Epidemiological Survey of Canine Distemper Virus Infection: Exploring the Link Between Virus Spread and Invasive Raccoon (Procyon lotor) Population Growth in Hokkaido, Japan
ABSTRACT
Invasive raccoons (Procyon lotor) naturalized in Hokkaido, Japan, potentially spreading infectious diseases. Canine distemper virus infection is a serious epizootic disease, for which the raccoon is one of the hosts. We investigated the virus's prevalence in Hokkaido's wild raccoons, using 611 serum samples collected from captured raccoons in 2007–2012, 2021, and 2022. Higher seropositivity rates were confirmed in 2007 (32.7%), 2021 (46.4%), and 2022 (46.8%) than in 2008–2012 (0.00%–6.06%), suggesting the occurrence of an epidemic in 2007, 2021, and 2022 and its disappearance in 2008–2012. However, the infection status has recently changed, with high seropositivity rates consecutively confirmed in 2021 and 2022. Logistic regression analysis was performed to investigate the relationships among the catch per unit effort (an index of animal population density), host and environmental factors, and antibody status. The catch per unit effort correlated with seropositivity in 2007. As for environmental factors, the forest area ratio had a weak influence on seroprevalence in 2007; however, the residential area ratio had a clear influence on seroprevalence in 2021 and 2022. The epidemic occurred in forested areas in 2007; nonetheless, recent raccoon population growth and habitat expansion may have caused widespread infections even around residential areas in 2021 and 2022. Continuous monitoring of the infection and reinforcement of raccoon control programs are necessary to avoid serious damage through disease transmission to sympatric native raccoon dog (Nyctereutes procyonoides) and fox (Vulpes vulpes) populations, as well as health consequences for domestic dogs (Canis familiaris).
1 Introduction
Invasive species have become a global concern due to their negative impact on local ecosystems and biodiversity. Additionally, these species can contribute to the spread of infectious diseases due to their high population density. As a result, numerous countermeasures have been implemented worldwide (Chinchio et al. 2020; Pyšek et al. 2020). In Japan, raccoons (Procyon lotor) are recognized as one of the most significant invasive species (Ikeda et al. 2004).
Raccoons are medium-sized mammals belonging to the order Carnivora. They are native to North America and were imported to Japan as pets until the 1990s. However, they are naturalized because of abandonment by owners or escape from facilities, and their population continues to grow in Japan (Ikeda et al. 2004; Hokkaido Government 2023). In Hokkaido, the annual number of captured raccoons was less than 5000 until 2010, while in 2020 and 2021, the number exceeded 25 000 for consecutive years (Hokkaido Government 2023). This invasive raccoon population growth can have serious direct and indirect effects on native species. Examples of direct impacts include competition with sympatric animals, such as raccoon dogs (Nyctereutes procyonoides) and foxes (Vulpes vulpes), and predation on rare native species, such as the Hokkaido salamander (Hynobius retardatus) and Japanese crayfish (Cambaroides japonicas) (Osaki et al. 2019; Oe et al. 2020). Examples of indirect effects include the transmission of infectious diseases to native species. Many studies on zoonotic diseases have been conducted because of public interest. To date, raccoons in Japan have been found to contract a variety of pathogens, including the Yezo virus (Kodama et al. 2021). Interest in epizootic diseases compared to zoonotic diseases has been limited, resulting in few detailed studies (Kameo et al. 2012; Aoki et al. 2017), largely due to their invisible and indirect impacts.
Canine distemper virus (CDV) infection is one of the most serious epizootic diseases for which raccoons are known hosts (Martinez-Gutierrez and Ruiz-Saenz 2016; Roelofs et al. 2023). CDV is a single-stranded RNA virus belonging to the genus Morbillivirus of the family Paramyxoviridae and has a wide range of hosts, primarily Carnivora. The clinical signs of CDV infection include fever and respiratory, gastrointestinal, and neurological symptoms (Appel and Summers 1995; Deem et al. 2000; Beineke, Baumgärtner, and Wohlsein 2015; Loots et al. 2017; Libbey and Fujinami 2023). The fatality rate of highly susceptible domestic dogs (Canis familiaris) is second only to that of rabies (Deem et al. 2000), which has been previously reported to be over 50% (Beineke et al. 2009; Mousafarkhani et al. 2023). An outbreak of CDV infection among highly susceptible wild animals can have a significant impact on local populations, such as lions (Panthera leo) in Tanzania (Roelke-Parker et al. 1996; Weckworth et al. 2020), island gray foxes (Urocyon littoralis catalinae) in Italy (Clifford et al. 2006; Timm et al. 2009), and Amur tigers (Panthera tigris altaica) living along the China–Russia border (Gilbert et al. 2015; Wang et al. 2023). Raccoons are considered the primary reservoir of CDV for other wild animals and domestic dogs in the United States (Roscoe 1993; Wostenberg et al. 2018; Taylor et al. 2021; Fitzgerald et al. 2022; Wilkes 2023). Raccoons generally prefer waterfronts but are highly adaptive to various environments owing to their omnivorous feeding habits (Ikeda 1999; Gehrt 2003). Given these characteristics, raccoons should be considered as potential transmitters of CDV epidemics in native species in a variety of environments.
In Japan, CDV infection has been documented as a significant cause of mortality in weasels (Mustela itatsi), masked palm civets (Paguma larvata), and badgers (Meles anakuma) (Machida et al. 1993; Yoshizawa and Watabe 2007; Kameo et al. 2012). Moreover, several raccoon dog deaths were reported in Kochi in 2006 (Yoshizawa and Watabe 2007) and Wakayama in 2007 (Kameo et al. 2012). High CDV seropositivity rates were also found in raccoons captured in Wakayama and Hyogo (Kameo et al. 2012). These previous reports suggest that CDV epidemics have already occurred in Japan and that monitoring CDV epizootics in wild animals is important to prevent their outbreak among susceptible wild native species and unvaccinated domestic dogs.
In Hokkaido, a decline in the raccoon dog population was confirmed between 2004 and 2013 near our study area, attributed to a scabies epidemic (Sashika et al. 2019). However, no studies on the CDV infection status have been conducted in Hokkaido yet, despite its potential impact on the local raccoon dog population. Therefore, we aimed to investigate the prevalence of CDV infections in Hokkaido wildlife in the past and present and to clarify the factors influencing the prevalence using raccoons as sentinel animals. First, we mainly investigated CDV antibody seroprevalence in raccoons in 2007 and 2008, when an epidemic of CDV infection was confirmed in the Japanese mainland (Kameo et al. 2012; Suzuki et al. 2015) and then compared the situation in 2021 and 2022. Furthermore, we investigated the distribution of CDV-seropositive raccoons for assessing CDV prevalence in wildlife. To clarify the factors influencing prevalence, the relationship between seroprevalence and three different factors (raccoon host factors, environmental factors, and an index of raccoon population density) was statistically analyzed. Additionally, RT-qPCR was performed to confirm viremia and viral shedding in wild raccoons.
2 Materials and Methods
2.1 Sample Collection
Raccoons are suitable sentinel animals for monitoring CDV infection among wild animals. Wild raccoon control programs in Japan facilitate obtaining samples from raccoons more easily than from other wild animals. Therefore, invasive raccoons were selected as the research target. We received raccoons that had been euthanized as part of the control program by the local Hokkaido government. Blood from euthanized animals was collected directly from the heart during necropsy, and the sera were separated. A total of 611 raccoon serum samples were obtained from wild raccoons captured in 2007–2012, 2021, and 2022. The 15 capture sites were divided into eight areas as follows: A: Tomakomai; B: Abira, Atsuma, and Mukawa; C: Naganuma, Yuni, and Chitose; D: Kitahiroshima; E: Iwamizawa, Bibai, and Kuriyama; F: Nanporo; G: Tobetsu and Tsukigata; and H: Shintotsukawa (Figure 1). All serum samples collected for the virus neutralizing (VN) test were heat-inactivated at 56°C for 30 mins and frozen at −20°C until use. Additionally, 67 serum samples and 36 rectal swabs were collected from raccoons captured in 2022 for RT-qPCR and frozen at −80°C until use. Table 1 and Figure 1 depict the number of samples in each area per year.
| Year | Area | VN test | RT-qPCR | |||||
|---|---|---|---|---|---|---|---|---|
| % | (N) | Serum | Rectal swab | |||||
| 2007 | A: Tomakomai | 21.1 | (19) | — | — | |||
| B: Abira, Atsuma, and Mukawa | 21.6 | (37) | — | — | ||||
| C: Naganuma, Yuni, and Chitose | 32.2 | (31) | — | — | ||||
| D: Kitahiroshima | 58.6 | (29) | — | — | ||||
| E: Iwamizawa and Kuriyama | 36.7 | (30) | — | — | ||||
| F: Nanporo | 10.0 | (10) | — | — | ||||
| Total | 32.7 | (156) | — | — | ||||
| 2008 | A: Tomakomai | 0.00 | (7) | — | — | |||
| B: Abira and Atsuma | 0.00 | (30) | — | — | ||||
| C: Naganuma, Yuni, and Chitose | 3.57 | (56) | — | — | ||||
| D: Kitahiroshima | 0.00 | (32) | — | — | ||||
| E: Bibai and Kuriyama | 5.36 | (56) | — | — | ||||
| G: Tobetsu and Tsukigata | 1.72 | (58) | — | — | ||||
| Total | 2.51 | (239) | — | — | ||||
| 2009 | D: Kitahiroshima | 3.13 | (32) | — | — | |||
| 2010 | D: Kitahiroshima | 3.23 | (31) | — | — | |||
| 2011 | G: Tobetsu | 6.06 | (33) | — | — | |||
| 2012 | G: Tobetsu | 0.00 | (30) | — | — | |||
| Total | 3.17 | (126) | — | — | ||||
| 2021 | F: Nanporo | 38.1 | (21) | — | — | |||
| G: Tobetsu | 100 | (3) | — | — | ||||
| H: Shintotsukawa | 50.0 | (4) | — | — | ||||
| Total | 46.4 | (28) | — | — | ||||
| 2022 | F: Nanporo | 42.9 | (28) | 0.00 | (17) | 0.00 | (18) | |
| G: Tobetsu | 54.2 | (24) | 0.00 | (40) | 0.00 | (8) | ||
| H: Shintotsukawa | 40.0 | (10) | 0.00 | (10) | 0.00 | (10) | ||
| Total | 46.8 | (62) | 0.00 | (67) | 0.00 | (36) | ||
- Note: All values represent percentages of positive samples. Numbers in parentheses indicate the number of samples. Different cities and towns were included in the study areas each year because the capture sites were selected based on raccoon traces and witness information every year, considering raccoon ecology and behavior, not on a municipal basis.
2.2 VN Test
As a first screening, the VN test for CDV KDK-1 (genotype Asia-1) was conducted by 75% plaque-reduction neutralization test (PRNT75) using the obtained serum samples and an established cell line, CRFK/cSLAM cells expressing canine SLAM, following the method of Nakano et al. (2009a). KDK-1 belongs to Asia-1, which was isolated in Japan in 2007.
CRFK/cSLAM cells were seeded in a 24-well plate (Nippon Genetics Co., Ltd., Tokyo, Japan) and cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco, Waltham, MA, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, MA, USA) and antibiotics at 37°C in 5% CO2 for 24 h. The viral solution was prepared to contain approximately 100 plaque-forming units (PFUs) of KDK-1 diluted with DMEM supplemented with 2% FBS. We added 35 µL of each serum sample to 315 µL of the viral solution to produce a 1:10 dilution and incubated at 37°C for 1 h. Next, 150 µL of the mixture was transferred to the cultured CRFK/cSLAM cells in each well of the 24-well plate. The plate was incubated at 37°C for 1 h, washed twice with FBS-free DMEM, and overlaid with DMEM containing 0.8% agarose and 10% FBS. The plate was then placed at room temperature (20−25°C) for 1 h, followed by incubation at 37°C in 5% CO2 for an additional 3–4 days. The cells were fixed with 5% buffered formaldehyde for 1 h, and the agarose layers were removed. Plaques were counted after staining with crystal violet. CDV-positive sera were evaluated by reducing the number of plaques by more than 75% compared with the mean number of plaques in the control wells.
As a second screening step, CDV-positive sera were used to determine VN titers. Sera were diluted 1:5 and serially diluted twofold in DMEM containing 2% FBS. Next, the diluted sera were mixed with equal volumes of a virus solution containing 100 PFU of KDK-1. Mixtures were incubated at 37°C for 1 h, added to CRFK/cSLAM, and PRNT75 was performed. VN titers were recorded as the highest dilution of serum samples that reduced plaques by more than 75% compared to the control wells without serum.
2.3 Age Group Classification
Captured raccoons were age-assessed using canine teeth or skulls and classified into three groups: juvenile (0 years), yearling (1 year), and adult (2 or more years). First, individuals with open root foramina were classified as 0 years old, whereas those with closed root foramina were deemed to be 1 year old or older (Junge and Hoffmeister 1980). For individuals from whom only canine teeth were collected, age groups were separated by counts of growth layer groups (GLGs) in the cementum following previously reported methods (Grau, Sanderson, and Rogers 1970; Isono et al. 2019). For individuals whose skulls could be collected, the ages of those with closed root foramina were determined using interfrontal and interpalatine sutures. If either suture could be identified, the individual was classified as 1 year old; if both sutures disappeared, the individual was categorized as 2 years old or older (Junge and Hoffmeister 1980; Kato et al. 2009).
2.4 RT-qPCR
Viral RNA was extracted from 67 serum samples and 36 rectal swabs collected from the raccoons captured in 2022. Total RNA was extracted from the serum samples using TRIzol LS (Thermo Fisher Scientific) according to the manufacturer's protocol. Viral RNA was extracted from rectal swabs using the MagMAX Viral/Pathogen II Nucleic Acid Isolation Kit (Applied Biosystems, Waltham, MA, USA) or NucleoSpin RNA Virus (TAKARA Bio Inc., Shiga, Japan), according to the manufacturer's instructions. A CDV standard was prepared from a viral solution containing approximately 104 PFU of KDK-1, and a log10 dilution series (104–10−1) was used to generate the standard curve. One-step RT-qPCR assays were conducted using primers and probes previously designated as CDV-Mix3 (Halecker et al. 2021), located in the highly conserved region of the P gene. The amounts of CDV RNAs were quantified using the One Step PrimeScript III RT-qPCR mix (TAKARA Bio Inc.) and (primer CDV4.1-F: 5ʹ-CTG TCR GTA ATC GAG RAT TCG A-3ʹ; primer CDV3-R: 5ʹ-GCC GAA AGA ATA TCC CCA GTT AG-3ʹ; and probe CD3.1-FAMas: FAM-ATC TTC GCC AGA RTC YTC AGT GCT-BHQ1) (Eurofins Scientific, Lancaster, PA, USA) on a qTOWER3 Real-time PCR Thermal Cycler (Analytik Jena AG, Jena, Germany) with the following thermal conditions: 52°C for 5 min, 95°C for 10 s, and 45 cycles of 95°C for 5 s and 56°C for 30 s, followed by measurement.
2.5 Statistical Analysis
Each year analyzed (2007–2012 and 2021–2022) was classified as having either high (>30%) or low (<10%) seroprevalence. Fisher's exact test was used to examine the differences in seroprevalence among raccoons over different years. Next, to estimate the factors associated with seroprevalence among raccoons, statistical analyses were conducted separately for the raccoon population density index, raccoon host factors, and environmental factors. First, as an index of raccoon population density, we calculated the catch per unit effort (CPUE = capture number × 100/number of traps × trap nights) for each 1 km × 1 km grid unit. Logistic regression analysis was performed using the CPUE as the explanatory variable and CDV antibody status (seropositive or seronegative) as the response variable. Areas H and G in 2021 and 2022 were excluded from this analysis because catch efficiency was extremely low in Area H, and farmers captured raccoons in Area G to prevent crop damage by raccoons, which is considered to affect the exact CPUE calculations. Next, Fisher's exact test was conducted using the sex and age of raccoons as host factors to test whether they were associated with CDV seroprevalence. For the environmental factors, four variables were used in the analysis: forest area ratio, agricultural area ratio, residential area ratio, and waterline length. Waterline data were obtained from Fundamental Geospatial Data 25000 (Ministry of Land, Infrastructure, Transport and Tourism, Japan), and other environmental variables were obtained from Report of Vegetation Survey on 6th and 7th National Basic Survey on Natural Environment (Ministry of the Environment, Japan). Each variable within a radius of 500 m from the raccoon capture point was extracted (Kuriyama et al. 2018; Okada et al. 2022) using the geographic information system software ArcGIS Pro 2.4.0 (Esri Japan Corporation, Tokyo, Japan). Buffer size was determined based on the reported raccoon home range size in Hokkaido (Kurashima and Niwase 1998; Ikeda et al. 2004; Abe, Ikeda, and Tatsuzawa 2006). The correlation coefficients were checked because the environmental factors in each buffer were considered to be correlated with each other. If the absolute value of the correlation coefficient was 0.7 or higher, these factors were considered strongly correlated, and either one was removed. Logistic multiple regression analysis was performed to test the association between CDV antibody status (seropositive or seronegative) and raccoon host factors with significant differences in the previous Fisher's exact test and environmental factors without highly correlated factors. The corrected Akaike information criterion (AICc) was used to evaluate the results of the logistic multiple regression analysis, considering the small sample size. If the AICc of the model with explanatory variables is lower than that of the null model, the prediction accuracy of the model is considered high (Burnham and Anderson 2004). The above statistical analyses were performed using Python 3.9.12 (Python Software Foundation, Wilmington, DE, USA).
3 Results
3.1 Seroprevalence of Antibodies to CDV
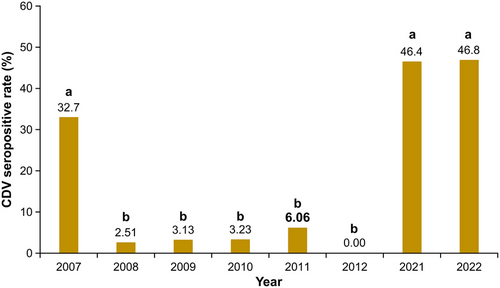
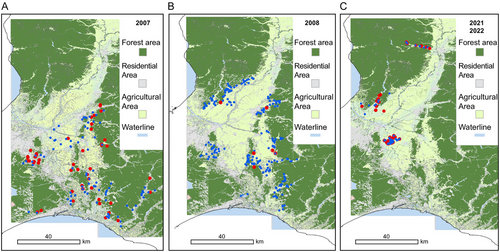
The CDV antibody seroprevalence for the entire study area was 32.7% in 2007, 2.51% in 2008, 3.13% in 2009, 3.23% in 2010, 6.06% in 2011, 0.00% in 2012, 46.4% in 2021, and 46.8% in 2022 (Table 1). The CDV seropositivity rates in 2007, 2021, and 2022 were significantly higher than those observed in 2008–2012 (p < 0.01; Table 1 and Figure 2). The CDV seropositivity rate was high in 2007 and subsequently low from 2008 to 2012. In contrast, in 2021 and 2022, the seropositivity rate was consistently high, reaching 45% or higher for two consecutive years (Figure 2). In 2007, although the positivity rate differed in each area, seropositive raccoons were confirmed in all areas, indicating CDV infection had already spread at that time (Table 1 and Figure 3). In 2021 and 2022, all three areas tested showed high seropositive rates of ≥ 38% (Table 1 and Figure 3). A 1.38-kg infant captured in Area F in 2021 tested positive for CDV antibodies. However, the data were excluded from statistical analysis because the infant was estimated to be approximately 1 month old based on its weight, and it was considered to have maternal antibodies (Paré et al. 1999).
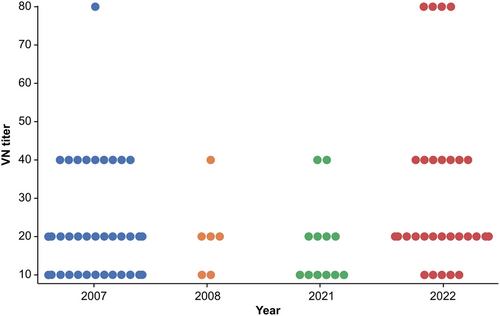
For the second screening, CDV-positive serum samples were examined to determine VN titers. VN titers showed variation based on the year and area of raccoon capture, ranging from 10 to 80 (Figure 4).
3.2 Relationship Between CDV Infection and Various Factors in Raccoons
Since the results showed high CDV seropositivity rates in 2007, 2021, and 2022, statistical analyses were conducted separately for 2007 and 2021–2022 to compare past CDV infections with the current situation. Data from 2021 and 2022 were combined for statistical analysis because the number of samples in each area was smaller than that in the 2007 data.
3.3 Relationship With CPUE
In 2007, the CPUE calculated for each 1 km × 1 km grid averaged 6.74, and the CPUE for each grid ranged from 1.19 to 19.1. For 2021 and 2022, the CPUE was calculated for each 1 km × 1 km grid in Area F, averaging 5.44, with the CPUE ranging from 3.13 to 10.9. Logistic regression analysis indicated an association between CPUE and CDV antibody status (seropositive or seronegative) in 2007, indicating that raccoons captured in higher CPUE areas were more likely to be seropositive (odds ratio = 1.49, 95% confidence interval [CI]: 1.07–2.08, p = 0.0190, Figure 5). In contrast, no association with CPUE was found in 2021 and 2022 (odds ratio = 1.17, 95% CI: 0.664–2.06, p = 0.586, Figure 5).
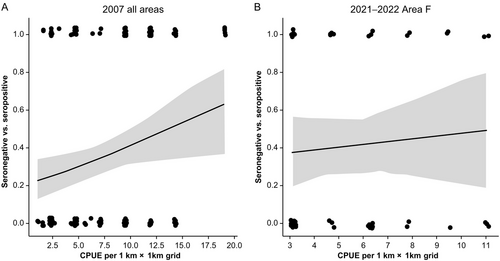
3.4 Relationships With Raccoon Host and Environmental Factors
Table 2 demonstrates the CDV seropositivity rates according to sex and age as raccoon host factors. The seroprevalence was significantly higher in adults (62.5%) than in juveniles (26.1%) in 2021 and 2022 (p < 0.05; Table 2). Although no significant difference in seroprevalence existed between raccoon age groups in 2007, the proportion of antibodies against CDV tended to increase with age (juveniles: 16.7%, yearlings: 31.9%, and adults: 35.7%; Table 2). Seroprevalence did not vary between sexes in all years (Table 2). Therefore, age data were used as explanatory variables in the logistic multiple regression analysis.
| 2007 | 2008 | 2021–2022 | |||||
|---|---|---|---|---|---|---|---|
| % | (N) | % | (N) | % | (N) | ||
| Sex | Male | 30.7 | (88) | 0.95 | (105) | 40.8 | (49) |
| Female | 35.3 | (68) | 3.73 | (134) | 53.7 | (41) | |
| Age | Juvenile (0 years) | 16.7 | (6) | 0.00 | (11) | 26.1* | (23) |
| Yearling (1 year) | 31.9 | (94) | 1.47 | (136) | 45.7 | (35) | |
| Adult (2 or more years) | 35.7 | (56) | 4.35 | (92) | 62.5* | (32) | |
| Total | 32.7 | (156) | 2.51 | (239) | 46.7 | (90) | |
- Note: All values represent percentages of positive samples. The numbers in parentheses indicate the total tested sample count. For 2021 and 2022, the data were combined because of the small sample size compared with 2007 and 2008.
- * p < 0.05.
Next, the absolute values of the correlation coefficients for the forest and agricultural area ratios were higher than 0.75 in all cases, and the agricultural area ratio was removed from the explanatory variables. Thus, the same explanatory variables (forest area ratio, residential area ratio, waterline length, and age) were used in the logistic multiple regression analysis to compare the association between CDV infection status and each factor between 2007 and 2021–2022. As a result, although no significant differences were found between CDV antibody status and each variable in 2007 (Table 3), the forest area ratio showed a weak influence on seroprevalence, indicated by a positive coefficient (odds ratio = 1.27, 95% CI: 0.888–1.83, p = 0.188, Table 3). Conversely, the residential area ratio distinctly impacted seroprevalence in 2021–2022, as evidenced by a significant positive coefficient (odds ratio = 2.20, 95% CI: 1.12–4.30, p = 0.0220; Table 3).
| 2007 | ||||||
|---|---|---|---|---|---|---|
| Coefficient | ||||||
| AICc | Intercept | Forest area ratio | Residential area ratio | Waterline length | Age | |
| 204.20 | −0.741 | 0.243 | −0.151 | 0.0972 | 0.151 | |
| Null | 199.20 | −0.722 | ||||
| Coefficient | SE | OR | 95% CI | p value | |
|---|---|---|---|---|---|
| Forest area ratio | 0.243 | 0.184 | 1.27 | 0.888–1.83 | 0.188 |
| Residential area ratio | −0.151 | 0.194 | 0.860 | 0.588–1.25 | 0.435 |
| Waterline length | 0.0972 | 0.187 | 1.10 | 0.764–1.59 | 0.603 |
| Age | 0.151 | 0.176 | 1.16 | 0.824–1.64 | 0.392 |
| 2021–2022 | ||||||
|---|---|---|---|---|---|---|
| Coefficient | ||||||
| AICc | Intercept | Forest area ratio | Residential area ratio | Waterline length | Age | |
| 120.68 | −0.115 | −0.166 | 0.788 | −0.400 | 0.436 | |
| Null | 126.41 | −0.134 | ||||
| Coefficient | SE | OR | 95% CI | p value | |
|---|---|---|---|---|---|
| Forest area ratio | −0.166 | 0.255 | 0.847 | 0.515–1.40 | 0.515 |
| Residential area ratio | 0.788 | 0.343 | 2.20 | 1.12–4.30 | 0.0220 |
| Waterline length | −0.400 | 0.309 | 0.670 | 0.366–1.23 | 0.195 |
| Age | 0.436 | 0.250 | 1.55 | 0.948–2.52 | 0.0800 |
- Abbreviations: SE: Standard error; OR: Odds Ratio; CI: Confidence interval.
- p-Values are shown for each factor.
Here, we focused on Area F (Figure 6), in which residential areas are surrounded by agricultural areas, which was commonly sampled in 2007 and 2021–2022. A low positivity rate of 10.0% (1/10) was observed in 2007, whereas a higher positivity rate of 36.7% (11/30) was observed in Area E, an adjacent forested area. However, Area F showed high seropositivity rates of 38.1% (8/21) in 2021 and 42.9% (12/28) in 2022 (Table 1 and Figure 6). Thus, a comparison of the relationship between CDV seroprevalence and environmental factors in the past and present showed different results, with the forest area ratio emerging as the primary factor in 2007 and the residential area ratio in 2021–2022.
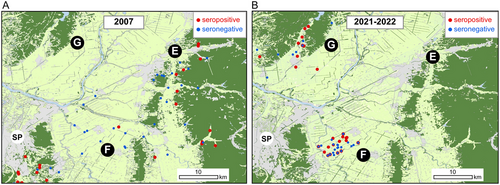
The model for seroprevalence in 2021–2022 exhibited a lower AICc value than the null model, indicating high predictive accuracy. In contrast, the results for 2007 were higher than those of the null model, suggesting a lower predictive accuracy compared to 2021–2022 (Table 3).
3.5 RT-qPCR
RT-qPCR was performed using serum and rectal swab samples obtained from raccoons captured in 2022, and all samples tested negative (Table 1).
4 Discussion
In this study, we investigated and compared the prevalence and factors of CDV infection spread status in the past and present among wild raccoons in Hokkaido for the first time. We found that the prevalence was high in three of the analyzed years (2007, 2021, and 2022), which was related to raccoon population density and weakly related to the forest area ratio in 2007, whereas it was significantly related to the residential area ratio in 2021–2022. Moreover, seroprevalence was high in older animals.
The initial screening results of the VN test demonstrated a CDV seropositivity rate in raccoons exceeding 30% in 2007, indicating widespread CDV presence in central and southern Hokkaido at that time. However, from 2008 to 2012, all six areas where the VN test was conducted showed low seropositivity rates. In addition, secondary screening results showed that VN titers in 2007 and 2008 were 80 or lower. A previous survey conducted in Wakayama showed a similar trend to that of this study, with a peak in the ratio of raccoons possessing antibodies against CDV in 2007 (approximately 60%), gradually declining after 2008 (approximately 30%), and remaining low from 2009 to 2012. The VN titers ranged from 10 to 5120 (Suzuki et al. 2015). A previous report suggests that CDV generally follows a “boom and bust” infection cycle as it relies on a dense population to persist in its enzootic state. In small wild animal populations, CDV epizootics are typically followed by a “fade-out,” as these populations lack sufficient susceptible animals for CDV to persist. Thus, the infection disappears until susceptible animals are reintroduced from outside sources (Wilkes 2023), which could suggest a typical temporal pattern observed in both Hokkaido (this study) and Wakayama (the previous study). Examining more samples between 2009 and 2020 is necessary to determine the epidemic cycle of CDV infections in Hokkaido. The low VN titers in 2007 suggest that the CDV epidemic had already entered a concluding phase in 2007 and nearly disappeared from 2008 to 2012.
In contrast, the first screening results in 2021 and 2022 showed that the CDV seropositivity rates remained high (nearly 50%) for two consecutive years, which is different from the trend mentioned in the previous study described above (Suzuki et al. 2015). The secondary screening results showed that the VN titers measured using samples from 2021 and 2022 were 80 or less, which was the same as those in 2007 and 2008. These results indicate that CDV infection could have also been prevalent among wild animals in recent years, and the present infection status may have changed from that in the past. Therefore, the low antibody titers in 2021 and 2022 could be explained by different reasons from 2007 to 2008. For example, mutant CDV strains could emerge in Hokkaido, potentially affecting the sensitivity of the method using KDK-1 (genotype Asia-1), as mentioned in previous studies (Lednicky et al. 2004; Clifford et al. 2006; Anis et al. 2018). Furthermore, a case was reported in North America where raccoons initially diagnosed as CDV-seropositive showed some becoming seronegative upon recapture, ranging from 1 month to 1 year later (Junge et al. 2007; Giacinti et al. 2023). Considering that virus exposure varies among wild animals, CDV antibodies may be maintained at low levels. To clarify if CDV mutation caused the low antibody titers in 2021 and 2022, isolating the virus from CDV-infected wild animal samples in Hokkaido and comparing it with genotype Asia-1, which was prevalent on the Japanese mainland in 2007, is necessary.
Previous studies have indicated that the density of the host population plays a crucial role in facilitating CDV spread (Ditchkoff, Saalfeld, and Gibson 2006; Wilkes 2023). This is because CDV can be transmitted through direct contact with infected animals or through the aerosolization of respiratory exudate, which contains the virus as well as other excretions and secretions like urine (Hoff et al. 1974; Deem et al. 2000; Loots et al. 2017). In our study, we used the CPUE as an index to measure the density of the raccoon population and examined its relationship with seroprevalence. The results demonstrated that, in 2007, raccoons in high-density areas were more likely to be CDV-seropositive. However, in 2021 and 2022, no relationship existed between CPUE calculated in Area F and seroprevalence, even though high CDV seropositivity rates (38.1% in 2021 and 42.9% in 2022) were confirmed. One potential explanation could be the difference in trapping periods: the periods were 21 days in 2007 but extended to 32 days in 2021 and 2022, resulting in an overall low calculation of CPUE for those years. Another possible reason may be the limited area analyzed in 2021–2022 compared with 2007. However, in Area F as a whole, the annual number of captured raccoons was 194 individuals in 2021 and 213 individuals in 2022, which was twice as high as the 93 individuals captured in 2007, indicating a significant population increase in recent years. Additionally, in Japan, raccoons showing symptoms of CDV infection have not yet been identified, and they are said to be relatively resistant (Nakano et al. 2009b). Therefore, when CDV infections are prevalent, raccoon populations do not decrease; instead, population densities continue to rise, potentially facilitating the transmission of CDV to susceptible animals. Therefore, monitoring the relationship between raccoon populations and infection status across broader areas will be crucial for future management efforts.
Next, considering CDV seroprevalence among raccoons as a clue for infection status among wild animals, environmental factors were examined. No significant associations were found in 2007; however, a weak positive influence was observed in the forest area ratio. However, a strong association was confirmed between the residential area ratio and CDV seroprevalence in 2021 and 2022, suggesting that CDV infection may have been prevalent among raccoons residing near residential areas in recent years. Raccoons prefer forest environments rich in water resources such as streams, ponds, and wetlands; however, they adapt to various environments, from agricultural lands to urban areas (Ikeda 1999; Gehrt 2003). Recent raccoon population growth has increased their natural habitat, allowing raccoons to spread the CDV in both residential and forested areas. This implies that the infection status has changed since the past, posing an increased infection risk for domestic dogs. Urban areas generally offer high-quality habitats due to supplemental food availability, leading to an aggregated distribution of raccoons and further facilitating disease transmission (Prange, Gehrt, and Wiggers 2003; Ditchkoff, Saalfeld, and Gibson 2006). Some studies analyzing landscape structures and CDV environmental dynamics have shown that the proximity of natural areas or urban-natural interfaces can facilitate CDV spread in wild foxes in Europe (Gras et al. 2018; Carella et al. 2022). Moreover, in a case reported in Italy in 2018, CDV was transmitted from wildlife to a domestic dog that had not been vaccinated, despite the widespread use of vaccination (Bianco et al. 2020). Our findings also serve as a warning, indicating that the risk of infection extends from forests to residential areas. Consequently, vaccination against CDV for domestic dogs must be strongly recommended.
Regarding raccoon host factors, we examined the association between raccoon sex and age with CDV infection. No sex differences were observed in any of the years in the present study. Similarly, many previous studies found no difference in CDV seroprevalence based on sex (Mitchell et al. 1999; Junge et al. 2007; Kameo et al. 2012; Aoki et al. 2017; Giacinti et al. 2023). This is thought to be because CDV is widely spread among raccoons through contact infection when males spend several days with multiple females during the mating season. CDV infection rates peak during the mating season in North America because of increased contact opportunities (Roscoe 1993; Giacinti et al. 2021; Taylor et al. 2021). Regarding age, raccoon adults were more likely to be seropositive than juveniles in 2021–2022. In addition, older raccoons tended to show higher seropositivity rates in both 2007 and 2021–2022. This tendency has also been reported in North America, possibly due to repeated or continuous exposure to the virus in older individuals compared to younger individuals (Mitchell et al. 1999; Giacinti et al. 2023). Also, maternal antibodies in raccoon pups were reported to gradually decline to negligible levels by the age of 20 weeks (Paré et al. 1999). Additionally, antibodies resulting from contact with the pathogen can persist for a year or more, which explains the higher seropositivity rates observed in older raccoons (Dall'Ara et al. 2023; Libbey and Fujinami 2023).
In this study, all RT-qPCR results were negative, although seropositive samples were confirmed using the VN test. RT-qPCR and RT-PCR have been used to detect CDV in many studies, but these have been mostly limited to susceptible animals that were either found dead or showing symptoms (Kameo et al. 2012; Rentería-Solís et al. 2014; Wostenberg et al. 2018; Bianco et al. 2020; Giacinti et al. 2021; Carella et al. 2022). However, no raccoons showing symptoms were confirmed in this study, as described previously (Nakano et al. 2009b). In general, animals infected with CDV have a limited period to retain or shed the virus (Sehata et al. 2015; Roelofs et al. 2023), whereas antibodies can last for several years (Dall'Ara et al. 2023). Considering these aspects, especially in wild animals, antibody testing can be better suited for screening than PCR, given the fact that it provides a longer-term history of viral exposure than PCR, which can only detect viruses during short periods of symptoms. However, antibody surveillance has limitations in determining the exact timing of virus infection in individuals. While CDV-neutralizing antibody titers can persist for several years, they tend to decrease over time, with variations among individuals (Junge et al. 2007; Giacinti et al. 2023). In addition, our findings may have been influenced by limitations in the animal species, study area, or study year. Although the present study focused on wild raccoons, which are considered best suited as sentinel species for surveillance, we hope to investigate the CDV infection status in susceptible species such as raccoon dogs and foxes, which have been confirmed to show symptoms of CDV. Additionally, previous studies targeting multiple species have reported varying seroprevalence among animal species within the same area (Kameo et al. 2012; Naidenko et al. 2018; Naidenko et al. 2019). Other animal species such as brown bears (Ursus arctos) and sika deer (Cervus nippon) have also been reported to be seropositive for CDV, suggesting the need for more extensive monitoring of infectious status in Hokkaido.
In conclusion, this study confirmed that CDV was as widespread in Hokkaido in 2007 as it was in mainland Japan. The prevalence of CDV infection has changed over the past 15 years, and one of the reasons for this change could be an increase in the raccoon population, which is the key to maintaining this infectious disease within a wide host range. In particular, raccoons in Japan are relatively resistant to CDV and are thought to be carriers that excrete CDV asymptomatically (Nakano et al. 2009b). Therefore, as the raccoon population increases, the frequency of host–host contact also increases, and raccoons act as reservoirs of CDV. Further, CDV poses a risk of spreading to sympatric raccoon dogs in forested areas, foxes in both forested and urban areas, and unvaccinated domestic dogs in residential areas. Wild raccoon dogs and foxes, in particular, may experience not just direct consequences like habitat and food competition from raccoons but also face the indirect threat of CDV transmission, potentially causing significant harm to local populations. Therefore, further studies are necessary to investigate the CDV infection status and population density trends among sympatric raccoon dogs, foxes, and invasive raccoons. To mitigate the impact on wild native species and domestic dogs, expanding the scope of our current raccoon control programs is essential, akin to the efforts carried out in 2007 and 2008 to reduce raccoon population density. Considering this indirect impact, continuous monitoring surveys for CDV infection are imperative, and genetic analysis of CDV should be conducted to confirm the status of the viral mutation.
Acknowledgments
We sincerely would like to thank the Bureau of Natural Environment Affairs of the Hokkaido Government and Tobetsu town for providing valuable samples and information. We are also deeply grateful to Dr. Keita Matsuno of the Risk Analysis and Management Department, International Institute for Zoonosis Control; Dr. Yutaka Watanuki and Dr. Orio Yamamura of the Marine Bio-resource and Environmental Science Department, Graduate School of Fisheries Sciences, Hokkaido University for kindly providing the opportunity to conduct the experiment; Dr. Norikazu Isoda of the Laboratory of Microbiology, Faculty of Veterinary Medicine, Hokkaido University for his valuable comments; Dr. Keisuke Yoshida of the Center for Teaching and Learning, Institute for the Advancement of Higher Education, Hokkaido University for his advice regarding statistical aspects; and Dr. Tsutomu Mano of the Hokkaido Research Organization for his advice regarding age determination. We would also like to express our sincere gratitude to all the members of the Wildlife Management Laboratory at Rakuno Gakuen University and some students at the Faculty of Veterinary Medicine, Hokkaido University for helping with age determination and to Ayako Fujimoto for continuously supporting our fieldwork. We would also like to thank Takemi Jin for providing a raccoon photo used in the graphical abstract. Moreover, we express our gratitude to FARMAGE, CO. LTD, Norihiko Ando, Hisashi Ishihara, Kyoko Kubo, Saki Kubo, Yukiko Kubo, and Manabu Tatokoro for their support of our research. We would like to thank Editage (www.editage.jp) for English language editing.




