Revealing the key signals in nestling begging behavior perceived by parent birds during parent–offspring conflict
Abstract
The parent–offspring conflict in avian species encompasses resource allocation and a balance necessary for survival for both parties. Parental investment is modulated according to various factors, among which begging is important. Endogenous hormones, particularly corticosterone (CORT), play a role in modulating begging behavior. However, most studies on hormonal regulation of begging behavior induced elevated hormone levels in the offspring through feeding or injections, thus, limiting our knowledge of the evolution of the parent–offspring conflict under natural conditions. In this study, we aimed to identify the key signals that parents respond to during interactions with their nestlings in the wild, considering factors such as endogenous hormone CORT, nestling age, and brood size, which may affect nestling begging behavior. Begging performance was evaluated by measuring the begging frequency and score of the red-whiskered bulbul (Pycnonotus jocosus), along with assessing CORT levels in feathers. CORT levels were significantly correlated with both the begging frequency and score of nestlings, while variables such as body mass and tarsus length did not influence parental feeding frequency. Additionally, factors such as the number of nestlings (brood size), age, and begging frequency were predictors of parental feeding frequency. Our findings indicate that begging frequency, nestling age, and brood size are signals that help navigate the intricacies of the parent–offspring conflict and that parents may rely on these key signals from the range of begging cues exhibited by nestlings to adjust their feeding strategies.
INTRODUCTION
Parent–offspring conflict refers to the mutual interaction between parents and offspring in allocating parental resources (Trivers 1974). While parents invest in offspring reproduction, they also incur costs that may reduce survival rates and future reproductive success (Owens & Bennett 1994; Alonso-Alvarez & Velando 2012). Therefore, life history theory predicts that, under natural selection, parents weigh the optimal strategy for investing in offspring reproduction against their fitness (Southwood 1977; Brown & Sibly 2006). When parents care for multiple offspring simultaneously, different strategies for allocating food resources may arise (Davis et al. 1999). For example, the “laissez-faire strategy” involves parents avoiding preferential feeding to offset or enhance the impact of asynchronous hatching on offspring (Neb & Selmi 2020). In contrast, the “brood survival strategy” (Lack 1947; Slagsvold et al. 1984; Pijanowski 1992) entails providing additional resources to the last-hatched chicks to enhance the overall survival of the brood (Brode et al. 2021). Nevertheless, when food resources are limited, parents tend to favor more advantaged chicks, a tactic known as the “brood reduction strategy” (Davis et al. 1999). However, this parental preference requires accurate observation of the chicks' growth status and food requirements (Hinde & Godfray 2011). Numerous characteristics of chicks, such as skin, beak, feather brightness, and ultraviolet (UV) feather color serve as signals for parental favoritism (Linville et al. 1998; Bize et al. 2006; de Ayala et al. 2007; Garcia-Campa et al. 2023). However, the most obvious signal is believed to be the begging behavior of nestlings, which directly reflects their food requirements and competitiveness (Rosivall et al. 2005; Zeng et al. 2023).
Nestlings adjust their begging behavior based on their own needs or the amount of food they have already received (Kilner & Johnstone 1997; Mock et al. 2011). Previous experiments have demonstrated that after being fed, the begging levels of the offspring decrease, but they increase when they are hungry (Wells 2003). As the “honest-signaling model” (Godfray 1991) predicts, parents respond actively to these reliable signals, meeting the demands of their offspring (Hinde & Kilner 2007; Smiseth & Moore 2008). However, not all nestling begging behaviors serve as honest signals; nestlings may exaggerate their needs by begging to acquire more resources (Godfray 1995; Caro et al. 2016). Moreover, parasitic nestlings, such as cuckoos (Cuculus spp.) and cowbirds (Molothrus spp.), may exhibit higher begging levels as supernormal stimuli to obtain more food (Soler 2017; Wang et al. 2020). Therefore, begging behavior may not only be a means of resolving the parent–offspring conflict but also represent an adaptive evolution in nestlings (Rodríguez-Gironés et al. 1996; Godfray & Johnstone 2000). Research on the factors influencing nestling begging behavior has two main aspects: (1) theoretical propositions suggest that competition among nestmates may promote the evolution of nestling begging behavior (Rodríguez-Gironés et al. 1996; Neb & Selmi 2020), a phenomenon supported by many experiments in which nestling begging behavior increases with the number of nest companions and begging intensity (Leonard & Horn 1998; Leonard et al. 2000). (2) Recent studies have proposed that hormones may also lead to changes in begging behavior, categorizing them into internally produced hormones (endogenous hormones) and hormones deposited by parents in eggs (maternal hormones) (Schwabl & Lipar 2002; Müller et al. 2007). Research on maternal hormones has primarily focused on testosterone (T), with experiments injecting T into egg yolks during the incubation period, which resulted in a higher begging intensity of the nestlings after hatching (Schwabl 1996; Eising & Groothuis 2003). Adding T to food shows a positive correlation between begging behavior signals and nestling T levels (Goodship & Buchanan 2007). However, some studies have found that adding T does not necessarily increase begging intensity (Boncoraglio et al. 2006; Müller et al. 2010). In other studies, a temporary increase in T concentrations inhibited begging, as observed for a semi-precocial bird species, the black-headed gull [Chroicocephalus ridibundus (Linnaeus, 1766)] (Boncoraglio & Groothuis 2013). Studies on endogenous hormones have primarily focused on corticosterone (CORT). Feeding or injecting nestlings with exogenous CORT causes a brief and moderate increase in CORT concentrations, and experimental findings demonstrate that elevated CORT levels can substantially enhance the begging rate of nestlings or extend the latency period before begging (Loiseau et al. 2008; Wada & Breuner 2008; Elderbrock et al. 2017).
Although the feeding quantity of some parent birds may vary with nestling begging intensity (Hinde & Kilner 2007; Smiseth & Moore 2008), studies have also revealed that hormones can stimulate an increase in nestling begging intensity without a corresponding increase in feeding by parent birds (Loiseau et al. 2008; Elderbrock et al. 2017). Therefore, the begging performance of nestlings was determined by the endogenous hormone CORT, but the chosen signals used by the parents to evaluate the food demand of nestlings remain unclear because the begging behavior may include several signals, some of which are exaggerated (Godfray 1995; Caro et al. 2016). Although parental birds must balance their investments between current offspring and future fitness, they can flexibly adjust their feeding frequency and preferences, potentially allowing them to play a dominant role in the parent–offspring conflict (Garcia-Campa et al. 2023). Therefore, an optimal strategy for parents is to evaluate the food demand of nestlings precisely by extracting an honest signal from their begging performance. Begging performance in nestlings is regulated by endogenous hormones. However, in experiments related to hormonal regulation of begging behavior, most involved elevated hormone levels in the offspring through feeding or injections. Studies investigating natural hormone levels focused on stressful environmental conditions such as food resource restriction on feather CORT (e.g., Patterson et al. 2014). These studies revealed the function of hormones in begging behavior, but they have not provided a comprehensive understanding of the evolution of the parent–offspring conflict under natural conditions, in which the begging intensity of nestlings is limited by their own conditions (Kilner & Johnstone 1997), while the parents’ provision capacity is also constrained because of the tradeoff between the nestlings’ and their own food needs (Parker et al. 2002). Furthermore, the conventional approach to detecting CORT levels entails measuring CORT hormones in the blood (Loiseau et al. 2008; Wada & Breuner 2008). However, the procedure for collecting blood samples can disturb and stress the birds, resulting in a transient elevation of hormone levels. Measuring CORT in feathers permits a simpler, less invasive sampling method (Bortolotti et al. 2009).
Studies examining the relationship between CORT levels in nestling feathers and their begging performance are scarce. Therefore, in this study, we investigated the natural CORT deposition levels in the feathers of nestlings to elucidate the relation between CORT and begging behavior in nestlings instead of incorporating artificial CORT interventions. We then determined the relationship between CORT levels and the begging behavior of nestlings, which comprises various behavioral displays and morphological traits, to reveal the key signals in nestlings during food provision by their parents. We hypothesized that some behaviors are honest signals while others may be redundant or exaggerated. Thus, we aimed to identify which nestling traits are perceived by parents as signals for feeding to provide insights into the evolution of the parent–offspring conflict.
MATERIALS AND METHODS
Study area and species
This study was performed during the breeding season (April–August) of 2021 in Nonggang village (23°39′N, 107°04′E), Guangxi Province, southwestern China (Bi et al. 2020). The area has a tropical monsoon climate with annual average temperatures of 20.8–22.4°C and annual average rainfall of 1150–1550 mm (Zhou & Jiang 2008; Yan et al. 2022). The primary vegetation types in this region include broadleaf forests, cultivated lands, shrublands, bamboo forests, and orchards with fruit trees. Notably, vast sugarcane (Saccharum officinarum) plantations dominate the open landscapes, serving as a major agricultural crop in the area (Yang et al. 2016). Potential nest predators in this region include snakes, the Indochinese green magpie (Cissa hypoleuca), the white-winged magpie (Urocissa whiteheadi), and the greater coucal (Centropus sinensis) (Feng 2019). The studied species was the red-whiskered bulbul [Pycnonotus jocosus (Linnaeus, 1758)], a resident species that builds open cup-shaped nests mostly in bush wood, corn, cane fields, or orchards. During the breeding season, female birds lay eggs consecutively over several days, depositing one egg per day. The average clutch size is 2.53 ± 0.51 eggs, predominantly comprising 2–3 eggs, with 4-egg clutches being rare (Li et al. 2015). After excluding predated and deserted nests, the valid data in this study included 49 bulbul nestlings from 20 nests, which were used to quantify begging behavior and CORT levels.
Quantification of begging performance
A mini camera (WJO3; Hisilicon, Shenzhen, Guangdong, China; https://www.hisilicon.com/cn), which was camouflaged by leaves, was used to record the begging behavior of nestlings and the feeding frequency of parents. Videos were recorded for 9 h from 8 AM to 5 PM on days when the nestlings were 3, 5, and 7 days old (a nestling was 0 days old on the day it hatched) because P. jocosus is diurnal and active in this period. We chose the three nestling age stages because: (i) at these ages, nestlings did not exhibit a stress response and alarm to human visitation based on our observation (older nestlings struggle and produce distress calls when the investigator touches them); (ii) 3-day-old nestlings started to present obvious begging behavior and their growing feathers allowed for sample collection; (iii) nestlings that were 9 days old or older were excluded because they exhibited a stress response and alarm to human visitation, which may impact their behavior and feather CORT levels. The nestlings were marked with silicone leg bands to facilitate individual identification, and the videos were played back in the laboratory to quantify their begging performance. Seven variables were considered as signals that may be used by parents to assess their nestlings during feeding. These include: (1) begging frequency (the frequency of begging during the investigation); (2) mean begging score, which followed a scoring rule from five begging behaviors of signal strength and three begging behaviors of non-signal strength by playing the video eight times slower than normal. The begging behaviors of signal strength refer to the behaviors that can be directly perceived by the parents, while the begging behaviors of non-signal strength are behaviors that affect food allocation but may not be perceived by the parents (Leonard et al. 2000; Kilner 2001; Rodrı́guez-Gironés et al. 2001; Heist & Ritchison 2016). The signal strength included open mouth (chicks open their mouths during the parents’ feeding), head up (chicks raise their heads during the parents’ feeding), stretching neck (chicks stretch their necks upward during the parents’ feeding), straighten up (chicks raise their bodies during the parents’ feeding), and multi-begging (chicks immediately beg for food again after receiving foods), while the non-signal strength included crowding behavior (chicks jostle each other before the parents’ feeding), priority food receiving (chicks receive food in the first priority during the parents’ feeding), and mis-begging (chicks display begging when the parents return without carrying food) (Leonard et al. 2000). The occurrence of each behavior was counted as one score, and the total score of each begging attempt represented the begging score. The mean begging score was calculated from all scores counted during the 9-h videos, while the begging frequency was the total frequency of begging during this time. We considered begging frequency because it is likely an honest signal with an obvious display and has a high cost for the nestlings (Kilner 2001; Lee et al. 2012). The begging score was used because it is an integrated signal that represents the total begging performance of nestlings (Rivers et al. 2013; Heist & Ritchison 2016). This allowed us to investigate whether the parents evaluated the performance of the nestlings using noticeable or integrated signals. (3) The CORT level was also considered a variable because it is a physiological determinant of begging performance (Romero et al. 2005). Four additional variables, including (4) age, (5) body mass, and (6) tarsus length of nestlings as well as (7) brood size, were considered for analyses, because these variables might also correlate with the nestlings’ performance. All variables above were measured or scored at the individual level. In addition, the position of each nestling in the nests and its leg bands were matched, thus allowing us to recognize its identity during video playback in the laboratory.
Feather sampling protocol
After recording 9 h of footage of each observed nest, the body mass and tarsus length of each nestling were measured, and feathers were collected (from 5 PM to 6 PM) for CORT determination. Body mass was measured using an electronic scale (CX-668B; Changxie Electronics, Wuxi, China; accurate to 0.01 g), and tarsus length was measured using a dial Vernier caliper (WiHa Inc., Eisenach, Germany; accurate to 0.02 mm). Feathers were collected after video recording to avoid any potential influence on the recorded begging behavior of nestlings. Feather sampling of all nestlings was performed in accordance with a uniform standard in which two symmetric and appositional feathers on their right and left secondary wing feathers were collected. The samples were stored in a 1.5-mL Eppendorf tube with information on individual identity. The tubes were transported to the laboratory for extraction and determination of CORT levels. The CORT levels were measured in the amount accumulated in the feather over time rather than the amount being produced immediately. Therefore, the CORT levels of 3-, 5-, or 7-day-old nestlings reflected the cumulative levels deposited up to those ages, rather than the levels on those exact days. Our method was the best approach given the properties of feather CORT and did not compromise our study objectives because: (i) feather samples were collected in intervals corresponding to the video recordings of 3-, 5-, and 7-day-old nestlings, allowing them to reflect changes in CORT levels with nestling age; (ii) feather samples were collected after the 9-h video recording on the same day, ensuring that this time-lag collection accurately represented the CORT levels relevant to the age of the nestling on that specific day. Additionally, we used feather sampling rather than fecal or blood sampling because we compared the begging performance of each nestling to its CORT level, and fecal excretion of the nestlings within a nest was not synchronous and blood sampling was considered more invasive than feather sampling, especially considering the sampling frequency (three times) within the 5 days (i.e., 3–7-day-old nestlings). In addition, we performed a pilot study of natural CORT levels in feather samples, which showed that feather sampling is feasible and less invasive.
Feather CORT extraction
Feathers from each tube were weighed using an analytical balance (CAV214C; OHAUS, Parsippany, NJ, USA; accuracy class: 0.0001 g), and CORT extraction was performed using the bird CORT enzyme-linked immunosorbent assay (ELISA) kit (Ca#SU-B95445; Shanghai Enzyme-linked Biotechnology, China) according to the manufacturer's instructions. We added a 1 g of the sample to 9 mL of phosphate-buffered saline (PBS; 0.01 mol L−1, pH = 7.2–7.4) using a pipette (Eppendorf, Germany). Subsequently, the samples were frozen in liquid nitrogen for 5 min, and a high-speed electric tissue grinder (OSE-Y10; Tiangen, China) was used to homogenize the feathers until the suspended particles were liquefied. Finally, all sample tubes were centrifuged (D3024R; Saiyataike, China) at 12 000 rpm for 15 min at 4°C for purification.
CORT level determination
Statistical analyses
Three generalized linear mixed models (GLMMs) were used to analyze data, in which stepwise backward elimination was performed to select the optimal models according to the Akaike information criterion. Before running the GLMMs, collinearity analyses were performed, in which the variance inflation factor (VIF) was calculated to measure the strength of correlation between predictor variables in each model. Generally, VIF = 1 indicates no correlation, VIF 1–5 indicates low to moderate correlation, and VIF > 5 indicates high correlation. For the variables where VIF was > 5, we included the one with the lowest VIF and excluded the others from the models. In model 1, the mean begging score was the response variable, and the effects of CORT levels, begging frequency, body mass, tarsus length, brood size, and nestling age were predictors. In model 2, the begging frequency was the response variable, and the predictors were the same as those in model 1, except that the mean begging score was used as a predictor instead of begging frequency. These two models were intended to reveal the predictors of begging performance (i.e., mean begging score or begging frequency) in nestlings. Model 3 was used to investigate the predictors of feeding frequency by the parents; thus, all variables related to nestlings, including the CORT level, begging frequency, and mean begging score and other variables in models 1 and 2 were included as predictors. Nest and nestling identities were used as random effects in the three models. According to collinearity analyses, three variables were highly correlated in the three models (VIF of body mass: 9–9.4; VIF of tarsus length: 12.3–12.5; VIF of nestling age: 4.8–5.4). The VIF values for all the other variables were < 2. Therefore, body mass and tarsus length were excluded from the GLMM analyses. Multiple comparisons between effect pairs in the models were achieved by comparing the estimated marginal means (also known as least squares means) with P-values adjusted using Tukey's method. The “car,” “lme4,” “stats,” and “emmeans” packages in R software for Windows (v. 4.2.2; R Foundation for Statistical Computing, Vienna, Austria) were used for collinearity analyses, GLMMs, stepwise backward elimination, and multiple comparisons, respectively.
RESULTS
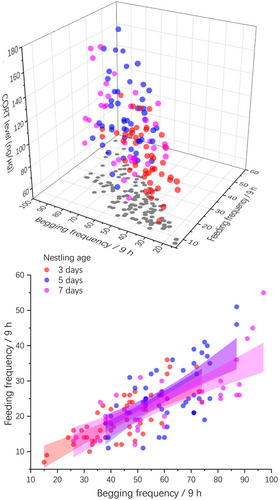
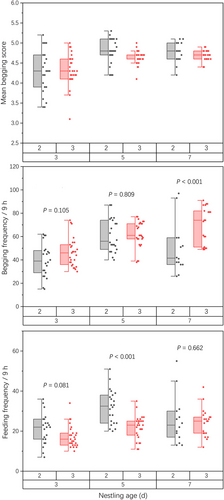
GLMM models 1 and 2, which investigated the effect of CORT levels and other nestling traits on begging performance, indicated that both begging frequency and score were predicted by the CORT level (begging score: F1,128.34 = 13.54, P < 0.001; begging frequency: F1,123.71 = 9.28, P = 0.003; Table 1 and Fig. 1). Nestling age also predicted the begging frequency and mean begging score, indicating that they changed with nestling age (Table 1). The begging frequency and mean begging scores of 5- and 7-day-old nestlings were higher than those of 3-day-old ones (Fig. 2 and Table 2).
| Model | Response variable | Effect | F | df1 | df2 | P-value |
|---|---|---|---|---|---|---|
| 1 | Begging score | CORT level | 13.54 | 1 | 128.34 | <0.001*** |
| Nestling age | 31.71 | 1 | 117.73 | <0.001*** | ||
| 2 | Begging frequency | CORT level | 9.28 | 1 | 123.71 | 0.003** |
| Nestling age | 42.08 | 1 | 115.83 | <0.001*** | ||
| 3 | Feeding frequency | Begging frequency | 417.31 | 1 | 128.1 | <0.001*** |
| Brood size | 115.11 | 1 | 48.73 | <0.001*** | ||
| Nestling age | 7.059 | 1 | 103.6 | 0.009** |
- Asterisks indicate significance at *P < 0.05, **P < 0.01, and ***P < 0.001. CORT, corticosterone.
| Contrast (nestling age/brood size) | Estimate | SE | df | t Ratio | P-value | |
|---|---|---|---|---|---|---|
| Response variable: mean begging score | ||||||
| Brood size 2 | Age 3 days versus 5 days | −0.53 | 0.10 | 127 | −5.13 | <0.001*** |
| Age 3 days versus 7 days | −0.50 | 0.12 | 127 | −4.26 | <0.001*** | |
| Age 5 days versus 7 days | 0.03 | 0.12 | 127 | 0.27 | 0.961 | |
| Brood size 3 | Age 3 days versus 5 days | −0.32 | 0.09 | 127 | −3.43 | 0.002** |
| Age 3 days versus 7 days | −0.37 | 0.10 | 127 | −3.76 | 0.001** | |
| Age 5 days versus 7 days | −0.05 | 0.10 | 127 | −0.55 | 0.847 | |
| Response variable: begging frequency | ||||||
| Brood size 2 | Age 3 days versus 5 days | −21.36 | 4.38 | 127 | −4.88 | <0.001*** |
| Age 3 days versus 7 days | −10.05 | 4.96 | 127 | −2.03 | 0.110 | |
| Age 5 days versus 7 days | 11.31 | 4.96 | 127 | 2.28 | 0.062 | |
| Brood size 3 | Age 3 days versus 5 days | −15.56 | 3.95 | 127 | −3.94 | <0.001*** |
| Age 3 days versus 7 days | −23.45 | 4.22 | 127 | −5.55 | <0.001*** | |
| Age 5 days versus 7 days | −7.89 | 4.22 | 127 | −1.87 | 0.152 | |
| Response variable: feeding frequency | ||||||
| Brood size 2 | Age 3 days versus 5 days | −11.05 | 2.28 | 127 | −4.84 | <0.001*** |
| Age 3 days versus 7 days | −4.38 | 2.59 | 127 | −1.69 | 0.212 | |
| Age 5 days versus 7 days | 6.66 | 2.59 | 127 | 2.57 | 0.030* | |
| Brood size 3 | Age 3 days versus 5 days | −4.78 | 2.06 | 127 | −2.32 | 0.057 |
| Age 3 days versus 7 days | −7.06 | 2.20 | 127 | −3.21 | 0.005** | |
| Age 5 days versus 7 days | −2.29 | 2.20 | 127 | −1.04 | 0.555 | |
| Nestling age (3 days) | Brood size 2 versus 3 | 3.82 | 2.17 | 127 | 1.76 | 0.081 |
| Nestling age (5 days) | Brood size 2 versus 3 | 10.09 | 2.17 | 127 | 4.64 | <0.001*** |
| Nestling age (7 days) | Brood size 2 versus 3 | 1.14 | 2.61 | 127 | 0.44 | 0.662 |
- Asterisks indicate significance at *P < 0.05, **P < 0.01, and ***P < 0.001. CORT, corticosterone.
In contrast, model 3, which tested the predictors of feeding frequency by the parents, showed that begging frequency rather than CORT levels and begging score was a significant predictor (F1,128.1 = 417.31, P < 0.001; Table 1). Brood size and nestling age also predicted feeding frequency (Table 1). The feeding frequency of 5-day-old nestling individuals from brood size 2 (i.e., two nestlings) was higher than that of nestling individuals from brood size 3 (i.e., three nestlings), whereas the begging frequency showed the opposite result for 7-day-old nestlings (Fig. 2 and Table 2). Both the feeding and begging frequencies were the highest for 5-day-old nestlings for brood size 2 but increased from 3- to 7-day-old nestlings for brood size 3 (Fig. 2 and Table 2). Finally, although the CORT level predicted the mean begging score and frequency, it did not change with brood size, with the only difference being that the CORT levels of the 5- and 7-day-old nestlings were higher than that of the 3-day-old ones (Fig. 2 and Table 2).
DISCUSSION
Previous studies have suggested that artificial interventions, including feeding nestlings CORT-rich food, application of CORT skin patches, and administration of CORT injections, have the potential to modify begging behavior (Loiseau et al. 2008; Wada & Breuner 2008; Smiseth et al. 2011). Nevertheless, under natural conditions, only a few studies have indicated that feather CORT concentrations are correlated with nutritional stress, with higher CORT deposition in nestlings from food-rich environments (Patterson et al. 2014). Notably, Patterson et al. (2014) found that feather CORT levels were lower under restricted food resources and correlated with feather mass and growth rate. They concluded that the feather CORT level was concentrated during growth. This is consistent with our results, which showed that both the CORT levels in feathers and nestling age significantly predicted the begging score and frequency of nestlings. Predominant nestlings that received more food resources are likely to grow faster and, thus, have higher feather CORT levels. Serving as the primary regulatory factor in the metabolic pathways underlying physiological stress response events (Romero et al. 2005), CORT levels likely constitute the physiological underpinnings of nestling begging behavior. However, in contrast to prior studies, we did not perform artificial interventions. Instead, we measured natural CORT levels in feathers to illustrate their relation to the nestlings’ begging behavior.
However, no correlation was observed between the begging score and frequency, implying that these signals do not have similar co-variations. One possible explanation is that the begging score, which reflects integrated begging signals, may embrace crowded and exaggerated begging performance to compete for food allocation (Mock & Parker 1998; Romano et al. 2013), which is caused by asynchronous hatching (Clark & Wilson 1981) or food resource pressure (Soravia et al. 2021). Consequently, the begging score signifies the priority of the offspring in acquiring parental provisioning, reflecting within-brood competition (Fig. 3). Studies have indicated that exogenous CORT can elicit nestling begging behavior, and interactions within the brood may obscure this effect; however, overall begging rates markedly escalate (Elderbrock et al. 2017), leading to enhanced parental provisioning rates (Kilner 1995). This implies that nestling begging frequency may represent a genuine cue for parental feeding decisions.

Furthermore, nestling age, which was highly correlated with the body mass and tarsus length, predicted the begging score and frequency. Older nestlings demonstrate notably elevated rates of both active and resting metabolism than younger nestlings; thus, energy expenditure in older nestlings is significantly correlated with their begging intensity (Leech & Leonard 1997). Nonetheless, the range of begging behavior (i.e., the ratio of active to resting metabolism) is similar between older and younger nestlings (Leech & Leonard 1997). This suggests that behavioral expressions in the begging score are accentuated as the nestlings mature, whereas begging frequency reflects the nestlings’ nutritional requirements more accurately. Similarly, older nestlings displayed begging more frequently, probably because they had developed better body conditions.
Nevertheless, subsequent analyses revealed that the begging frequency, but not the begging score, predicted parental feeding. This further emphasizes the distinction between the signal of begging frequency and the integrated signals of the begging score, possibly attributed to the incorporation of exaggerated begging and within-brood competition behaviors in the latter. Such exaggerations may become more pronounced with an escalation in competitiveness. Conversely, the begging frequency likely serves as an honest indicator of nestlings’ food requirements and proficiently predicts parental feeding frequency. An alternative explanation is that nestling begging frequency is influenced by parental provisioning rates to attain optimal reproductive decisions (Horsfall 1984; Hinde et al. 2010). Moreover, CORT levels fail to predict parental feeding, conceivably due to their impact on the overall performance of nestlings. This includes various behaviors captured in begging scores and as a physiological factor underpinning begging (Romero et al. 2005). Consequently, the CORT level could not be detected by the parents as a signal. CORT levels govern numerous begging behaviors in nestlings, some of which may correlate with nestling competition manifested through behaviors such as pecking, jostling for advantageous begging positions, or exaggerated begging (Mock & Parker 1998; Romano et al. 2013). In contrast, nestling begging frequency delineates the food requirements that parents utilize to fine-tune their efforts in providing food (Loiseau et al. 2008).
Additionally, the results indicated that brood size predicted parental feeding frequency. The feeding rate per nestling diminishes with increasing brood size, implying that nestlings from larger broods manifest increased begging intensity (Leonard et al. 2000; Zeng et al. 2023), owing to their augmented food requirements. Previous studies have indicated that the parental feeding frequency tends to increase with nestling age (Bi et al. 2020; Ye et al. 2021; Zhang et al. 2024). However, some studies have reported divergent findings, indicating no association between nestling age and parental feeding frequency (Maher 1996; Laiolo et al. 1998; Hampl et al. 2005). Nonetheless, the findings suggest that nestling age can predict the frequency of parental feeding. This could be attributed to the fact that older nestlings require more substantial food intake than younger ones, especially as their growth rate accelerates, demanding increased energy consumption (Steen et al. 2012). Furthermore, the begging signals of nestlings may exhibit age-related variations; as nestlings mature, the quantity of hunger-coded call features increases (Marques et al. 2011), thereby augmenting the parental capacity to gauge nestling hunger levels using these enriched signal characteristics (Leonard & Horn 2006). One limitation of this study is that we were unable to visually distinguish between the sexes because of the monomorphic nature of our study species. Therefore, future investigations on sexually dimorphic avian species are required to elucidate the roles, strategies, and distinctions of different sexes in parent–offspring dynamics.
In conclusion, our study indicates that CORT levels in nestling feathers serve as predictors of the begging frequency and score, which highlights that CORT levels act as an important physiological base for the begging performance in nestlings. Older nestlings were better in begging performance and nestmate competition, probably because they had developed better body conditions. Furthermore, we found that parent birds utilize begging frequency rather than the begging score, as well as the nestling age and brood size, as reliable information for food provision. This illustrates that the begging frequency, nestling age, and brood size are key signals to navigate the parent–offspring conflict. Finally, to our knowledge, this is the first study to investigate the natural CORT levels of nestlings by feather sampling. This method was feasible and convenient for field work, and we suggest applying it as the standard to measure natural CORT levels of nestlings in future studies.
ACKNOWLEDGMENTS
We thank Jiangwen Wu for his assistance with the fieldwork. This research was funded by the Education Department of Hainan Province (project number: HnjgY2022-12) and the National Natural Science Foundation of China (project number: 32260127).




