Microhabitat and adhesive toepads shape gecko limb morphology
Abstract
Different substrates pose varied biomechanical challenges that select specific morphologies, such as long limbs for faster running and short limbs for balanced posture while climbing narrow substrates. We tested how gecko locomotion is affected by the microhabitat they occupy and by a key adaptation—adhesive toepads—through analyzing how those are related to limb morphology. We collected microhabitat and toepads data for over 90% of limbed gecko species, and limb measurements for 403 species from 83 of the 121 limbed gecko genera, which we then used in phylogenetic comparative analyses. Our data highlight the association of adhesive toepads with arboreality, but a phylogenetic analysis shows that this relationship is not significant, suggesting that these traits are phylogenetically constrained. Comparative analyses reveal that pad-bearing species possess shorter hindlimbs and feet, more even limb lengths, and lower crus: thigh ratios, than padless geckos, across microhabitats. Saxicolous geckos have the longest limbs and limb segments. This is probably influenced by selection for long strides, increased takeoff velocity, and static stability on inclined surfaces. Terrestrial geckos have more even hind- and forelimbs than arboreal geckos, unlike patterns found in other lizards. Our findings underline the difficulty to infer on microhabitat–morphology relationships from one taxon to another, given their differing ecologies and evolutionary pathways. We emphasize the importance of key innovation traits, such as adhesive toepads, in shaping limb morphology in geckos and, accordingly, their locomotion within their immediate environment.
INTRODUCTION
Locomotion imposes strong selective pressures on animals (Hildebrand et al. 1985), as the physical environment poses many challenges for animal movement. For instance, living in trees involves climbing on variably inclined surfaces of differing diameters, whereas cursorial locomotion is more common on open ground, which is generally perceived as more dangerous (Lima & Dill 1990; Arnold 1998; Irschick & Garland 2001). Accordingly, each locomotion type requires specific morphological adaptations for better biomechanical performance, for example, longer limbs for faster running, while shorter limbs keep the organism's center of mass closer to the substrate, enhancing climbing ability, particularly on narrow surfaces (Losos 1990a,b; Van Damme et al. 1997; Arnold 1998; Zaaf et al. 1999; Zaaf & Van Damme 2001; Spezzano & Jayne 2004; Kilbourne & Hoffman 2015; Kubo et al. 2019). Substrate specialists are expected to evolve morphologies that facilitate efficient movement in it (Zaaf et al. 1999).
Geckos (Gekkota), alongside the limbless dibamids, are the sister clade to all other lizards (Title et al. 2024). Geckos, the most speciose lizard group, comprise almost 2300 species in seven families (Uetz et al. 2023). In six families, all species have well-developed pentadactyl limbs, while in the seventh (Pygopodidae), all species have strongly reduced limbs or no limbs. Geckos occupy varied habitats worldwide and are versatile in their locomotory technics and morphological phenotypes (Zaaf & Van Damme 2001; Meiri 2019, 2024; Grismer et al. 2021; Norris et al. 2021; Riedel et al. 2024). Habitat preferences of gecko clades are reflected in their elaborate foot structures, that is, the adhesive toepads of many taxa (Peattie 2008; Riedel et al. 2021; though often congenerics occupy different habitats, e.g. Grismer et al. 2020, 2021). Analyzing these foot structures can help associate locomotion-related morphological changes with habitat and microhabitat shifts (Russell 1979; Losos 1990b, 2009).
Many gecko lineages, as well as anoles and a few species of skinks (Scincidae), evolved adhesive toepads, which are a putative key innovation for the evolution of arboreality in some lizards (Russell & Gamble 2019; Miller & Stroud 2022). Adhesive toepads have been acquired and lost several times throughout gecko evolution (Gamble et al. 2012; Russell & Gamble 2019). “Pad-bearing” and “padless” geckos can be closely related but rarely occur in the same genus (notable exceptions being the padless Lucasium damaeum, Chondrodactylus angulifer, and Pachydactylus rangei, all having multiple pad-bearing congeners; Gamble et al. 2012; Russell & Gamble 2019). Unlike other pad-bearing lizards, geckos must use active hyperextension; that is, they curl up their toe tips to disengage their feet from the surface after adhesion (Russell 1975, 2002; Irschick & Higham 2016). This action slows stride frequency and might result in a trade-off between better clinging ability and faster running (Russell & Higham 2009; Higham et al. 2015; Naylor & Higham 2019). Adhesive toepads might be burdensome on nearly horizontal surfaces because they delay the raising of the foot during running owing to the active hyperextension (Russell & Higham 2009). On some surfaces, for example, sand, toepads may become clogged with soil, losing their adhesive efficiency (Bauer & Russell 1991). Befittingly, adhesive toepads were often lost in species evolving a terrestrial lifestyle (Russell 1979; Bauer & Russell 1991; Higham et al. 2015; Grismer et al. 2020; Lajmi et al. 2020).
There are various hypotheses regarding how limb morphology changes with microhabitat. Cursorial animals are expected to have adaptations for increased running speed (Cartmill 1985). Cursorial mammals, for example, horses and antelopes, possess long limbs, especially the foot and distal limb elements (antebrachia, crura). These increase stride length and shift the limb's center of gravity closer to the hip joint, to facilitate leg swing and save energy (Lull 1904; Taylor et al. 1974; Kilbourne & Hoffman 2015; Kubo et al. 2019). Climbing animals, on the other hand, risk falling (Cartmill 1985). Accordingly, some scansorial animals evolved adaptations, such as prehensile limbs and tails (e.g. Khannoon et al. 2014; Spinner et al. 2014), to keep a firm grip on the surface. Others evolved short limbs to keep their center of mass as close as possible to the surface (Cartmill 1985). However, Foster et al. (2018) suggested that longer limbs and a higher fore to hindlimb ratio could improve climbing animals’ ability to evade predators due to increased stride length and static stability. Inhabitants of densely vegetated habitats could benefit from shorter limbs, which reduce the effects of obstructions on movement speed (Foster et al. 2018).
Based on such biomechanical assumptions, several hypotheses have been explored regarding the links between lizard limb morphology and microhabitat. Many studies considered locomotion as either scansorial or cursorial. Ground-dwelling species (henceforth “terrestrial”) are often expected to have relatively short forelimbs, long hindlimbs, and high crus:thigh ratios for more efficient sprinting (Losos 1990b; Sinervo & Losos 1991; Arnold 1998; Zaaf et al. 1999; Zaaf & Van Damme 2001). Conversely, limbs of scansorial species (climbing trees or rocks) are expected to be relatively short and more equal in size with a low crus:thigh ratio, increasing stability while moving on narrow or steeply inclined surfaces (Losos 1990a,b; Arnold 1998; Zaaf et al. 1999; Zaaf & Van Damme 2001; Schwarz et al. 2021). Additionally, fore- and hindlimbs of equal sizes could be important for scansorial lizards to match stride lengths, and potentially increase thrust from the forelimbs, required in upward locomotion (Arnold 1998).
Recently, there has been an increasing recognition that locomotion should be considered at finer scales with regard to the microhabitat type. For instance, transitions to rock dwelling were found to be associated with increased lizard limb lengths (Revell et al. 2007; Grismer & Grismer 2017; Kaatz et al. 2021). Longer limbs increase running speed over open or rocky terrain, whereas dense (as opposed to open) terrestrial or arboreal habitats have been mostly related to shorter, subequal limbs, likely to ease locomotion through dense vegetation (Vitt et al. 1997; Arnold 1998; Melville & Swain 2000; Revell et al. 2007; Goodman et al. 2008; Foster et al. 2018; Norris et al. 2021; Riedel et al. 2024). Similarly, shorter proximal limb segments (brachium, thigh) can be more beneficial for moving in vegetation because they reduce the lateral extent of the limbs that impede movement through complex habitats and may increase maneuverability therein (Bauer et al. 1996; Herrel et al. 2002; Kolbe & Losos 2005; Olberding et al. 2016; Hagey et al. 2017a). In contrast, longer proximal limb segments increase stride length while keeping the body close to the surface in steeply inclined rocky habitats, thus increasing both running speed and stability (Revell et al. 2007). Overall, arboreal lizards have been found to possess relatively shorter hindlimbs than both saxicolous and terrestrial ones (Williams 1983; Vitt et al. 1997; Hagey et al. 2017a; but see Riedel et al. 2024). Long distal hindlimb segments, that is, the crus and hindfoot, are more prevalent in ground-dwelling lizards; they increase stride length and enhance propulsion when running (Lull 1904; Snyder 1954; Losos 1990b; Zaaf et al. 1999; Herrel et al. 2001, 2002). However, the biomechanical advantage of longer feet to increase propulsion might be reduced in pad-bearing geckos because they detach the digit tips first, using active hyperextension (Russell 1975; Zaaf et al. 1999). Accordingly, longer forefeet in pad-bearing geckos have been associated with arboreality, rather than with rock or ground-dwelling (Hagey et al. 2017a). Elstrott and Irschick (2004) showed that anoles perching higher in the canopy have larger adhesive toepads and better clinging ability, which suggests that large feet, which potentially increase the adhesive surface, might improve grip on narrow branches.
Adhesive toepads might thus mediate the relationship between limb morphology and microhabitat. This relationship has mostly been addressed from the perspective of the coevolution of toepads and claws (e.g. Zani 2000; Crandell et al. 2014; Naylor & Higham 2019; Yuan et al. 2019) or clinging ability on variable slopes and perch diameters (e.g. Spezzano & Jayne 2004; Bloch & Irschick 2005; Russell & Higham 2009). Kulyomina et al. (2019) found that padless geckos have longer limbs than those with adhesive toepads, but their study relied on a very small sample of padless species (nine padless species in four families, none of which were arboreal, against 103 pad-bearing species). Many studies (e.g. Vanhooydonck & Van Damme 1999; Zaaf & Van Damme 2001; Higham et al. 2015; Olberding et al. 2016; Foster et al. 2018; Kulyomina et al. 2019; Schwarz et al. 2021; but see Norris et al. 2021; Riedel et al. 2024) largely failed to find a relationship between lizard limb morphology and microhabitat use.
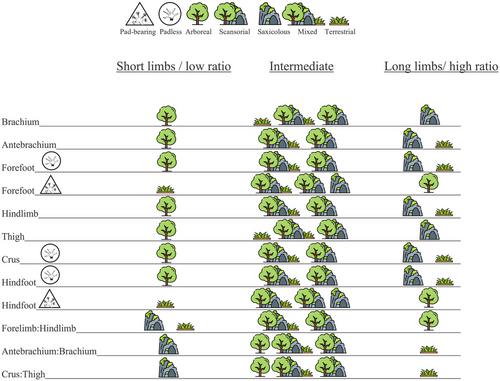
We studied the relative effects of adhesive toepads and microhabitats on limb morphology in 403 species from 83 of the 121 limbed gecko genera worldwide, testing six hypotheses: (1) Because adhesive toepads are a putative key adaptation for arboreality, we hypothesize that they are most prevalent in arboreal geckos and least abundant in terrestrial geckos. (2) If adhesive toepads are associated with arboreality, which requires movement in dense vegetation while keeping the climber's center of mass close to the substrate, pad-bearing geckos will have shorter limbs than padless geckos. (3) Long limbs increase running performance in rocky and open terrains, whereas vegetated microhabitats were associated with short and subequal limbs. Hence, we hypothesize that arboreal geckos have shorter hindlimbs and more even fore and hindlimbs, than saxicolous and terrestrial geckos. (4) Rock climbers increase running performance owing to elongated proximal limb segments and ground dwellers owing to elongated distal segments, whereas vegetated microhabitats may favor short and even limbs. Consequently, we hypothesize that arboreal geckos have shorter and more even limb segments (antebrachium vs brachium; crus vs thigh) than saxicolous and terrestrial ones; saxicolous geckos have long proximal limb segments and low crus:thigh and antebrachium:brachium length ratios, and terrestrial geckos have intermediate proximal limb segments and high crus:thigh and antebrachium:brachium ratios. (5) Similarly, we hypothesize that, among padless geckos, arboreal species have shorter distal limb segments (crura, feet) than geckos from saxicolous and terrestrial microhabitats. (6) While a larger adhesive surface area of the foot could improve clinging ability, active hyperextension may hinder movement on plain surfaces. Therefore, we hypothesize that among pad-bearing geckos, climbers (i.e. arboreal, saxicolous, or both) have longer feet than terrestrial ones, with arboreal species having the longest, and terrestrial species, the shortest feet. In hypotheses 3–6, we expect geckos occupying more than one microhabitat (i.e. not specializing solely on either arboreal, saxicolous, or terrestrial microhabitats) to have intermediate patterns (Fig. 1).
MATERIALS AND METHODS
Data collection
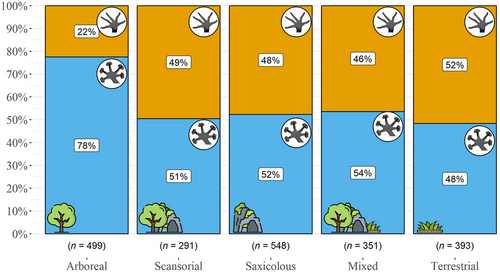
We compiled a dataset of gecko toepads' presence and microhabitat use for 2082 (93%) limbed gecko species (see Supporting Information 1), following the taxonomy in the September 2023 version of the Reptile Database (Uetz et al. 2023). We treated species as either possessing adhesive toepads or not, following Gamble et al. (2012), Hagey et al. (2017b), Russell and Gamble (2019), and personal communication with Daniel Jablonski and Ramamoorthi Chaitanya (2023). We allocated each species a microhabitat, based on the datasets of Meiri (2024) and Riedel et al. (2024). We classified Gekko iskandari and Luperosaurus cumingii as arboreal, following Brown et al. (2000) and Gaulke et al. (2007), respectively. We defined five substrate uses: “arboreal” (i.e. climbing on any type of vegetation; 499 species), “saxicolous” (i.e. climbing of any type of rock; 548 species), “scansorial” (i.e. both arboreal and saxicolous; 291 species), “terrestrial” (i.e. ground-dwelling; 393 species), and “mixed” (terrestrial and either arboreal, or saxicolous, or both; 351 species).
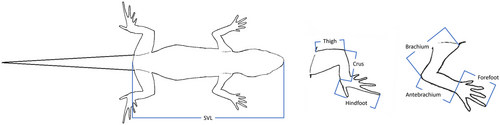
We collected morphological data for 403 of the abovementioned species (87 arboreal, 74 saxicolous, 73 scansorial, 85 terrestrial, and 84 from mixed microhabitats), comprising 83 out of 121 extant gekkotan genera (excluding the legless pygopodids). We collated published morphological data for 366 species and supplemented these with our own measurements for 45 species (eight of which also have data from the literature) of living specimens and ones preserved in the collections at the Steinhardt Museum of Natural History in Tel Aviv, Israel. The leading author measured all specimens, using a digital caliper at 0.1 mm precision. She measured the length of the snout to vent (SVL), brachium (the upper arm, from the shoulder to the elbow), antebrachium (the lower arm, from the elbow to the base of the palm), forefoot (from the tip of the longest finger to the base of the palm), thigh (the upper leg, from the body wall to the knee), crus (the lower leg, from the knee to the base of the heel), hindfoot (from the tip of the longest toe to the base of the heel), forelimb, and hindlimb (calculated as the sum of their segments mentioned above; Fig. 2). See Supporting Information 2 for the morphological dataset.
We used only adult specimens with complete morphological data. We examined whether preservation influences limb and body length, by comparing our measurements of four specimens of Hemidactylus turcicus (family: Gekkonidae) brought to the Steinhardt Museum of Natural History (for an unrelated project; INPA capture permit number 2022/43057) before and after preservation. We conducted Bayesian linear mixed models, designed to account for repeated measurements, to test for differences between live and preserved specimens. We found no differences between these two groups except for thigh length (see Supporting Information 3). Therefore, we assumed that potential differences between live and preserved specimens were minimal, compared with the differences observed across species.
In addition to the eight limb measurements, we calculated the length ratios of crus:thigh, antebrachium:brachium, and forelimb:hindlimb, to test our hypotheses 3–5. While the use of ratios is considered in the literature as an inappropriate practice for controlling for trait allometry with body size (Glazier 2022), the ratios we use here are almost isometric and serve as our variables of interest, rather than as a means of correcting for body size influence on a trait.
Data analysis
For our analyses, we used R software, ver. 4.3.2 (R Core Team 2023). We calculated species-specific means for all morphological traits. To reduce heteroscedasticity and normalize the residuals’ distributions, we log-transformed the measurement data (the ratios of crus:thigh, antebrachium:brachium, and forelimb:hindlimb were normally distributed and thus not transformed). We estimated the phylogenetic signal (Pagel's λ) of each morphological trait using “phylosig” function in “phytools” package (Revell 2012). We fitted a phylogenetic generalized least squares (PGLS) regression model for each limb segment length, or ratio, as the response variable, with microhabitat and adhesive toepads' presence as explanatory variables (N = 403 species). We performed the analyses using “pgls” function in “caper” package (Orme 2018). We tested for normality of residuals and absence of heteroscedasticity, by examining the model diagnostic plots. Pagel's λ of each modeled trait was estimated via the maximum likelihood method. The abovementioned approach enables accounting for phylogenetic dependence among species by adding a phylogenetic distance matrix to the models, based on the most complete time-calibrated phylogenetic tree available for squamates (Tonini et al. 2016). All the 403 species included in the analysis were present in Tonini et al.’s (2016) phylogenetic tree. Many of the species locations in this phylogenetic tree are based solely on taxonomy, which might bias the inferences of comparative studies such as ours (Rabosky 2015). Therefore, we conducted sensitivity analyses, running our models on a sample of species (N = 381) present in a molecular phylogenetic tree by Title et al. (2024). Because many of our hypotheses rely on the evolutionary relationship between adhesive toepads and arboreality, we chose pad-bearing arboreal as the reference category. To compare the effects among the other microhabitats, we repeated the analyses after changing the reference from pad-bearing arboreal to an alternative combination of toepads and microhabitat each time. To account for the allometry of limbs with body size, we added SVL as a covariate to all the models. To control for the expected proportion of type I errors in multiple hypotheses testing, we used the Benjamini–Hochberg procedure to calculate the false discovery rate and adjust P-values (Benjamini & Hochberg 1995). Additionally, we performed a principal component analysis (PCA) of the relative length of the eight limb measurements (as a proportion of SVL). We then used the first two principal components, explaining 90% of the variance, as response variables in a PGLS, with toepads' presence and microhabitat as predictors (see details in Supporting Information 4).
We reconstructed ancestral states of adhesive toepads' presence and microhabitats using the “corHMM” R package (the only package currently capable of ancestral state reconstruction of discrete traits on polytomous phylogenetic trees; Beaulieu et al. 2013). We evaluated models of toepads and microhabitat evolution with different transition rates, via the “corHMM” function, and selected models with the lowest AIC scores. The compared inter-state transition rates comprised alternating rates (i.e. distinct for each pair of states) and constrained rates, defined as both equal to all and symmetrical (i.e. equal within trait pairs but differing between them). States were considered ancestral if their maximum posterior probability (MAP) at nodes, calculated via “corHMM” function, exceeded two-thirds, that is, being at least twice as likely as the next candidate (Slavenko et al. 2022). When no trait reached such posterior probability, we analyzed the results qualitatively, noting the states with the highest probabilities. To ascertain the tempo of toepad and microhabitat evolution, we evaluated the node ages obtained via “tree.age” function in the “dispRity” package (Guillerme 2018). For additional details see Supporting Information 5.
RESULTS
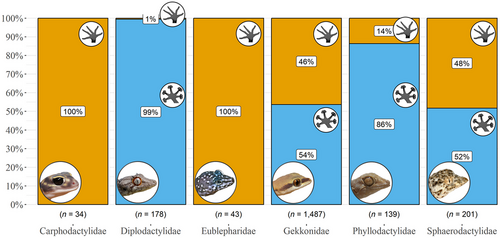
The reported results follow the order of our hypotheses as stated in the introduction. First, adhesive toepads are much more common in arboreal geckos than in species from other microhabitats (Fig. 3; Table S1, Supporting Information 6). However, this pattern turns non-significant once phylogeny is accounted for (Table S2, Supporting Information 6), meaning that the presence of toepads, the microhabitat, or both, are phylogenetically constrained. Nevertheless, while all eublepharids and carphodactylids are padless, both pad-bearing and padless species are present in the Gekkonidae (the largest family of geckos), Sphaerodactylidae, Phyllodactylidae, and Diplodactylidae (Fig. 4). The most recent common ancestor of all limbed geckos (node age = 99.3 Ma) was reconstructed as padless with strong support (MAP = 0.98) and likely terrestrial (MAP = 0.66). Among the most recent common ancestors of each gecko family, pad-bearing ancestors were climbers in either saxicolous or mixed microhabitats, whereas specialization on terrestriality occurred only in padless ancestors (Table S3, Supporting Information 6; Figs S1,S2, Supporting Information 5). Notably, terrestriality appears to be ancestral in the two oldest families, Eublepharidae (node age = 86.7 Ma; MAP = 0.74) and Sphaerodactylidae (node age = 85.3 Ma; MAP = 0.82; Table S3, Supporting Information 6), both of which had padless ancestors (MAP = 0.1 and 0.99, respectively).
There is a strong phylogenetic signal in all measurements (Pagel's λ ≥ 0.92; see Table S4, Supporting Information 6), antebrachium:brachium (λ = 0.73) and the forelimb:hindlimb (λ = 0.74) ratios, but it is much weaker in the crus:thigh ratio (λ = 0.56). Carphodactylids have longer than average forelimbs, antebrachia, and crura, while eublepharids possess relatively short forefeet (relative to SVL; Supporting Information 7). Both families comprise, on average, the largest geckos (i.e. longest SVLs), with no species with SVL below 61 mm in our dataset (Supporting Information 2). Diplodactylids have relatively short hindlimbs and thighs, whereas phyllodactylids possess longer brachia and thighs. The lowest ratios of crus:thigh and antebrachium:brachium are found in phyllodactylids and sphaerodactylids (eublepharids also possess a relatively low antebrachium:brachium ratio, closer to the mean), whereas the highest ratios are in carphodactylids and diplodactylids. In gekkonids, there is high variation in all traits among species. All families have forelimb:hindlimb ratios both lower and higher than the mean, with only the carphodactylids showing a slight tendency for a higher-than-average ratio.
In the PGLS models, geckos lacking adhesive toepads had significantly longer hindlimbs than pad-bearing species, regardless of microhabitat use (P ≤ 0.005, R2 ≥ 0.83; Fig. 5; Table S5, Supporting Information 6), for each of the limb segments (especially the hindfeet; Fig. 6) and for their sum. The forelimbs of padless geckos were not significantly longer than in pad-bearing species (P > 0.05, R2 ≥ 0.73), but their forefeet were significantly longer (P = 0.04, R2 = 0.84; Fig. 5). Pad-bearing geckos thus have more even limb lengths, that is, higher forelimb to hindlimb length ratio, but still lower than one (P < 0.001, R2 = 0.05; Fig. 5). However, the models explained little of the variation in limb ratios; for example, only 10% of the variation in the crus:thigh ratio (P = 0.005), while for the antebrachium:brachium ratio, there was no significant difference between pad-bearing and padless geckos (P = 0.65, R2 = 0.02).
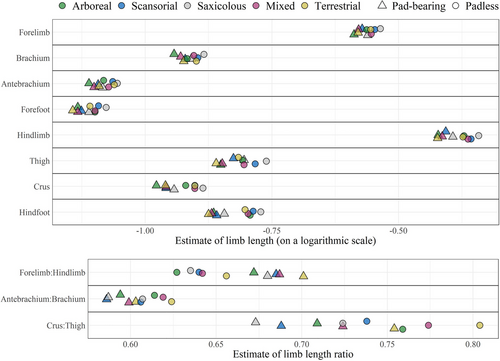
Limb lengths of pad-bearing and padless geckos differ across microhabitats but maintain similar relationships regardless of pad mode (Fig. 5). Within both pad-bearing and padless geckos, saxicolous species have the longest limbs, with the hindlimbs significantly longer than those of geckos from all other microhabitats and forelimbs significantly longer than those of geckos from mixed and arboreal microhabitats. Notably, terrestrial geckos have the highest forelimb:hindlimb ratio (i.e. most even-sized limbs), significantly higher than that of arboreal species. Species from other microhabitats show intermediate ratios, not significantly different from the above extremes (Fig. 5).
The proximal limb segments, thigh and brachium, are generally the longest in saxicolous species, in both pad-bearing and padless geckos. The thighs of scansorial geckos are significantly longer than those of all, except saxicolous species, whereas terrestrial geckos have the shortest thighs (though not significantly different from mixed and arboreal microhabitats). The only significant difference in brachium length is between arboreal (shortest) and saxicolous (longest) species (Fig. 5).
The crus:thigh ratio is highest in terrestrial species in both pad-bearing and padless geckos, lowest in saxicolous species (though not significantly different from scansorial) and intermediate in geckos from arboreal and mixed microhabitats (Fig. 5). There are no significant differences in antebrachium:brachium ratios among microhabitats (Fig. 5).
The longest distal limb segments, crura and antebrachia, in both pad-bearing and padless geckos are found in saxicolous species, but these are significantly longer only from those of arboreal species, which have the shortest distal segments. Species from mixed microhabitats have intermediate crura, significantly longer than in arboreal species, but not significantly different from other microhabitats (Fig. 5). The longest feet (fore and hind) in both pad-bearing and padless geckos are found in saxicolous (though not significantly longer than in scansorial) species, and the shortest hindfeet in terrestrial microhabitats (though not significantly shorter than in microhabitats other than saxicolous; Fig. 5). The PCA and the other sensitivity analyses yielded qualitatively similar results (Supporting Information 4 and Table S6, Supporting Information 6, respectively).
DISCUSSION
Geckos are found in many habitats. About a fifth of the species are terrestrial, a quarter are saxicolous, another quarter are arboreal, and the rest occupy more than one habitat (Meiri 2019, 2024; Grismer et al. 2021; this study). Accordingly, geckos exhibit diverse adaptations for the particular requirements of these habitats (e.g. Russell 1979; Bauer & Russell 1991). A notable adaptation in lizards, which occurs only in geckos, anoles, and a few skinks, is the possession of adhesive toepads (Miller & Stroud 2022). Adhesive toepads enhance clinging ability, which is key for climbing steep surfaces, but at the same time impede locomotion speed, thus constraining cursorial locomotion (Russell & Higham 2009; Naylor & Higham 2019; Miller & Stroud 2022). Accordingly, we found that the presence of toepads was associated with arboreality, but one or both of these traits were also phylogenetically constrained (hypothesis 1; Fig. 3; Tables S1,S2, Supporting Information 6). Reconstructing the ancestral state of toepads for over 1400 gecko species, we found that the absence of toepads is ancestral in geckos and that adhesive pads originated 12 times and were lost 11 times, independently in multiple lineages (Table S3, Supporting Information 6; Fig. S1, Supporting Information 5), in line with previous studies (Gamble et al. 2012). Although the ancestors of the oldest lineages were padless and likely terrestrial (Table S3, Supporting Information 6; Figs S1,S2, Supporting Information 5), many terrestrial geckos, such as the genera Diplodactylus, Rhynchoedura, most Lucasium species, and many Pachydactylus species, have adhesive toepads (yet greatly reduced; Lamb & Bauer 2006; Gamble et al. 2012), while many climbing forms, such as the genus Cyrtodactylus, particularly the fully arboreal C. brevipalmatus group (L.L.G., personal observation), and the fully arboreal Mediodactylus orientalis (U.R. and S.M., personal observation), are padless.
Here, we linked, for the first time, locomotory adaptations in limb morphology of geckos with the microhabitats they use, while taking into account potential differences due to the presence or absence of adhesive toepads (but see Riedel et al. 2024, for a comprehensive analysis of the genus Cyrtodatylus, the most speciose and entirely padless gecko genus). We found that padless geckos have longer hindlimbs than pad-bearing geckos (hypothesis 2; Fig. 5). Such long hindlimbs are probably advantageous for increased running and jumping ability, higher static stability, and lower energy cost of travel (Losos 1990b; Sinervo & Losos 1991; Arnold 1998; Zaaf et al. 1999; Zaaf & Van Damme 2001; Foster et al. 2018). Short and even fore- and hindlimb lengths, on the other hand, can be beneficial both for balance in climbing (Sinervo & Losos 1991; Arnold 1998; Zaaf et al. 1999; Zaaf & Van Damme 2001) and for maneuverability within complex microhabitats (Arnold 1998; Herrel et al. 2002; Kolbe & Losos 2005; Goodman et al. 2008; Olberding et al. 2016; Hagey et al. 2017a; Riedel et al. 2024). While such tradeoffs between different aspects of locomotion, for example, climbing versus running performance, occur in some lizard taxa, they are absent in others. For instance, an association between shorter forelimbs and cursorial locomotion occurs in many lizard clades, for example, Agamidae, Corytophanidae, Anolidae, Iguanidae, Phrynosomatidae, and Teiidae (Snyder 1949, 1954; Losos 1990b; Irschick & Jayne 1998, 1999; Herrel et al. 2001, 2002). In contrast, limb proportions do not differ between cursorial and scansorial locomotion in lacertids (Vanhooydonck & Van Damme 2001), lygosomine skinks (Goodman 2007), and geckos (Zaaf & Van Damme 2001; Higham et al. 2015; Kulyomina et al. 2019). Moreover, while locomotion on broader substrates (e.g. wide branches) was associated with longer limbs in anoles (Kolbe & Losos 2005), the opposite was found for geckos (Hagey et al. 2017a; but see Riedel et al. 2024). Such complexity of morphology–microhabitat relationship is highlighted by Johnson et al. (2005), who suggest that the observed morphology in the genus Pachydactylus (family Gekkonidae) may be more related to evolutionary history than to their locomotory mode. Consequently, limb morphology patterns across microhabitats might differ significantly between geckos and other lizard clades, owing to their distinct ecologies. For instance, unlike other lizards, most geckos are nocturnal (over 70% of all geckos vs less than 10% of other lizards) or active at dim light conditions (Röll 2000; Gamble et al. 2015; Meiri 2019); therefore, inferences from diurnal lizards might be less relevant for geckos. Indeed, our findings did not support the predicted adaptations for climbing (hypothesis 3): Terrestrial species had more even fore- and hindlimbs than climbing geckos, with arboreal species exhibiting the lowest forelimb:hindlimb ratios (Fig. 5). The differences between arboreal and terrestrial species were significant and consistent across analyses even if they only explained a small portion of the variation in the forelimb:hindlimb ratio (R2 = 0.05). The benefit of extended vision range for terrestrial species, resulting from an elevated locomotory posture on subequal elongated limbs (Werner & Broza 1969) may explain this result. The greater visual range might be adaptive for nocturnal geckos as well, some of which exploit the moon (or artificial) light for the detection of potential prey and predators (Nordberg & Schwarzkopf 2022). Additionally, some nocturnal geckos were found to have lower maximum sprint speeds than diurnal ones, regardless of light conditions (possibly as an adaptation for the reduced spatial and temporal visual acuity at dim light; Higham & Schmitz 2019), which might suggest that selective pressure on limb length for running speed is weaker in nocturnal species.
Our results for limb and segment lengths highlight the hypothesized differences between the more vegetated, arboreal, and the less vegetated, saxicolous, habitats (hypotheses 3–6). Saxicolous species had the longest limbs and limb segments, significantly longer than in arboreal geckos (Fig. 5; Table S5, Supporting Information 6). Scansorial geckos had intermediate limb lengths, though only their thighs were significantly shorter than those of strictly saxicolous species and longer than those of strictly arboreal species. Additionally, although arboreal geckos did not differ much from those in microhabitats other than saxicolous, they possessed significantly shorter crura than those in mixed microhabitats (Fig. 5; Table S5, Supporting Information 6), in accordance with hypotheses 4 and 5. While short, evenly-sized limbs can improve arboreal lizard balancing on perches narrower than the body (e.g. narrow branches; Cartmill 1985; Losos 1990b; Van Damme et al. 1997; Arnold 1998; Zaaf et al. 1999; Zaaf & Van Damme 2001; Spezzano & Jayne 2004), this adaptation might be less relevant for geckos, which are on average smaller than other lizards (Meiri 2019), or for climbers on broad substrates, such as tree trunks or rocks (Williams 1983; Spezzano & Jayne 2004). However, a large lateral extent of the limbs can interfere with the lizard's movement through dense vegetation in arboreal and some terrestrial microhabitats, thus possibly reducing the benefits of long limbs for low-energy locomotion (Herrel et al. 2002; Kolbe & Losos 2005; Olberding et al. 2016; Hagey et al. 2017a; Riedel et al. 2024). In contrast, in inclined saxicolous habitats, longer proximal limb segments, which are kept mostly parallel to the surface in sprawling gait, could allow for increasing stride length while keeping the body close to the surface during climbing (Revell et al. 2007; Grismer & Grismer 2017). Furthermore, overall long limbs can save energy and increase jumping distance (Emerson 1985; Toro et al. 2004), which might be advantageous for predator escape behavior. We suggest that increased limb length in saxicolous geckos might be driven by several mechanisms: first, the absence of constraints associated with narrow substrates (i.e. thin branches) and complex habitats (i.e. dense vegetation); second, longer limbs may increase stride length, and thus locomotion speed, and enable low energy travel; third, longer limbs can increase takeoff velocity, thus increasing jumping distance; fourth, long limbs kept close to the substrate can increase climber's static stability. This way, elongated limbs, in combination with a flattened body structure, can improve sprinting and climbing ability of saxicolous geckos, for example, the padless Cyrtodactylus eisenmanae and C. grismeri (Grismer & Grismer 2017) and the pad-bearing Ptyodactylus dhofarensis and P. orlovi (Nazarov et al. 2013).
While there are clear differences in limb length between saxicolous and arboreal geckos, other potential differences between microhabitats remain obscured due to the data resolution. Microhabitat categories could be defined on a finer scale, based on soil type (e.g. firm ground vs fine sand), rock type (e.g. karst vs granite), vegetation type (e.g. trees, shrubs, grass, or no vegetation), perch diameter (e.g. tree trunk vs crown vs twigs), and incline (steep vs planar) (e.g. Williams 1983; Russell & Higham 2009; Olberding et al. 2016; Grismer et al. 2020; Riedel et al. 2020; Grismer 2021; Riedel et al. 2024). Such fine-scale categorization might produce clearer patterns of limb lengths across microhabitats, especially between terrestrial microhabitats of varying complexities, for example, open versus densely vegetated. However, such fine-scale microhabitat data are missing for most gecko species, and a special data collection effort should be made for future inquiries, for example, for the presence of anole-like “ecomorphs” (Williams 1983; Beuttell & Losos 1999; Grismer et al. 2020, 2021).
Our results indicated a strong relationship between microhabitat type and hindlimb proportions, a relationship notably absent in forelimb proportions (Supporting Information 8, Fig. j). Similarly, the forelimbs, brachium, and antebrachium lengths and their proportions did not significantly differ between pad-bearing and padless geckos (Table S5, Supporting Information 6). Hence, we suggest that the selection of forelimbs in geckos may be relatively weak. By contrast, longer distal hindlimb segments can be particularly beneficial for movement on plain terrestrial surfaces (Arnold 1998). This can also be true in pad-bearing geckos, for example, Tarentola mauritanica, whose adhesive toepads are inactivated on horizontal substrates (Russell & Higham 2009), thus seemingly neutralizing the speed limitation imposed by active hyperextension when releasing the toepads from the substrate at each pace. This could explain our finding that terrestrial geckos have the highest crus:thigh ratios, while saxicolous the lowest, regardless of adhesive toepads' presence. This result partially supports our hypotheses (4 and 5) that longer distal segments are an adaptation for longer stride size in locomotion over land, while longer proximal segments are an adaptation for rock climbing.
Long feet can be advantageous for pad-bearing geckos, for increasing the adhesion surface, and enhancing clinging capacity (Elstrott & Irschick 2004). Padless geckos, on the other hand, strongly rely on their claws for climbing steep surfaces (Zani 2000; Naylor & Higham 2019). However, claws alone might be insufficient for maintaining a firm grip, especially when shifting the body position during locomotion. For example, while some padless karst-dwelling Cyrtodactylus geckos can walk completely inverted using claws only, granite-dwelling Cyrtodactylus geckos cannot even hang on steep surfaces (L.L.G., personal observation). Therefore, long feet in padless climbing species might increase their foot area and thus enhance grip strength on steep surfaces due to higher frictional force (Cartmill 1985; Fuss & Niegl 2012). Increased friction with the substrate can be especially important for longer-legged climbing species, for example, chameleons which often have their center of mass farther from the substrate during locomotion (e.g. da Silva & Tolley 2013; Khannoon et al. 2014; Spinner et al. 2014). Therefore, hypothesis 5, according to which padless geckos from arboreal habitats have shorter feet than those from less vegetated microhabitats, was partially supported by our results, with saxicolous geckos having significantly longer feet than those from all microhabitats (except scansorial), but arboreal and terrestrial geckos have feet of similar length. Additionally, there was partial support for hypothesis 6 that climbing pad-bearing geckos possess longer feet, with saxicolous, rather than arboreal, species having the longest feet, while all geckos, except saxicolous, having feet of similar length (Fig. 5). Accordingly, we suggest that long fore- and hindfeet could be useful for rock climbers regardless of the presence of adhesive toepads.
Some cursorial lizards reach a high running speed at a lower energetic cost by employing digitigrade foot posture, reducing contact with the ground, and increasing stride length (Irschick & Jayne 1999). Other species might reduce contact with the surface by running on their hindlimbs only (e.g. Clemente et al. 2008), or by having smaller feet, which may be favored for additional reasons discussed below. Gecko's preferred body temperatures are low relative to other lizards, regardless of time of day or night (Autumn et al. 1999; Meiri 2019); consequently, desert-dwelling geckos, such as the genera Nephrurus, Stenodactylus, and Teratoscincus (Bauer & Russell 1991), might also benefit from small feet for reducing contact area with hot soil (Mosauer 1935; Huey & Pianka 1977; Stevenson 1985; though they might not emerge if the surface is too hot). These genera, among other desert-dwelling geckos, have evolved special scale structures on their palms and digits, as well as swollen plantar surfaces, that prevent sand from clogging their feet (Russell 1979; Bauer & Russell 1991). Other sand-dwelling lizards use comparable foot adaptations for burrowing (Lamb & Bauer 2006; Zheng et al. 2020), and some geckos, such as Pachydactylus rangei, possess webbed feet, which increase feet surface area without increasing their length and serve as an adaptation for burrowing and locomotion in dunes (Lamb & Bauer 2006). Regardless of such adaptations, while increased foot surface area can reduce large animals’ sinking while moving on loose soils by widening weight distribution, long feet might not be of such value for geckos, which can avoid sinking in the substrate due to their light body mass (Bauer & Russell 1991). In summary, running speed does not seem to be the sole or even the main driver of foot length evolution in geckos.
A potentially interesting aspect, which was not incorporated in this study, is sexual size dimorphism. Male and female lizards can behave differently during the breeding season. In some species, including geckos, males defend a breeding territory from competing males, whereas females do not (Petren et al. 1993; Briggs 2012; Campo & García-Roa 2014; Irschick & Higham 2016). As a result, in such species, there could be a selection for males that can run faster to confront other males and banish them quickly from their territory; accordingly, males would have longer limbs than females (Olberding et al. 2016). Hence, future studies could address species’ ecology to uncover such patterns.
Finally, we based our analyses on a dichotomy of pad-bearing versus padless species, but we lacked sufficient data to address additional adaptations for climbing, such as claw length and shape, adhesive toepad surface and microstructure (and thus clinging performance), and prehensile or adhesive tails (e.g. Bauer 1998; Griffing et al. 2021). Claws have an integral role in climbing and were found to have varying impacts on clinging performance, based on their length, shape, and substrate type, in both pad-bearing and padless geckos (Zani 2000; Crandell et al. 2014; Naylor & Higham 2019; Yuan et al. 2019). The same rationale can be true for adhesive pad size and structure (e.g. Russell & Gamble 2019) and for prehensile or adhesive tails (e.g. Bauer 1998). Therefore, future comparative studies should consider the size and structure of both claws and adhesive mechanisms, once such data are largely available.
CONCLUSION
Analyzing over 90% of limbed gecko species, we highlight the association of adhesive toepads with arboreality. However, adhesive toepads are phylogenetically constrained, which reduces the significance of this relationship. Our large-scale analysis of most gecko genera reveals that pad-bearing species possess shorter hindlimbs and feet, more even limb lengths, and lower crus:thigh ratios than padless geckos, regardless of the microhabitat they occupy. Notably, our results emphasize the long limbs of saxicolous geckos versus all other microhabitats. Nevertheless, our findings reveal a complex pattern of the relationships between microhabitat and gecko limb lengths, with some relationships being concordant with findings for other lizard clades, while others being either insignificant or reversed. This may imply that adaptations to seemingly similar needs can vary among species with different ecologies and evolutionary histories. Hence, future macroecological studies on lizard locomotion across various microhabitats should incorporate key innovation traits, such as the presence of adhesive toepads, species’ ecology, and phylogenetic affinities.
ACKNOWLEDGMENTS
The authors would like to thank Karin Tamar for the help and advice on measuring geckos and using the Tel Aviv University collections in the Steinhardt Museum of Natural History; Gal Ribak, Shahar Dubiner, and Noa Halevy for reviewing early drafts of the manuscript; Daniel Jablonski for providing high-resolution photos of specimens and advice on genera Altiphylax and Alsophylax; Ramamoorthi Chaitanya for advice on genus Dravidogecko and discussion of the manuscript; Guy Haimovitch, Hai Ngo Ngoc, Jonathan Ben Simon, Jules Farquhar, Salvador Carranza, and Simon Jamison for providing high-resolution gecko photos for Fig. 4 and for the graphical abstract. This work has partially been funded by the United States-Israel Binational Science Foundation (No. 2021030 to S.M. and U.R.) and the Australian Research Council (LP170100012 to S.M. and D.G.C.; FT200100108 to D.G.C).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the supporting information of this article.
All applicable institutional and national guidelines for the care and use of animals (Hemidactylus turcicus) were followed (INPA capture permit number 2022/43057).




