Increased nest temperature during winter does not affect residual yolk metabolism of hatchling painted turtles (Chrysemys picta)
Abstract
Rising global temperatures have a wide range of effects at organismal, population, and ecosystem levels. Increased winter temperatures are expected to alter the energetics of species that are dormant during this time. Hatchling painted turtles (Chrysemys picta) spend their first ∼8 months in shallow nests on land, where they putatively rely on residual yolk reserves to fuel energetic demands during this period of inactivity before they emerge in the spring. We performed a laboratory experiment to characterize changes in residual yolk quantity in hatchling C. picta and experimentally tested the effect of temperature on residual yolk, hatchling size, and survival over the winter brumation period. We manipulated winter nest temperature by simulating two natural thermal regimes (“low” vs “high” treatments) and one regime that approximates warmer temperatures expected by 2100 (“future” treatment). Because high temperature increases metabolism, we predicted that the future temperature treatment would decrease the amount of residual yolk remaining by the end of winter and reduce hatchling mass and survival. Residual yolk over winter did not differ from that before winter, and the temperature had no effect on the quantity of residual yolk or hatchling survival by the following spring. However, hatchlings that experienced future temperatures lost more mass over winter than those from the other treatments. These results correspond with previous work indicating that residual yolk does not fuel the energetic needs of hatchlings during winter. The effect of future warming temperatures on body mass may have negative consequences during energetically demanding activities during spring emergence and dispersal.
INTRODUCTION
The effects of warming temperatures on biotic systems are well documented and raise substantial concern about the local and global impacts of climate change (Parmesan 1996; Walther et al. 2002; Blois et al. 2013). The impacts of rising temperatures vary among organisms depending on specific aspects of their physiology, life history, and ecology. For example, increased temperatures over the past several decades have advanced the timing of spring reproduction in plants (Anderson et al. 2012), caused shifts in the onset of migration in many birds (Cotton 2003), and increased energy expenditure of ectothermic organisms (Dillon et al. 2010; Sinervo et al. 2010). These responses to changing temperatures could have negative effects at individual, population, and ecosystem levels. Ectotherms, in particular, are susceptible to these negative effects because most of their physiological functions are temperature-dependent.
While predicted warming due to climate change will vary among different regions of the Earth, evidence indicates that temperature has increased most rapidly in polar regions (IPCC 2007). Organisms at these latitudes may be particularly vulnerable as they have specific adaptations that enable them to cope with relatively cold winter temperatures, which could be maladaptive in warming winter climates. Moreover, increases in summer temperatures may have negative physiological effects that are further exacerbated when organisms continue to experience increased temperatures during autumn and throughout winter dormancy. In many reptiles, for example, summer temperatures greatly affect the thermal environment within nests in ways that influence the development of different morphological, behavioral, and physiological traits of offspring (Noble et al. 2018); these traits, in turn, might have different fitness consequences during early life depending upon the winter thermal environment. Early life stages of most temperate organisms have specific behavioral, morphological, cellular, and physiological adaptations that enable survival under “typical” winter conditions (Storey & Storey 1990; Mohr et al. 2020). However, responses to warming temperatures during winter may have negative consequences on the ability of overwintering animals to survive these challenging environments (Moss & MacLeod 2022).
Most organisms that overwinter in bodies of water or burrow deep underground experience relatively stable temperatures over this time (DeGregorio et al. 2012). However, species that hibernate on land near the ground surface will have greater exposure to rising temperatures during winter, and the level of that exposure could vary considerably among individuals (Packard 1997; Huey et al. 2020). Hatchlings of the painted turtle (Chrysemys picta) are a prime example. The range of this temperate species extends as far north as southern Canada (Ernst & Lovich 2009), and most populations at northern latitudes experience considerable within- and among-year variation in temperature (Weisrock & Janzen 1999; Nagle et al. 2000; Hedrick et al. 2021). Females emerge from their aquatic environments to construct shallow nests (bottom nest depth ranges 6–12 cm) on land where they lay their eggs in late spring and early summer (Janzen & Morjan 2001; Morjan 2003; Refsnider et al. 2013). While females typically choose nesting areas with relatively little canopy cover, shade cover varies among nests and results in substantial variation in temperature (Janzen 1994; Janzen & Morjan 2001; Pruett et al. 2019). Embryos develop over the summer and hatching occurs in late summer or early fall. Rather than immediately emerging from their nest (like many other turtle species), the hatchlings remain inside their shallow nest from late summer through winter, and emergence is delayed until the following spring when they disperse to an aquatic habitat (Gibbons & Nelson 1978; Mitchell et al. 2013). Thus, winter at this early life stage is spent inside a shallow nest that frequently experiences subzero temperatures (Costanzo et al. 1995; Packard 1997; Weisrock & Janzen 1999; Murphy et al. 2020); the hatchlings survive these low temperatures either by supercooling or tolerating some level of tissue freezing (Costanzo et al. 1995). Importantly, while cold temperatures reduce metabolism over winter, the residual yolk that embryos internalize before hatching has traditionally been considered a source of energy needed to sustain the young turtles during the winter months (Congdon & Gibbons 1990; Tucker et al. 1998; Filoramo & Janzen 1999; Costanzo et al. 2008). Despite this traditional perspective, however, some evidence indicates that residual yolk is used mostly prior to winter and is a relatively unimportant energy source during winter dormancy (Muir et al. 2013). In addition, the observation that the amount of residual yolk is similar between fall- and spring-emerging hatchlings supports this claim (DePari 1996). Presumably, rising winter temperature should increase hatchling metabolism, thereby increasing the rate of residual yolk use (Willette et al. 2005) or the use of other sources of energy (e.g. lipids) over this time (Costanzo et al. 2004).
Our primary objectives are to characterize how residual yolk quantity changes during winter in hatchling C. picta and to evaluate the consequences of elevated temperatures on residual yolk, body size, and survival near the end of the winter brumation period. Previous work demonstrates that most metabolic demands during winter of hatchling C. picta are fueled by lipids or carcass reserves, rather than residual yolk (Muir et al. 2013). In line with these findings, we hypothesized that residual yolk reserves contribute little, if any, to maintenance during winter and, therefore, should not change over winter even under increased temperatures. Our first experiment was designed to quantify the effects of three ecologically relevant winter temperatures; while our study follows a similar approach to that of Muir et al. (2013), our experiment differs in that we used fluctuating temperatures (rather than constant temperatures) that reflect thermal patterns from natural nests at our study site, and we use a thermal regime that simulates warmer conditions that approximate future temperatures under a climate change scenario. Our second experiment was designed to document incremental patterns of yolk use and changes in body mass over the course of the season by comparing clutch mates housed under common conditions but sampled at different time points throughout the winter season. If residual yolk does not provide a direct energy source that sustains hatchlings during winter (Muir et al. 2013), we predict that the quantity of residual yolk will remain stable over the season. However, because of increased metabolism under high temperatures, the survival and body size of hatchlings in spring will be lowest for individuals exposed to increasingly warmer winter temperatures.
MATERIALS AND METHODS
Collection and husbandry of hatchling turtles
The hatchling turtles used in this study were obtained from two different projects conducted at the Thomson Causeway Recreation Area in Thomson, Illinois, USA, in the summer of 2009. Details about egg incubation and hatchling collection are described elsewhere (Schwanz et al. 2010; Mitchell et al. 2013), but we highlight relevant information here. We performed two experiments using hatchling turtles collected at different times in 2009.
Experiment 1: effect of winter temperature on residual yolk, body mass, and survival
Hatchling turtles for this experiment (n = 171) were unused individuals from another study (Mitchell et al. 2013). These hatchlings came from eggs that were collected on August 17, 2009, from 40 previously identified nests at our field site (Mitchell et al. 2013). Egg collection was at a time when offspring were close to hatching; indeed, many eggs were pipped at this time, indicating that most of the incubation occurred naturally in the field. All eggs/hatchlings were brought to Iowa State University and unpipped eggs were placed in plastic shoeboxes with moistened vermiculite (−150 kPa) and maintained in an incubator (constant 28°C) where they hatched several days later. Eggs were monitored twice daily for pipping, at which point, we placed a bottomless paper cup over the egg to ensure that we could identify which hatchlings came from which eggs. All hatchlings were weighed and measured (carapace and plastron length) within 12 h of hatching and then housed individually in covered 0.47-L plastic cups containing moist vermiculite.
These hatchlings were housed at room temperature (∼20°C) until October 27, 2009, and then put in incubators set at 15°C. On October 30, hatchlings were weighed and moved to plastic individual cups (473 mL) that contained moist sand (4% water by mass of dry sand). We then gradually turned down the incubator temperature to 8°C by November 4, 2009, at which point hatchlings were moved to one of three overwinter temperature treatments (see below).
A subset of hatchlings (n = 61) were euthanized at the beginning of the experiment (October 30, 2009) to quantify pre-winter relative yolk mass. Electronic balances were used to weigh hatchling turtles (to 0.01 g) and to obtain wet and dry mass of residual yolk (to 0.0001 g). The remaining hatchlings (n = 110) were randomly assigned to three overwinter temperature treatments (Fig. 1). The “low” and “high” treatments approximated overwinter temperatures within nests that we have measured during previous years (Weisrock & Janzen 1999; Mitchell et al. 2013), and the “future” temperature treatment was set to 2–5°C higher than the other two treatments to simulate thermal conditions expected by the end of the 21st century (more details below), which allowed us to quantify the effects that future winter temperatures will likely have on hatchlings. We shifted the temperatures of the incubators about every 50 days to simulate the cooling and warming conditions of natural nests during the early, middle, and late periods of winter in the field. The temperature of the future treatment was determined by adding 3.5°C to 4°C to the mean value of the low- and high-temperature treatment during the early, middle, and late periods of winter; this thermal increase is within the temperature range predicted within the region of our study site (Sinha & Cherkauer 2010; Wuebbles et al. 2021). Fig. 1 shows the temperatures at which each incubator was set for three intervals during winter, as well as the actual temperature profile measured from iButtons (model DS1921G-F5) that were placed inside cups with hatchlings. The hatchlings that were assigned to treatments were weighed and euthanized at the end of the experiment (April 2, 2010) to quantify differences in post-winter yolk mass among temperature treatments.
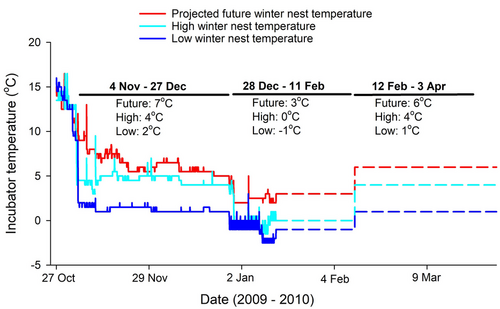
Our future temperature treatment is within the range of the predicted air and soil temperature increase by the year 2100 in Illinois, USA. The average daily air temperature in Illinois is predicted to increase between 2.2°C and 5°C under a low emission scenario and between 4.4°C and 7.8°C under a high emissions scenario by the year 2100 (Wuebbles et al. 2021). The prediction for the low emission scenario in Illinois is similar to a global climate projection under four different socioeconomic and CO2 emission scenarios (Raftery et al. 2017). More importantly, for hatchling turtles that spend the winter underground, soil temperatures at 10 cm depth during the winter season are predicted to increase by about 2–4 °C in the region of our study site (Sinha & Cherkauer 2010). Thus, the temperature of our “future” treatment fits well with these predicted thermal patterns and represents a very plausible estimate for nest temperatures at the end of the century.
Experiment 2: residual yolk and body mass variation over winter
Hatchlings in the second experiment (n = 60; 10 clutches with 6 individuals each) were collected on September 19, 2009, from 10 previously identified nests; these were part of a long-term study on nesting ecology (Schwanz et al. 2010; Janzen et al. 2018). All eggs had hatched by the time of collection, and the hatchlings were taken to the laboratory at Iowa State University. These hatchlings were kept in 473-mL plastic cups that contained a dampened paper towel and were maintained at room temperature (∼20°C); sibling turtles were kept in the same cup together. On November 10, 2009, all the hatchlings were weighed and one individual from each clutch (n = 10 total) was euthanized and dissected, and then internalized yolk was removed and weighed. The remaining 50 hatchlings were placed in individual plastic cups (473 mL) with moistened sand (4% water by dry mass of sand) within the same incubator as those in the high-temperature treatment described above. To quantify incremental changes in internalized yolk mass over winter, one randomly chosen individual from each clutch (i.e. 10 individuals) was sampled approximately every 25–50 days until the end of the experiment (sampling dates were November 29, December 24, January 18, February 12, and April 3). All cups with moist sand were weighed at the beginning of the experiment, and the sand was rehydrated at approximately 1–2-month intervals to maintain constant water content over the duration of the experiment. We also weighed each cup on the sampling dates for each hatchling to quantify sand hydration; cup mass never declined by more than 3.7% (mean = 1.1% ± 1.4% SD), indicating minor fluctuations in water loss from the sand during the experiment.
Hatchlings from both experiments were euthanized by injection of sodium pentobarbital. Hatchlings were dissected and all internalized yolk was removed. The yolk was placed on a weighing tray to measure wet mass, then dried at 48°C to a constant mass (typically 3–4 days later) to measure dry mass. All protocols were approved by the Iowa State University Animal Care and Use Committee.
Statistical analyses
All statistical analyses were performed with the lme4 package in R (Bates et al. 2015; R Core Team 2022). For the first experiment, hatchling survival (scored as alive vs not alive by the end of the study) was analyzed as a binomial dependent variable in a generalized linear mixed model that compared the three winter treatments. General linear mixed models were used to quantify the effect of winter temperature on hatchling body mass, the quantity of residual yolk, and its water content that remained in the turtles at the end of the experiment. These analyses also included the individuals that were dissected prior to the winter treatments, which served as a comparison of residual yolk and body mass before and after winter. The residual yolk was assessed as the dry yolk mass, and this analysis included hatchling body mass as a covariate in the models. The water content of the residual yolk was the difference between the wet and dry mass of the yolk, and wet yolk mass was a covariate in the model. For the analyses of body mass after winter, the initial body mass prior to winter was included as a covariate. In a complementary analysis, we calculated the change in body mass over winter (i.e. the difference in mass at the start vs the end of the experiment) and used this value in a generalized linear mixed model to quantify the effect of temperature treatment on the change in body mass, using initial mass as a covariate. Interactions between temperature treatment and hatchling body mass were never statistically significant and were removed from the models. The clutch was a random effect in all models.
For the second experiment, linear mixed models were used to assess the effect of time (categorical independent variable with six levels: at day 1, 25, 50, 75, 100, 150) and pre-winter body mass (continuous independent variable) on body mass and residual yolk mass over winter. Interactions between the two independent variables were never statistically significant and were removed from the final model. Individuals were not repeatedly sampled since they were euthanized at a pre-determined time during winter, but sibling relationships among clutch mates were accounted for by using the clutch as a random effect.
RESULTS
Experiment 1: effect of winter temperature on residual yolk, body mass, and survival
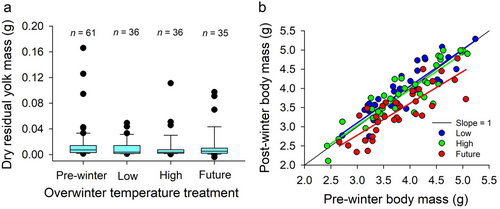
Winter temperature did not influence hatchling survival (93.6% overall survival; P > 0.733 for all comparisons among treatments) or the quantity of residual yolk remaining after winter (F3,121.6 = 1.30, P = 0.278; Fig. 2a). Moreover, the quantity of residual yolk remaining within hatchling C. picta after winter did not differ from that before winter for any treatment (all β < 0.0075, P-values > 0.086). Larger individuals had less residual yolk at the end of winter than smaller individuals (β = −0.012 ± 0.003 SE, P < 0.001), and this pattern did not differ among treatments (F3,120.3 = 0.234, P = 0.873). Hatchlings from the low (β = 0.059 ± 0.05 SE, P = 0.260) and high (β = −0.021 ± 0.05 SE, P = 0.685) temperature treatments at the end of winter did not differ in body mass from those euthanized at the start of the experiment, but individuals from the future treatment that approximated temperatures predicted by 2100 had reduced mass compared to all other treatments (β = −0.351 ± 0.05, P < 0.001; Fig. 2b). Similarly, the decline in body mass over winter was significantly greater in hatchlings from the future winter treatment compared to the low (β = 0.407 ± 0.07 SE, P < 0.001) and high treatments (β = 0.331 ± 0.07 SE, P <0.001). The water content of residual yolk at the end of brumation did not differ among winter temperature treatments or from individuals measured prior to winter (F3,133.27 = 1.425, P = 0.238).
Experiment 2: residual yolk and body mass variation over winter
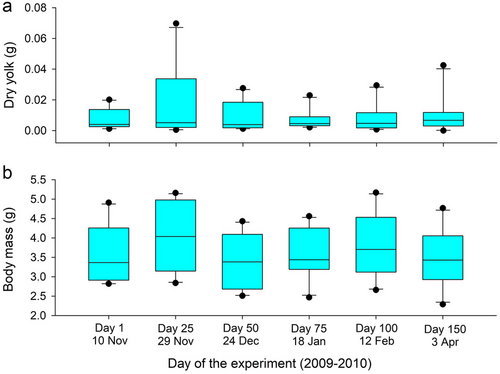
Consistent with the results from the temperature experiment, the amount of residual yolk in hatchlings did not differ across time periods during winter (F5,45.4 = 0.850, P = 0.526). Indeed, the amount of residual yolk in turtles did not differ for any pairwise comparison among time increments (Fig. 3a). Hatchling body mass varied across time increments (F5,45.1 = 6.1; P < 0.001), but there was no discernable pattern, and these changes were small (Fig. 3b); only differences between days 0 and 25 (β = 0.198 ± 0.09 SE, P = 0.033) and days 0 and 50 (β = −0.237 ± 0.09 SE, P = 0.011) were statistically supported.
DISCUSSION
Our primary objective was to quantify the effect of temperature on the yolk metabolism of hatchling painted turtles during winter brumation. We show that temperature did not affect yolk metabolism during winter even when thermal conditions were within the range predicted under a climate change scenario by 2100 (Wuebbles et al. 2021). Indeed, the amount of internalized residual yolk in hatchling C. picta remained relatively constant over winter. Nevertheless, individuals from the treatment that approximates future temperatures lost more mass over winter than those from the other treatments. These results indicate that residual yolk is not directly used to sustain hatchling turtles during winter, and it is likely that other sources of energy will be used to fuel metabolism under warming winter temperatures in the future. These results are consistent with previous work on C. picta showing strong evidence that the metabolism of residual yolk ceases prior to winter and that energy from other substrates is used to sustain hatchlings during winter (Muir et al. 2013). Consequently, projected temperatures could have important consequences on hatchling body mass prior to nest emergence in the spring.
The functional importance of residual yolk varies among taxa. In some reptiles, for example, residual yolk has no effect on offspring growth or survival (Radder et al. 2007; Van Dyke et al. 2011; Guo et al. 2023). Yet, residual yolk positively affects growth rates in other reptiles (Troyer 1983; Pandav et al. 2006) and could benefit other energy-demanding post-hatching activities (e.g. nest emergence, dispersal; Kraemer & Bennett 1981; Troyer 1983). In addition, about 86% of residual yolk is consumed by neonatal red-eared sliders (Trachemys scripta elegans) between hatching and post-winter nest emergence (Tucker et al. 1998), indicating that residual yolk is used at some point during the period when hatchlings remain in their nest; because the study by Tucker et al. (1998) did not distinguish between changes in residual yolk before versus during winter, hatchling T. s. elegans may use most of their residual yolk prior to winter similar to that of C. picta (Muir et al. 2013). Indeed, in C. picta, residual yolk may be most important during the period soon after hatching when temperatures are substantially warmer than the winter conditions used in our experimental treatments. Hatching occurs in August and September, which is a time when ambient temperatures are still quite high, and nest temperatures can reach as high as 33°C at our study site (Murphy et al. 2020). These high temperatures should increase the hatchling metabolism of residual yolk resulting in nearly depleted yolk stores by the time winter begins. For example, a study of C. picta from Nebraska shows that most residual yolk is used by October, well before the onset of winter temperatures (Muir et al. 2013). Our data also show that the average mass of wet residual yolk (0.033 g) at the onset of our treatments (i.e. November) is 81.4% lower than that reported immediately after hatching (0.177 g; Costanzo et al. 2003), indicating that most of the residual yolk is metabolized before winter. Thus, our results are consistent with the interpretation of Muir et al. (2013) that residual yolk is used to sustain hatchlings during pre-winter months rather than during the winter.
Given that most residual yolk is used by the onset of winter, rising temperatures should increase the likelihood of yolk depletion before emergence in spring. Indeed, in a similar study on C. picta, hatchlings exposed to relatively warm winter temperatures had higher metabolic rates, emerged with reduced residual yolk, and had abnormal body conditions (Muir et al. 2013). While our experimental temperature treatments revealed no effect on post-winter residual yolk, the effect reported by Muir et al. (2013) was only evident due to the warmest treatment; the residual yolk from their cold and mild temperature treatments did not differ from each other. Notably, however, their warm treatment was substantially higher than our future temperature treatment, which likely explains the difference between our results. Our warmest treatment that simulated future temperatures was about 5.3°C on average, whereas the warmest average temperature used by Muir et al. (2013) was 15°C; although maximum winter temperatures in nests can reach 15°C at our study site (Murphy et al. 2020), a constant winter temperature of 15°C is substantially higher than what is typically experienced at northern latitudes and much higher than that expected under climate change scenarios. In addition, it is possible that turtles enter a brumation state at the onset of winter and reduce metabolism (or possibly suppress metabolism as demonstrated in other reptiles; Dubiner et al. 2023); this may explain the lack of differences in the quantity of residual yolk observed in our temperature treatments, but active metabolic suppression may not occur at substantially higher temperatures like that used by Muir et al. (2013).
Although residual yolk was unaffected by winter temperature in our study, body mass declined in hatchlings in our future temperature treatment. This pattern may be explained by the increased metabolism of hatchlings under this warmer temperature, but rather than drawing upon yolk stores, hatchlings may have been using other energy reserves to sustain them at these warmer temperatures. Indeed, hatchling C. picta catabolize carcass-derived substrates (e.g. carbohydrates and lipids) to supply energy during overwinter dormancy (Nagle et al. 1998; Muir et al. 2013). Alternatively, reduced mass in our future temperature treatment may reflect water loss from the hatchlings; however, we argue against this possibility because the change in substrate hydration in our experiment was minor (1.1% on average), the water content of residual yolk was not affected by temperature, and previous work shows no change in hydration state of hatchling C. picta over winter (Muir et al. 2013). This reduced body mass, coupled with little residual yolk by spring (Tucker et al. 1998; Muir et al. 2013), indicates that hatchling turtles that experience warming winter temperatures would be in poor condition by springtime, which could affect energy-demanding activities, such as emergence and dispersal from nests (Moss & MacLeod 2022). Indeed, overland dispersal to water around our study site could take 1–9 days (Warner & Mitchell 2013), which could be fueled in part by the remaining residual yolk in spring. Moreover, warming temperatures in the future could plausibly induce early spring emergence (Murphy et al. 2020), which in turn (coupled with reduced body mass) might affect the vulnerability of hatchlings to predation and adverse weather conditions (Paitz et al. 2007; Warner & Mitchell 2013).
Although hatchling C. picta have adaptations to cope with cold winter temperatures, reduced temperatures during winter may be more likely to impact overwinter survival than warming winter temperatures. Hatchlings can survive subzero temperatures by tolerating tissue freezing or by supercooling (Costanzo et al. 1995); these strategies are critical at our study site where temperatures can drop well below freezing and ground frost can penetrate far deeper than the average depth of C. picta nests (Wendland 1997). Even with the ability to survive these cold temperatures, the risk of overwinter mortality, directly or indirectly, increases with decreasing nest temperatures (Weisrock & Janzen 1999; Nagle et al. 2000; Costanzo et al. 2004; Murphy et al. 2020), particularly if nests are not insulated by snowfall (Nagle et al. 2000; Costanzo et al. 2004). Because many reptiles suppress metabolic processes under cold temperatures (Dubiner et al. 2023), if we had reduced winter temperature in our experiment, yolk metabolism likely would not have differed much from that under the treatments used here. However, mortality due to freezing may have increased. Future research that examines the effects of lower and warmer temperatures is warranted (e.g. Muir et al. 2013), as it will help identify critical thresholds of cool and warm temperatures that have energetic and survival costs, and in turn, help to improve predictions of the effects of changing winter conditions (e.g. temperature and snowfall).
Our results indicate that increases in winter temperature due to climate change have no effect on yolk stores during winter, but negatively affect body mass by the time hatchling turtles emerge from their nests in spring. We argue that the greatest impact of warming temperature will likely be during the periods of egg incubation and the pre-winter post-hatching period. Indeed, numerous studies demonstrate strong effects of temperature during embryonic development on a broad range of fitness-related traits in C. picta (Janzen & Morjan 2002; Telemeco et al. 2013; Bodensteiner et al. 2019; Warner et al. 2020) and other ectotherms (Noble et al. 2018; O'Dea et al. 2019). In addition, as noted above, the conditions that hatchlings experience between hatching and winter (i.e. late summer and autumn) should have the largest effect on yolk metabolism, rather than the conditions during winter. Consequently, embryonic and pre-winter environments will likely affect the condition of hatchlings when they enter the winter months, and these negative effects on hatchling conditions will only be exacerbated if winter temperatures continue to rise. Although our study does not assess the relative effects of temperature exposure at these different times of early development, the impact of rising winter temperatures under climate change may depend on environmental conditions experienced at multiple early life stages. Understanding these interactive effects across these different life stages will provide insight into the complex consequences of rising global temperature at organismal and population levels.
ACKNOWLEDGMENTS
We thank R. Alverio, A. Durso, J. Maciel, M. Piñon, A. Reedy, J. Strickland, and J. Ward for field and laboratory assistance. Permission to work at the field site was provided by the US Army Corps of Engineers, Illinois Department of Natural Resources (permit #NH10.0073) and the US Fish and Wildlife Service (permit #32576-0A022). This research was approved by the Iowa State University IACUC (protocol #12-03-5570-J) and supported by NSF grant LTREB DEB-0640932 to F. J. Janzen.




