Mountain gazelles’ (Gazella gazella) males use mutual dung middens in favorable locations
Graphical Abstract
INTRODUCTION
Dung middens (also dung hills, dung latrines, stud piles, or fecal piles) are spatially fixed points where animals regularly deposit urine and feces. Most of the animals that are known to construct dung middens are odd- and even-toed ungulates (Walther et al. 1983). They range in size from the Kirk's dik-dik, Madoqua kirkii (Ono et al. 1988), to the white rhinoceros, Ceratotherium simum (Marneweck et al. 2018). Yet, this behavior has also been observed in carnivores (Darden et al. 2008; Rodgers et al. 2015), as well as primates (Eppley et al. 2016). In antelopes, middens are constructed mostly by males (in dik-dik and klipspringer, Oreotragus oreotragus, both sexes deposit in the midden, Macdonald 1984). A communal form of social depositing was found in several species; for example, in the European badger, Meles meles, and the white-footed sportive lemur, Lepilemur leucopus (Charpentier et al. 2008).
There have been several hypotheses explaining the purpose of constructing dung middens: to serve as an information center (Rodgers et al. 2015; Eppley et al. 2016), to announce estrous in females (Marneweck et al. 2017), to over-mark and display superiority (Marneweck et al. 2018), and to mark a territory to be defended (Wronski et al. 2013). Middens that are deposited at the edge of the territory are often thought to mark the territories' boundaries, such as displayed by the oribi, Ourebia ourebi (Brashares & Arcese 1999a).
While dung middens are often associated with territory markings, there has not been a consensus on how to define a territory. For example, in a 1935 paper that referred to birds, Mayr (1935) debated this question at length and eventually defined a territory as “an area occupied by one male of a species which it defends against intrusions of other males of the same species and in which it makes itself conspicuous.” When it comes to ungulates, a territory might hold other definitions. For example, Walther et al. (1983) define a territory for Antiloinae and likely for some other ungulates as “a place in which an animal lives for a variable period of time and around which that animal has established a subjective boundary.” They further suggest that “The territorial status of the owner as well as the existence of the territorial boundaries are indicated by the behavioral symptoms,” such as “intolerance of, or at least dominance over conspecifics the same sex within the boundaries…” Bowyer et al. (2020) recently divided territoriality into three types: pair, lek, and polygynous resource.
In many antelopes, the resource defense polygyny theory of midden depositing seems to be the most appropriate explanation, including for the oribi (Brashares & Arcese 1999a; Ezenwa 2004; Wronski et al. 2013). According to this theory, polygynous males will deposit in dung middens to mark a territory that they will defend; this territory, presumably a high-resource area, may attract females and thus increase the potential of these males to mate with more females and increase their fitness. Yet, there is insufficient data to support this explanation for midden depositing. To provide evidence for the resource defense polygyny theory, data should show, for example, that the deposits in the midden honestly announce and reflect the quality of the defecating male, as has been demonstrated in the glandular secretions of the white-footed sportive lemur (Charpentier et al. 2008). Another line of reasoning is that the territory marked by the polygynous male increases its fitness; this was recently found for a grassland songbird (Nelson et al. 2020). In both scenarios, the territory should encompass resources that are defendable and sufficiently worthy for the territory holder to expend effort to defend it; for example, in the Japanese serow, Capricornis crispus (Kishimoto & Kawamichi 1996).
The mountain gazelle (Gazella gazella), distributed in the Levant and the Arabian Peninsula (Wronski et al. 2010), is socially grouped into territorial adult males; female groups of various sizes, with or without juveniles; and groups of bachelor males (Baharav 1974; Grau & Walther 1976; Dunham 1999). Mountain gazelles are considered unimale polygynous, whereby a single male defends a core area with several females (Dunham 1999), using dung middens to mark its territory (Walther et al. 1983). However, gazelle territories have never been related to high-resource patches, as might be assumed by their territorial behavior and the resource defense polygyny theory described above. We, therefore, examined this hypothesis using the locations of dung middens assigned to specific individuals in a semi-natural setup, where resources are distributed unevenly. We associated specific dung middens with specific haplotypes that mark territories, using DNA markers. We hypothesized that dung middens would be clustered in high-quality patches, and they would increase in number toward the breeding season; only males would deposit in middens; and a midden would be used by a single male.
MATERIALS AND METHODS
Research site and experimental structure
The study was performed at a long-term ecological research (LTER) site in the Kedoshim Forest, Israel in 2016–17 (further details in Supportive Text S1, Supporting Information).
We selected an area of approximately 30 ha of mature planted Pinus halepensis forest for the study. The forest area was uniform in terms of tree age (40 years) and management history. A grid of 70 × 70 m2 (0.5 ha) was laid out over the entire research area (30 ha), defining 23 plots representing continuous and homogeneous forest cover. Each plot was randomly assigned to one of the following pine-thinning treatments: (i) clear-cut (0 trees ha−1); (ii) intensive thinning (100 trees ha−1); (iii) moderate thinning (300 trees ha−1); (iv) no thinning (control: ≈ 560 trees ha−1) (see Supportive Text S2, Supporting Information for more details). Thus, the experimental design comprised four thinning treatments, each with five to six replicate 70 × 70 m plots serving as the basic experimental unit (Fig. S1, Supporting Information).
Do dung middens cluster in high-quality patches and increase in number as the breeding season approaches?
We constructed a map of grouped dung pellets (to account for the total activity distribution pattern of the gazelles in the site; we did not distinguish between male [non-territorial] and female dung pellets) and dung middens by surveying on foot all the plots and the areas beyond (up to 100 m from the forest edge) in each of the 3 months (May, August, and October) (see Supportive Text S3, Supporting Information). We collected three to four single fecal pellets from each of the midden piles, stored them in an Eppendorf tube on ice, and then put them into storage in a −20°C freezer for later genetic analysis of haplotypes.
To evaluate whether the distribution of dung middens was affected by the total number of dung pellets in the study area (a proxy for the activity of all individuals and their preferred plot use), we fit a linear mixed model (lme4 R package; Bates et al. 2015) to the data, while also taking into account the treatment (tree thinning) and season (See Supportive Text S4, Supporting Information).
Identified gazelle haplotypes (see genetic analysis section) and the middens were uploaded as layers to GIS with ArcGIS 10. 2 (ESRI). We used the same software to calculate the territory's sizes and the degree of overlap among them. The minimum putative-territory size was calculated using the polygon formed by a line connecting all the peripheral middens of a single haplotype (Minimum Bounding Geometry–Convex Hull).
The number of middens deposited and the number of haplotypes identified in middens at each of the forest-thinning treatment plots were both compared using one-way ANOVA (Analysis of variance) with thinning treatment as the explanatory variable for both (see Supportive Text S5, Supporting Information). We further established the sex of the individuals deposited in the middens, by monitoring some of the dung middens (randomly chosen) with six trophy trap cameras (see Supportive Text S6, Supporting Information).
Is a midden used by a single male?
In middens where there was clearly more than one pile of feces, we sampled each pile separately and stored them as described above until genetically analyzed for haplotypes. Only fresh (based on color and shape) feces were collected.
Genetic analysis
For DNA extraction, we used a Qiagen kit, according to the manufacturer's instructions (QIAamp Fast DNA Stool Mini Kit). We assessed the genetic diversity using five microsatellite markers that were adapted from cattle, Dorcas gazelle (Gazella dorcas), sheep, and goats (see Supportive Text S7 and Table S1 in Supporting Information for further details).
RESULTS
Genetic typing
We found a total of 93 active dung middens at our research site (65 in May, 54 in August, and 69 in October; most dung middens were repeatedly used throughout the year). Based on the genetic analysis (see more details in Supportive Text S8, Supporting Information), we were able to identify 8–11 different haplotypes of males at the research site.
Do dung middens cluster in high-quality patches and increase in number as the breeding season approaches?
The distribution of the middens was not uniform. Spatial autocorrelation analysis found a positive autocorrelation between the thinning treatments and the distribution of the dung middens (Moran's I Index = 0.68, Z = 4.684, P = 3×10−6), indicating a clumped distribution. The average number of middens (active and inactive) in the clear-cut plots was 1 ± 0.45, whereas in the 100 trees ha−1, 300 trees ha−1, and 560 trees ha−1 (control) plots, the average number of middens was 2.7 ± 0.33, 2.7 ± 0.53, and 3.2 ± 0.57, respectively (one-way ANOVA: F3,19 = 5.04, P = 0.01, Fig. 1a; Fig. S2, Supporting Information). The thinning treatment had a significant effect on the number of active middens (two-way ANOVA: F11,68 = 2.202, P = 0.027). We found no interaction between season (month) and the number of active middens in the different thinning-treatments plots.
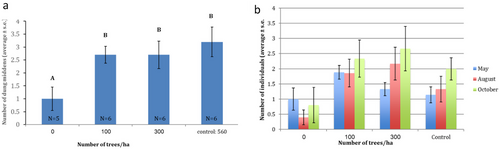
A similar trend, though not supported statistically, was found in the number of identifiably different haplotype markings in those dung middens. In all three seasons, there is a trend for more individuals to deposit middens in the 100 and 300 trees/hectare plots, fewer in the control, and even fewer in the clear-cut plots (Fig. 1b).
The number of dung middens was significantly affected by the number of group pellets (a proxy for the activity of all individuals and their preferred plot use), the treatment, and their interaction (Type II Wald test applied to the results of the linear mixed effects model: χ2(1) = 7.99, P = 0.004; χ2(3) = 8.67, P = 0.033; and χ2(3) = 12.54, P = 0.005, for the number of group pellets, treatment, and their interaction, respectively; Fig. S3 and Table S2, Supporting Information). By contrast, the season did not have a significant effect on the number of dung middens. An interaction analysis of the linear mixed model revealed that the effect of the number of group pellets on the number of dung middens did not differ between the two medium thinning treatments (100 and 300 trees ha−1), though the number of dung middens was consistently higher at 100 trees ha−1 compared to 300 trees ha−1. The clear-cut (0 trees ha−1) treatment had both the smallest amount of group pellets and the lowest number of dung middens. The effect of the number of group pellets on the number of dung middens was strongest for the control treatment (560 trees ha−1), though the overall number of group pellets was much smaller compared to that in the two medium (100 and 300 trees ha−1) thinning-treatment plots (Fig. S4, Supporting Information).
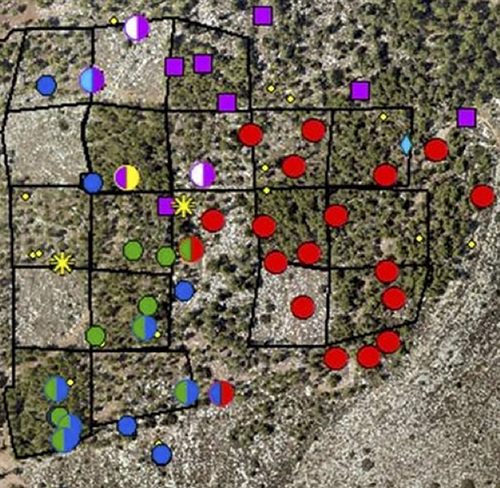
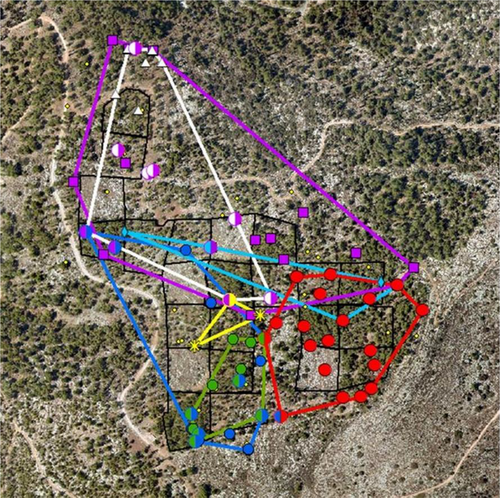
Usually, the over-marked middens (having more than one pile of feces) were found at the border between territories (Fig. 2; see also Figs S4–S6, Supporting Information). Not all individuals appeared to hold territories in the area throughout the year. Moreover, the size of the territories changed over the year and seemed to be increasing approaching October (although it was not statistically significant).
Do only males deposit in middens
In all the camera-shot events (about 240), only males and no females were observed to deposit feces in the middens. Females were observed to approach the dung middens between two and four times a week, smell them, and then move on. Some females slept next to the dung middens. Other species that approached the middens and smelled the deposit were the golden jackal (Canis aureus, N = 8), red fox (Vulpes vulpes, N = 3), dog (Canis lupus familiaris, N = 2), and striped hyena (Hyaena hyaena, N = 2).
Is a midden used by a single male?
We found that some of the dung middens were marked within a short period of time (within 1 or 2 weeks) by more than one individual. Those middens had a clumped distribution (variance/average, I = 1.86). The proportion of these over-marked middens increased from spring to summer to autumn, based on both conservative (difference of three bases or more between alleles) and conventional (difference of two bases or more between alleles) calculations (Fig. S7, Supporting Information).
DISCUSSION
The mountain gazelles in this study showed a clear preference for marking forested patches and avoided marking open patches, where there were shrubs and herbs but no tree cover (Fig. 1a; Figure S2, Supporting Information). Mountain gazelles’ habitat consists of plains and hills with herbaceous plants, shrubs, and undergrowth. They are not common in dense forests or chaparral (Macdonald 1984; Mendelssohn & Yom-Tov 1999; Yom-Tov 2016); therefore, we were surprised to find such a clear affinity for the forested patches. Using an exceptionally polymorphic locus, we were able to identify 8–11 male haplotypes and thus to find out that more individuals deposited in the plots with 100 and 300 trees per hectare than in the control (560 trees per hectare) and in the clear-cut patches (Fig. 1b). Apparently, mountain gazelles carefully choose where to establish their dung middens (Attum et al. 2006). We suggest that the thinned forest patches of 100 and 300 trees ha−1 (LAI ≈ 1–1.6) provide shade, which is an important resource for mammals in the Levant, especially during the hot summer months. These patches may also have an advantage over the non-thinned patches, where tree density is likely overly dense for mountain gazelles to roam. It is also possible that the preference for forest patches of all gazelles, males, and females (Fig. S3, Supporting Information), is affected by the danger of predation.
Marking activity increased from May to October (Fig. 1b; Fig. S7, Supporting Information), the onset of the mating season of the mountain gazelle in this geographic location (Baharav 1983; in some other locations, mountain gazelles may breed throughout the year, Dunham 1999). A similar pattern was found for the Chinese water deer, Hydropotes inermis, where a higher deposit rate was observed during the mating season (Sun et al. 1994). The increase in deposit behavior at the onset of the breeding season, along with the positive correlation of depositing dung middens in the preferred roaming patches of all the gazelles in the site, and the use of some dung middens by more than one male, provide strong support for associating the dung-midden deposits with the resource defense polygyny (Bowyer et al. 2020). The gazelles perceive the 100 and 300 trees ha−1 patches as resources worth defending because they will potentially increase their fitness, as would be expected from territorial species (Petrie 1984). Another piece of supporting evidence for the view that dung middens are signs of territories is that many of the middens that were double-marked were found at the boundaries of the areas where males deposited in middens (Fig. 2). The mountain gazelles deposited in middens both within and on the periphery of their territories, similar to the pattern of midden depositing of the black rhinoceros, Diceros bicornis (Tatman et al. 2000) and oribi (Brashares & Arcese 1999b), but unlike that of mountain gazelles roaming the desert of the Negev (Walther et al. 1983), or that of the Chinese water deer, where marking occurred more along the borders than inside the territories (Sun et al. 1994). This difference in spatial depositing patterns, even within the same species, may depend on the habitat. In this study, the gazelles occupied a forest habitat where peripheral middens will not be as effective as in open spaces to mark the territories, thus the advantage of marking the periphery as well as the core of the territory.
It has been proposed that double-marking helps to maintain group cohesion in social ungulates (Pluháček et al. 2019). However, scent over-marking has also been proposed as a way to mark territories and as a display of dominance (Ferkin 1999; Hurst & Rich 1999). Over-marking in ungulates has been described in the oribi (Brashares & Arcese 1999b) as a pattern in which males over-mark females' urine and feces. Recently, Apps et al. (2022) showed that African wild dogs (Lycaon pictus) share a scent-marking latrine at the borders of their home ranges. The present study shows an ungulate's male-over-male marking that can be attributed to specific individuals at what appears to be a territory border in preferred patches. We cautiously use the term “territory border” since like other studies using this term, we did not show that gazelles have been engaging in active defense along these specific boundaries (which could be a wide border stripe, rather than a border line, Walther et al. 1983); however, we bring support that allows us to infer, based on their dung-midden distribution, that the middens deposited on the periphery mark a territory border.
The results show that some males deposited in more middens than others (e.g. the haplotype marked red in Fig. 2). This may be a sign of superiority as well as the need to defend a highly valued patch. Similar observations and explanations were offered for the common duiker, Sylvicapra grimmia (Lunt & Mhlanga 2011), where prime males were found to defecate more than females and more than other males. Indeed, it appears that the red haplotype in this study holds the largest area of preferred habitat (5 plots of 100 and 300 trees ha−1). Interestingly, none of the shared middens were found in the less-favored habitats—a finding that further supports a resource defense polygyny theory (Emlen & Oring 1977).
In this study, we used the minimum convex polygon to delineate the gazelles’ territories based on their active dung middens (Fig. 2). Therefore, the overall picture of males’ territories seems to be complex: some territories are exclusive, and some seem to be overlapping or even engulfed by other territories. These results might suggest that territories were still not fully established in October. Another explanation that cannot be ruled out is that two males had the same haplotype. Since the studied population showed a relatively high level of inbreeding (F > 0), relatives (e.g. brothers) may show the same haplotype. Furthermore, we cannot rule out that the mountain gazelle's dung middens, and especially the double-marked middens, may have other purposes than border markings.
The territorial males are the guarantors of reproduction, whereas the territorial behavior is adaptable to environmental conditions (Walther et al. 1983) and specific habitats. We conclude that unlike many antelopes, which prefer open plains and short grass areas, the mountain gazelle not only visit artificially planted pine forests (Mendelson 1974), but thinned pine forests may also provide a preferred habitat for mountain gazelle reproduction, an important factor when considering forest management.
ACKNOWLEDGMENTS
We thank Avi Bar-Masada for his help with the statistics, Lior Hasid for software assistant, Chanoch Zoref for on-ground help, and the staff of Beit Margolin Oranim. Partial funding support was received from the Israeli Forest Service (KKL).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest and no competing interests.
Open Research
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this published article in Supporting Information.