A long-term study on the impact of climatic variables on two common nest-dwelling ectoparasites of the Eurasian blue tit (Cyanistes caeruleus)
The institution, Museo Nacional de Ciencias Naturales has an agreement with Wiley so the manuscript will be open access.
Abstract
We explored the potential influence of temperature and precipitation on the abundance of two nest-dwelling ectoparasites (blowflies and mites) of Eurasian blue tits (Cyanistes caeruleus) during a period of 21 years and compared the results with those of a shorter period. The abundance of blowflies was negatively related to precipitation, which could prevent flies from locating their host, and laying date. In addition, blowflies were positively related to brood size (more food implies more parasites) and the interaction between precipitation and temperature. The highest abundances of blowfly pupae were attained in conditions of increasing precipitation and decreasing temperature, which should be more common at the beginning of the bird breeding season. Mites were significantly and positively related to laying date and the interaction between average precipitation and temperature but only for the larger dataset. Higher abundances of mites were related to intermediate values of temperature and precipitations, conditions that are found at the end of the breeding season. These results imply that optimal conditions for both parasites differ, with blowflies preferring earlier breeders and colder and more humid conditions than mites. Thus, the effects of the climatic conditions studied on parasite abundances are non-monotonic and can vary with years and parasite species. Finally, the fact that average temperature and precipitation decreases across the years of study is probably due to the advancement in Eurasian blue tit laying date because we calculated those variables for the period of birds’ reproduction. This earlier nesting does not affect parasite abundance.
INTRODUCTION
Under the current scenario of climate change with increasing temperatures and more variable weather, a spread of diseases to temperate areas due to more favorable climatic conditions for parasites is expected in the near future (Patz et al. 2005; Campbell-Lendrum et al. 2015; Rocklöv & Dubrow 2020). However, some studies have suggested that sharp increases in temperature could have detrimental effects on parasites reducing their numbers and impact on hosts (Castaño et al. 2018; Castaño-Vázquez et al. 2021; Albert et al. 2023).
The study of the effects of climate on parasite incidence is important, not only because of the potential effects of climate on the spread of diseases but also because of their effect on hosts via effects on resources, habitat, density, etc. (see Dunn & Møller 2019 for the case of birds). This could produce ecological non-monotonicity effects on populations as described by Zhang et al. (2015). That is, the effects of abiotic and biotic factors could not be monotonic (i.e. either positive, negative, or neutral), but with positive and negative effects depending on several circumstances often closely related to spatial and temporal scale processes which may explain why ecosystems are frequently highly variable and unpredictable in both space and time (Zhang et al. 2015). There is growing evidence that non-monotonic effects of environmental factors and inter-specific interactions can significantly influence the dynamics and stability of populations, communities, and ecosystems (see, e.g. Commander & White 2020; Lundblad & Conway 2020; Dai et al. 2022).
The predicted extension of higher temperatures in temperate zones that could generate an extension of parasitic diseases (see Caminade et al. 2019 and references cited therein) can also occur at altitude so that mountainous areas reach milder temperatures over time and therefore allow the spread of diseases (Chapa-Vargas et al. 2020). Parasitic arthropods could be profoundly impacted by changes in climatic conditions because virtually all arthropods need humid and hot conditions to complete their cycles, as they are not homeothermic beings (see, e.g. Guarneri et al. 2002; Caminade et al. 2019; Rizzoli et al. 2019). Thus, we expect that relatively high temperatures and a certain level of humidity will favor the development of ectoparasites, while at least in the case of insects that fly to locate their hosts, it is possible that heavy rainfall makes it difficult to locate hosts (Castaño-Vázquez & Merino 2022). On the other hand, climate change can not only favor the spread of diseases and pathogens but can also lead to the decrease and even the extinction and disappearance of some of them, for example, in cases of parasites with a narrow thermal tolerance (Cizauskas et al. 2017).
Although it is possible that environmental conditions necessary for the development of the disease approach to optimal conditions for parasites, these new conditions may also facilitate the extension of susceptible host populations from other zones or may generate more susceptible hosts due to the climatic stress they suffer, thus changing the interaction between parasites and hosts (Cohen et al. 2017). If this environmental stress is sufficient, it could also prevent the generation of long-term resistance and keep susceptible individuals in the population, hindering the possibility of generating adequate immune reactions to infection.
On the other hand, birds can also try to counteract the effects of climatic conditions (see Mainwaring et al. 2021) by varying their behavior at least to a certain level, for example, by changing to more elevated breeding areas where climatic conditions are now more favorable (Tingley et al. 2012; Freeman et al. 2018) or changing breeding dates to adjust to milder climatic conditions (Samplonius et al. 2018; Whitenack et al. 2023). This, in turn, could have an effect on parasites that would have to face a phenological mismatch with respect to their hosts (see e.g. Saino et al. 2009, McDevitt-Galles et al. 2020).
Long-term datasets that include enough variation in climatic conditions are needed to better understand their potential effects on the distribution and incidence of parasites. However, these kinds of long-term datasets are scarce, with few but important exceptions based on different types of datasets. Some of these studies are based on historical reconstructions and/or data obtained from literature on climatic conditions and disease incidence (Garamszegi 2011; Tian et al. 2017; Zonneveld et al. 2024) while others use data obtained from natural history collections (Wood et al. 2023). In addition, some studies are based on data directly collected for relatively long periods in the same area (Hauber et al. 2021; Mennerat et al. 2021; Castaño-Vázquez & Merino 2022).
We have been studying wild bird–parasite interactions for more than two decades in a deciduous forest in central Spain focusing on insectivorous bird species breeding in nest boxes (see Merino et al. 2000; Tomás et al. 2007; Moreno et al. 2009; Martínez-de la Puente et al. 2011; Castaño-Vázquez et al. 2020). Several parasites affect birds during breeding in our study area, and in the case of Eurasian blue tits [Cyanistes caeruleus (Linnaeus, 1758)], data on nest-dwelling ectoparasites have been gathered across years for different purposes (Castaño-Vázquez & Merino 2022). In a previous study, we analyzed the effect of climatic conditions on different parasites affecting Eurasian blue tits during a decade selecting a period of study including data from as many different species of parasites as possible, thus allowing a study of interactions between birds, parasites and climatic conditions for that period (see Castaño-Vázquez & Merino 2022). Here, we have selected a longer period of study (up to 21 years) for two nest-dwelling parasites, the blowfly Protocalliphora azurea and the mite Dermanyssus sp. and compare the results of analyses using both datasets to explore variations in relationships between parasite abundances and climatic data when data from more years are analyzed. In addition, we explore if changes in birds' breeding dates in response to climatic conditions could potentially counteract the effects of parasites on birds by forcing the latter to develop under unfavorable temperature and precipitation conditions.
Usually, direct adverse effects on the nestlings by mites and blowflies are apparent only when numbers are high. However, frequent bleeding can affect nestling growth via loss of blood and, in some cases, even produce death of nestlings (Merino & Potti 1995; Hurtrez-Boussès et al. 1997; Merino & Potti 1998). In addition, infestations by these parasites may have an indirect influence on nestling fitness because parasitism slows down the development and growth of nestlings (Merino 2010), ectoparasites could have more deleterious effects on nestlings in poorer condition (Christe et al. 1998), and the longer the time that birds stay in the nest, the more they are at risk of predation (Scholl et al. 2019). In addition, mites can transmit blood parasites like Trypanosoma (Macfie & Thomson 1929) or Lankesterella (Chagas et al. 2021) to nestlings, thus causing additional detrimental effects to them. Moreover, in Eurasian blue tits, parents usually pay the costs of parasitism by increasing feeding efforts and suffering elevated stress (Merino & Potti 1995; Bańbura et al. 2004). On the other hand, nest-dwelling parasites are usually more abundant in nests with more nestlings as they represent their food source (Merino 2010; Castaño-Vázquez & Merino 2022).
Here, we explore the variation in the abundance of blowflies and mites in the nests of Eurasian blue tits across years in relation to average temperature, precipitation, and their interaction during the period of nestling rearing. We expect a positive influence of temperature and humidity on ectoparasites up to a certain level but also an interaction among these climatic variables on ectoparasites because usually the increase in temperature is associated with a decrease in humidity and vice versa (Castaño et al. 2018). In addition, we check whether abundances of parasites have changed across the years of study due to a change in climatic conditions in the area because increases in temperature during the spring in the last decades have been reported (Sanz et al. 2003; González-Braojos et al. 2017; Castaño-Vázquez & Merino 2022). Finally, we also explored whether changes in breeding dates by birds are related to changes in climatic conditions in the area and if these changes can affect ectoparasite abundance.
MATERIALS AND METHODS
For this study, we used data collected during the Eurasian blue tits' breeding seasons including between 2002 and 2023 in a Pyrenean oak (Quercus pyrenaica Willd, 1805) deciduous forest located in Valsaín (Segovia, central Spain, 40°53′N, 4°01′W, 1200 m a.s.l.). A Eurasian blue tit population breeding in wooden nest boxes has been studied in this area since 1991, and although the first studies on bird–parasite interactions began in 1994 (Fargallo & Merino 1999), they were established continuously from 1999. Nest boxes were in the same location across the years, and some were used by birds in several years. Each breeding season, nest boxes were periodically inspected to determine reproductive parameters including laying date, clutch size, and hatching date (Merino et al. 2000; Tomás et al. 2007). Every year, at the end of the breeding season, nest boxes were emptied so that each year the birds had to build a new nest. We conducted experimental manipulations on birds with different targets every year. Thus, for this study, we only used data from control not manipulated nests to avoid any potential effect of experimental manipulations on the abundance of parasites in nests. Data for blowflies were obtained from a total of 438 nests corresponding to 21 different reproductive seasons (all years between 2002 and 2023 except 2018). In the case of mites, data from 362 nests corresponding to 18 years were included in this study (all years between 2002 and 2023 except 2003, 2004, 2012, and 2018).
Climatic data
Data on daily average air ambient temperature and rainfall were obtained from the Spanish National Meteorology Institute station of Segovia (40°56′43″N, 4°07′35″W), located approximately 9.9 km from the study area. Weather data from this station have been previously used in studies of the effects of climatic conditions on birds in our study area (Lobato et al. 2006; Martínez-de la Puente et al. 2009).
Studied ectoparasites
Blowflies are a common nest-dwelling parasite of birds with more than 40 species described in the Holarctic region (Sabrosky et al. 1989). After pairing, adult female flies locate nests of their hosts to lay between 15 and 75 eggs close to the cup of the nest. In the following 24 h, the eggs hatch, and hematophagous larvae look for food. Larvae spend the day hidden in the nest material to avoid being eliminated by the adult bird and during the night feed on nestlings' blood. After about 4 days (it can vary between 3 h and 33 days depending on the Protocalliphora species), the larvae pupate for 5 to 7 days protected among the nest material. After that period, adult flies emerge from the pupae and a new cycle begins (Bennett & Whitworth 1991).
There exist 23 species of hematophagous Dermanyssid mites (Roy & Chauve 2007). The life cycle could vary slightly among species and hosts, but in cavity nester birds like the Eurasian blue tit (C. caeruleus), it begins with mites being transported to nests by birds becoming infested when visiting potential nest cavities or by contact with other infested birds. Mites lay their eggs in the nest material, and after hatch, first instar six-legged larvae that do not feed emerge. The next two steps in the life cycle produce eight-legged hematophagous nymphal stages after molt. Finally, the second nymphal stage molt to adult and after pairing, fed females lay their eggs on the bird nest. Eggs hatch after 2–3 days and the cycle begins again (Sparagano et al. 2014).
Ectoparasite survey
Once nestlings fledge (usually 20- or 21-day post-hatching), nests were collected in sealed plastic bags and stored at 4°C from 2 to 4 days previous to be transported to Berlese funnels to extract mites. Nests were placed in funnels for 48 h, under conditions of constant temperature and light provided by lamps (60 W) placed 6 cm above the nest (Merino & Potti 1996). The abundance of Dermanyssid mites (only one species was detected) was estimated by counting them in the material obtained from the funnels under a binocular loupe (Merino & Potti 1995). Nests were later dismantled and blowfly pupae counted.
Statistical analyses
For each nest and year included in the study, we calculated the data of average temperature and precipitation for the period between hatching till the day of nestling fledge (about 21 days), because it is during this period when ectoparasites develop in the nests. First, we explored whether several variables (abundances of both parasites, temperature, precipitation, brood size, and laying date) increased or decreased over the years using Spearman rank correlations. Kruskal–Wallis tests were used to explore differences in parasite abundances between years. We used these tests because those variables did not fit into a normal distribution, even by transforming the variables.
In analyses to explore relationships between parasite abundances and different climatic and bird variables, there could be an effect of the use of the same nest box by birds in different years. This is the case if parasite distribution varies consistently across the study area. To avoid this potential problem, we used generalized linear mixed models (GLMM) of repeated measures with negative binomial distribution and log-link function. The abundance of parasites (blowfly pupae or mites) was used as dependent variables (one model for each parasite). Laying date (date of laying of the first egg measured as the date of April), brood size (included as a continuous variable), average precipitation, average temperature, and the interaction between average temperature and precipitation were introduced as covariables. Nest-box identity and year were accounted for as random factors. Models were reduced to significant variables by backward stepwise elimination. The negative binomial distribution is used because it adjusts to the aggregated distribution of parasites. The same analysis was also conducted on the dataset of blowflies and mites used in Castaño-Vázquez and Merino (2022), which includes only 10 years of data (2008–2017).
We use the Akaike information criterion (AIC) to compare the fit of the models with and without the interaction term (temperature × precipitation) for both datasets. The AIC is an estimator of prediction error and, thereby, the relative quality of statistical models for a given set of data. Given a collection of models for the data, AIC estimates the quality of each model, relative to each of the other models. Thus, AIC provides a means for model selection. The information criterion is based on Log-likelihood-2, and models with lower information criterion values are those with a better fit. In addition, although the time in the season as measured by laying date may also influence parasitism, this variable could be correlated with brood size, and temperature and relative humidity have also been shown to be negatively correlated with each other (see, e.g. Castaño et al. 2018). Thus, we checked the variance inflation factor (VIF) for these variables (brood size, laying date, temperature, and relative humidity) in multiple correlation analyses with each of the parasite variables. VIFs estimate how much the variance of a coefficient is “inflated” because of linear dependence with other predictors. That is, the VIF provides an index that measures how much the variance of an estimated regression coefficient is increased because of collinearity. In all cases, the VIF values were below the conventional threshold of 2, indicating minimal multicollinearity concerns (see O'Brien 2007). Thus, we included all of these variables in mixed model analyses.
Graphics and statistical analyses were performed in SPSS (IBM SPSS Statistics for Windows, Version 26.0, Released 2019; IBM Corp., Armonk, NY, USA) and Microsoft Excel (Versión16.66.1; 2022). This study complies with current European legislation on experimental procedures with animals (2010/63/UE). Annual ringing permissions were provided by Junta de Castilla y León.
RESULTS
The average temperature during the periods of birds breeding in the years of study was 16.92°C ± 2.44°C SD (range: 11.55–22.97, N = 439) and average precipitation was 1.35 mm ± 1.01 mm SD (range: 0–4.74, N = 439). The average laying date measured as the date of April was 28.74 ± 8.69 SD (range: 12–62, N = 439) and the average brood size was 8.29 ± 1.67 SD (range: 2–13, N = 439). The average abundance of blowfly pupae was 22.08 ± 19.30 SD (range 0–95, N = 438), and the average mite abundance was 602.83 ± 1650.34 SD (range: 0–18546, N = 362).
Blowfly and mite abundance showed annual variation across the study years (Kruskal–Wallis's tests, H = 138.882, N = 438, P < 0.001 and H = 107.499, N = 362, P < 0.001, respectively; Fig. 1). Abundances of blowfly pupae and mites increased across the years of study (rs = 0.221, n = 438, P < 0.001 and rs = 0.207, n = 362, P < 0.001, respectively). Temperature and precipitation during the period of development of parasites in nests decreased across the years of study (rs = 0.263, n = 439, P < 0.001 and rs = 0.170, n = 439, P < 0.001, respectively). Laying date also decreased with years (rs = 0.483, n = 439, P < 0.001) but brood size did not vary significantly with years (rs = 0.050, n = 439, P = 0.297).
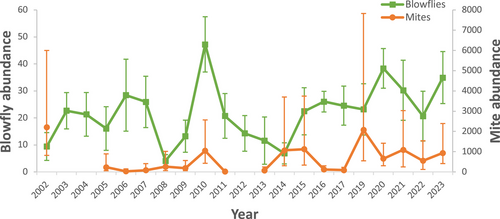
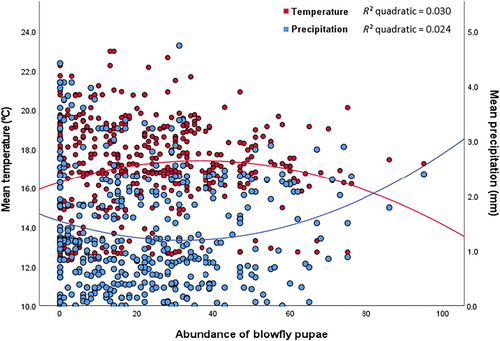
In the case of blowflies, the best-fit models (GLMM) were those including the interaction between temperature and precipitation both for the larger dataset (AIC = 1306.159 vs 1323.001) and the 10 years dataset (AIC = 2931.269 vs 2966.354). Blowfly abundance was negatively and significantly related to average precipitation and laying date, but positively to the interaction between average precipitation and temperature (Table 1). The interaction indicated that the abundance of blowfly pupae increased with slight increases in temperatures and decreases in precipitation till a certain level where abundance increased with decreased temperature and increased precipitation (see Fig. 2). That is, in warm dry seasons, blowfly pupae abundances were low to medium, and in colder and humid seasons, blowfly abundances varied from medium to high (Table 1; Fig. 2). In addition, a positive relationship between brood size of Eurasian blue tits and blowfly pupae abundance existed (see Table 1). The results obtained by using the 10 years dataset were similar (Table 2) except for the laying date, which was not significant in this case.
| Blowflies | Mites | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fixed effects | Coef. | SE | F | df | P-value | Coef. | SE | F | df | P-value |
| Corrected model | 2.507 | 0.320 | 28.673 | 4, 433 | <0.0001 | 0.994 | 0.571 | 17.337 | 2, 359 | <0.0001 |
| Avg. temp. | 0.020 | 0.036 | 0.316 | 1, 432 | 0.574 | −0.121 | 0.128 | 0.903 | 1, 356 | 0.343 |
| Avg. precipitation | −1.583 | 0.195 | 66.187 | 1, 433 | <0.0001 | −0.214 | 0.558 | 0.147 | 1, 357 | 0.702 |
| Brood size | 0.137 | 0.027 | 25.606 | 1, 433 | <0.0001 | 0.130 | 0.079 | 2.699 | 1, 358 | 0.101 |
| Laying date | −0.021 | 0.005 | 16.288 | 1, 433 | <0.0001 | 0.091 | 0.017 | 29.673 | 1, 359 | <0.0001 |
| Temp. × Prec. | 0.088 | 0.012 | 52.135 | 1, 433 | <0.0001 | 0.021 | 0.009 | 6.087 | 1, 359 | 0.014 |
- The results of the models including the interaction term between temperature and precipitation are shown for both parasites because they were the best fitted models as compared to models without the interaction (see text). A negative binomial distribution and log-link function were used. Backward stepwise was used to reduce the model to the significant variables in each analysis. The value for each variable in the final model or just before to be eliminated is shown. Significant differences (P < 0.05) are marked in bold.
| Blowflies | Mites | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fixed effects | Coef. | SE | F | df | P-value | Coef. | SE | F | df | P-value |
| Corrected model | 1.797 | 0.342 | 22.848 | 3, 225 | <0.0001 | 1.432 | 0.567 | 27.959 | 1, 205 | <0.001 |
| Avg. Temp. | 0.071 | 0.063 | 1.295 | 1, 223 | 0.256 | 0.052 | 0.098 | 0.277 | 1, 202 | 0.599 |
| Avg. precipitation | −1.900 | 0.277 | 47.018 | 1, 225 | <0.0001 | 0.119 | 0.136 | 0.767 | 1, 204 | 0.382 |
| Brood size | 0.149 | 0.038 | 15.229 | 1, 225 | <0.0001 | 0.107 | 0.096 | 1.247 | 1, 203 | 0.265 |
| Laying date | −0.009 | 0.007 | 1.574 | 1, 224 | 0.211 | 0.095 | 0.018 | 27.959 | 1, 205 | <0.001 |
| Temp. × Prec. | 0.101 | 0.017 | 37.892 | 1, 225 | <0.0001 | - | - | - | - | |
- The model for blowflies includes the interaction term between temperature and precipitation because it was the best fitted model as compared to the model without the interaction. In the case of mites, results for the best fitted model (that without the interaction) are shown (see text). A negative binomial distribution and log-link function were used. Backward stepwise was used to reduce the model to the significant variables in each analysis. The value for each variable in the final model or just before to be eliminated is shown. Significant differences (P < 0.05) are marked in bold.
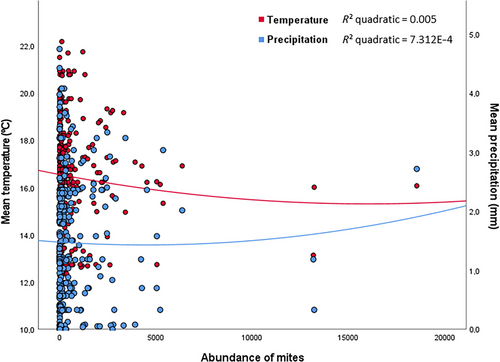
In the case of mites, the best-fit models (GLMM) were those including the interaction between temperature and precipitation for the larger dataset (AIC = 2931.269 vs 2966.354) but the one without the interaction for the 10 years dataset (AIC = 1371.835 vs 1387.866). For the larger dataset, mite abundance was significantly and positively related to laying date and the interaction between average temperature and precipitation, indicating a higher abundance of mites at intermediate temperatures and precipitation (Table 1; Fig. 3). That is, the highest abundances of mites were attained in relatively warm and humid years (Fig. 3). However, for the 10 years dataset, the only significant variable retained by the model was the laying date (Table 2).
DISCUSSION
Mites and blowflies showed significant variation across years, indicating that the incidence of these parasites is variable from one year to another. However, significant relationships for fixed effects are different for both parasites. Among the variables explaining variation in blowfly abundance is brood size. This is not surprising as nestlings represent the food source for these parasites (see also Castaño-Vázquez et al. 2022). On the other hand, blowfly pupae abundance showed a negative relationship with precipitation as previously reported (Merino & Potti 1996; Castaño-Vázquez & Merino 2022; Maziarz et al. 2022) but the significant interaction between precipitation and temperature implies that the intensity of the effect of precipitation depends on thermal conditions. Rainfall could increase the difficulty for blowflies to locate their hosts because adult blowflies search for bird nests by themselves, being more exposed to climatic conditions. However, temperature used to be positively related with development of blowflies in nests (Dawson et al. 2005; Mennerat et al. 2021; Castaño-Vázquez & Merino 2022), especially when high ambient temperatures prevailed in the late nestling stage (Maziarz et al. 2022; Stierhoff et al. 2024). The significant interaction between temperature and precipitation on blowfly pupae abundance suggests a slight positive effect of warm and dry seasons on abundances, but the highest abundances were attained in colder and wetter seasons. That is, abundances of blowflies increased with temperature till about 17°C and with decreasing precipitation to about 1 mm, but further increases in abundances were attained for lower temperatures and higher precipitations (see Fig. 2). In this respect, Dawson et al. (2005) and Mennerat et al. (2021) showed that the number of blowflies increased from 20°C and began to decrease from 25°C. In addition, Musgrave et al. (2019) found that an increase in precipitation could provoke a negative impact of blowflies on Western bluebird (Sialia mexicana Swaison, 1832) fledging success. Similarly, in the same study, these authors found a positive effect of temperature on blowflies, which affected negatively the fledging success of ash-throated flycatchers [Myiarchus cinerascens (Lawrence, 1851)]. Therefore, these results suggest that there exists a considerable variability in the effects of climatic conditions on parasitism between host species. In the case of blowflies in our study, we find a negative relationship with laying date, indicating that abundances of this parasite decrease during the birds' breeding season. However, in the model for the 10 years dataset (see Table 2), there are no significant relationships between laying date, temperature, and abundance in blowfly pupae. Thus, by using a larger dataset, we find a different effect on blowfly pupae abundance.
Mites do not usually reach high numbers in Eurasian blue tits nests, at least in our population (an average of about 600 but varying from 0 to more than 18 000, see above), but tens of thousands can be found in nests of other cavity nesters in the same area, as is the case of pied flycatchers (Ficedula hypoleuca, see also Merino & Potti 1995). This difference does not seem to be due to the different nest material composition (barks in flycatchers vs mosses in tits; see Moreno et al. 2009) but perhaps to the time of breeding because flycatchers are migratory birds that usually arrive later to the breeding grounds when climatic conditions could be more favorable for mite reproduction and development (warmer and drier conditions) (Nordenfors et al. 1999). We found a positive relationship between laying date and mite abundance, thus implying that conditions for late breeders favored mite abundances (see also Castaño-Vázquez & Merino 2022). However, the interaction between average temperature and precipitation shows that higher mite abundances are produced at intermediate temperatures (about 18°C) and precipitation (about 2 mm; Fig. 3), and at least in pied flycatchers, lower temperature and higher precipitation affect population growth of mites inside nests positively (Merino & Potti 1996). Whatever the reason, Eurasian blue tits do not appear to be the preferred hosts for mites in our study area.
The fact that, when we use a shorter period of study for mites, the significant relationships between mite abundance and the interaction between precipitation and temperature disappear implies a less consistent effect of climatic conditions on mites, which can vary from one year to another, probably also depending on the host species. For example, Dube et al. (2018) suggested that mite population growth in avian nests was influenced by high temperatures and low humidity in a barn swallow (Hirundo rustica erythrogaster Boddaert, 1783) breeding season, but in other studies on different bird species, mite population growth was favored in conditions of high temperature and humidity (Burtt et al. 1991). It is also surprising that the number of nestlings was not related to mite abundance, as they also feed on nestlings. This could be partly due to the fact that both apparently favorable climatic conditions for mites and declining brood size occur at the end of the Eurasian blue tit breeding season. In other words, the increase of mites due to climatic conditions could be reduced due to the concurrent decline in brood size, rendering a lack of relationship between mite abundance and number of nestlings.
Our results imply the relationships between the abundance of blowflies or mites and average temperature and precipitation are non-monotonic (see Zhang et al. 2015) due to the influence of one variable on the other and probably on the optimal conditions of temperature and humidity for the development of parasites (see Figs 2, 3). The relationships between abundance of parasites and climatic conditions vary from one year to another and depending on the number of years analyzed some tendencies could also vary.
Interestingly, abundances of both parasites increase with years while precipitation and temperature decrease. However, the increase in parasite abundances is apparently not due to an increase in brood size (that is, higher food abundance for parasites), which did not vary significantly with years in spite of the potential effects of even slight climatic differences in Eurasian blue tits breeding success (Garrido-Bautista et al. 2023). Average temperatures in spring in our study area have been increasing in recent years (Sanz et al. 2003; González-Braojos et al. 2017; Merino et al., unpublished data), and the reduction in temperature with time observed in our study could be related to the advancing laying date reported here, since we analyzed the average climatic variables during development of nestlings for each nest and not during the complete season. The advance in breeding by Eurasian blue tits imply lower temperature and precipitation during parasite development but does not reduce parasite incidence, implying that parasites are able to adapt to climatic variations and laying date variations across years. The negative significant relationship between blowfly abundance and laying date also indicates that earlier nesting does not appear to affect blowflies negatively. However, in the case of mites, the relationship with laying date is positive, indicating that early bird breeding is not favorable for them. In spite of this, mites still increase with years. Anyway, this tendency toward an advancement of breeding by Eurasian blue tits will have a limit imposed probably by the availability of food, and it will hardly affect parasite abundance, except in the case that the birds will be forced to reproduce in climatic conditions that are far from optimal for parasites (Fig. 2). It is difficult for this scenario to occur because Eurasian blue tits feed on insects that may also need climatic conditions probably not too different from those affecting mites and blowflies.
Overall, our results showed that climatic variables could interact, affecting abundance of parasites in a nonlinear way varying with time, periods of study, and interactions between variables for different parasite species.
ACKNOWLEDGMENTS
We thank the National Museum of Natural Sciences (MNCN) and “El Ventorrillo” Biological Field Station (EVEB) for providing the facilities for this research. The Junta de Castilla and León authorized the ringing and handling of birds. Work in the field area was done with permission from J. Donés and J. García Gámez (Director of “Montes de Valsain”). This study was funded by MCIU/AEI/10.13039/501100011033/, “ERDF A way of making Europe” (project PGC2018-097426-B-C21), and CSIC (project 2022 AEP023). Between 2002 and 2023, several researchers worked with us on the study of blue tits and their parasites, and we also greatly acknowledge their contribution to obtaining the data analyzed here. M.M. thanks Leticia Gómez for her support and initial suggestions in a previous version of this work. We also thank Laura Barrios for her priceless statistical advice. We acknowledge support of the publication fee by the CSIC Open Access Publication Support Initiative through its Unit of Information Resources for research (URICI).




