroscopy21, 22 but was not an independent predictor of radiologist detection of dysplasia in our study. The only independent predictors of group allocation were the presence of a lower reference LCEA, an aspherical femoral head and a history of injury.
A large proportion of our cohort presented with a history of injury while other series reported the presentation being more typically insidious in onset.5 This discrepancy is likely accounted for by the presence of a universal insurance scheme in New Zealand, the Accident Compensation Corporation (ACC), which covers the cost of imaging and treatment of posttraumatic injuries.23 In our study, history of injury was associated with a decreased likelihood of the dysplasia being reported (31% compared to 64% of the unreported cases). Cognitive biases have been shown to influence the interpretation of information24, 25 and may explain this association. The framing effect is a recognized cognitive bias which leads to a different conclusion depending on the clinical information provided.24 Although the accuracy of image interpretation is generally improved by clinical history,26 in this case, the history of injury may have resulted in selective looking25 for a bony injury and inattention blindness27 leading to a failure to identify the dysplasia. Even radiologists, despite their expertize evaluating images, can experience inattention blindness.28 The dysplasia was so severe in some cases it is hard to imagine it being overlooked, however, it is possible it was omitted from the report because it was not deemed relevant in the context of trauma. Additionally, there may be reluctance to jeopardize the patient's ACC claim as pre-existing conditions can result in denial of cover, even if asymptomatic.
In this study, the mean time from radiograph to surgery was almost 1 year. Most referrals were from other medical specialists who were unlikely to be the primary health care provider for the individual suggesting a longer duration of symptoms. These findings are in keeping with reports of prolonged time between onset of symptoms and diagnosis of dysplasia in skeletally mature individuals described in the literature.5, 6 There was no significant difference in the interval between the radiograph being performed and referral to a hip surgeon depending on whether the dysplasia was reported. A possible explanation for this is that in our cohort, many cases were referred by other orthopaedic surgeons who would routinely review the radiographs themselves. Assessment of the radiographs by the operating surgeon would also account for the lack of any delay between the referral radiograph and surgery in the not reported group. Surprisingly, the ‘not reported’ group had a shorter interval between diagnosis of dysplasia and surgery. This may reflect a tendency for patients to have surgery in private hospitals, where wait times are shorter, when they are covered by ACC (due to a history of trauma).
This study has several limitations. The assessors were aware that this was a cohort who had undergone PAO, introducing biases in the assessment of radiographic parameters. This potentially led to overcalling of dysplastic morphology; however, the interrater reliability was moderate to excellent and demographics of our cohort are very similar to other PAO series.4, 6 Additionally, the identification of symptomatic dysplasia in this cohort is based on the surgeon's clinical diagnosis. It is acknowledged that it can be challenging to determine whether symptoms are primarily caused by instability, particularly in mild/borderline dysplasia.3, 13, 22 Nevertheless, the radiographs are a starting point where any features that could contribute to instability should be documented.
In conclusion, in this cohort with symptomatic dysplasia, the dysplasia was not reported in almost half the cases. The cause for this is likely multifactorial. The strong association between a history of trauma and failure to report dysplasia in our study is an important reminder that clinical information can cause cognitive biases reinforcing the importance of a systematic approach to reporting. In this cohort, the only radiological features predicting detection of dysplasia by radiologists were a lower LCEA and the presence of an aspherical femoral head. Hip dysplasia should be considered in all adolescents/young adults presenting with hip pain and assessment of pelvis radiographs should include evaluation of acetabular coverage and other parameters of dysplasia including an active search for any malalignment.
Conflict of interest
The authors have no conflict of interest to declare.
Acknowledgement
Open access publishing facilitated by The University of Auckland, as part of the Wiley - The University of Auckland agreement via the Council of Australian University Librarians.
Appendix I
Methods for radiographs measurements
| Feature | Description | Reference/source |
|---|---|---|
| Shenton's line | Interrupted if the inferior femoral head neck contour and superior border of the obturator foramen do not form a smooth arc | Mascarenhas et al. (2019). Imaging Methodology for Hip Preservation: Techniques, Parameters, and Thresholds |
| Posterior wall sign | On best fit circle femoral head centre FHC lies lateral to the posterior wall | Reynolds et al. (1999). Retroversion of the acetabulum: a cause of hip pain |
| LCEA | Vertical line through the FHC perpendicular to inter tear drop line and a line drawn from FHC to the lateral edge of the weight-bearing surface (sourcil) | Tibor et al. (2013). Two or more impingement and/or instability deformities are often present in patients with hip pain |
| Acetabular index | Angle formed by a transverse axis of pelvis and a line through the medial and lateral edge of the acetabular roof | Mascarenhas et al. (2019) Imaging Methodology for Hip Preservation: Techniques, Parameters, and Thresholds |
| The Femoro-Epiphyseal Acetabular Roof (FEAR) index | Angle between line from the most lateral to the most medial point of the straight central third of the femoral head physeal scar and a line is rom the most medial and lateral points of the sourcil | Wyatt et al. (2017). The Femoro-Epiphyseal Acetabular Roof (FEAR) index: a new measurement associated with instability in borderline hip dysplasia? |
| Femoral neck-shaft angle | Angle formed by a line extending through centre of femoral head along axis of femoral neck with an intersecting line drawn along axis of femoral shaft | Beltran et al. (2013). Imaging evaluation of developmental hip dysplasia in the young adult |
Open Research
Data availability statement
Research data are not shared.




