Seasonal remodeling of visceral organs in the invasive desert gecko Tarentola annularis
Abstract
In winter, many reptiles have a period of inactivity (“brumation”). During brumation there is no energetic intake, therefore there would be an advantage to reducing energetic expenditure. The size of energetically costly organs, a major determinant of metabolic rate, is known to be flexible in many tetrapods. Seasonal plasticity of organ size could serve as both an energy-saving mechanism and a source of nutrients for brumating reptiles. We studied a population of an invasive gecko, Tarentola annularis, to test for seasonal changes in activity, metabolic rate, and mass of various organs. The observed period of inactivity was December–February. Standard metabolic rates during the activity season were 1.85 times higher than in brumating individuals. This may be attributed to decreased organ mass during winter: heart mass decreased by 37%, stomach mass by 25%, and liver mass by 69%. Interestingly, testes mass increased by 100% during winter, likely in preparation for the breeding season, suggesting that males prioritize breeding over other functions upon return to activity. The size of the kidneys and lungs remained constant. Organ atrophy occurred only after geckos reduced their activity, so we hypothesize that organ mass changes in response to (rather than in anticipation of) cold winter temperatures and the associated fasting. Degradation of visceral organs can maintain energy demands in times of low supply, and catabolism of the protein from these organs can serve as a source of both energy and water during brumation. These findings bring us closer to a mechanistic understanding of reptiles’ physiological adaptations to environmental changes.
INTRODUCTION
Low temperatures pose a limit on reptile locomotion (Bennett 1980; Crowley 1985; Hertz et al. 1988; Lailvaux 2007) and digestion (Cowles & Bogert 1944; Naulleau 1983; Wang et al. 2002; Bonnet et al. 2013), and therefore constrain their foraging ability. Consequently, energetic intake is reduced seasonally in regions with even mildly cold winters and many species stop feeding until spring. Such seasonal fasting poses a risk of starvation, giving rise to seasonal adaptations such as fat accumulation (Goldberg 1972; Gavaud 1983) and reduction of energy expenditure (Hailey & Loveridge 1997; Christian et al. 1999). The seasonal cessation of activity in reptiles, in some ways similar to mammalian hibernation and insect diapause, is termed brumation (Mayhew 1965). A recent study (Dubiner et al. 2023) demonstrated that temperature-independent reduction of metabolic rates during brumation is common to the majority of Israel's reptile species, across all squamate families, in both the Mediterranean and desert biomes. Despite the ubiquity of this phenomenon, there is no mechanistic explanation at present for its occurrence.
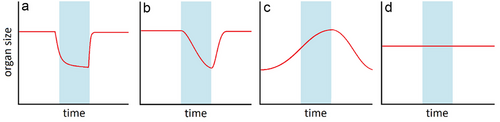
Much of the variation in whole-organism resting metabolic rates is explained by the relative size of energetically costly internal organs (Daan et al. 1990; Konarzewski & Diamond 1995; Secor & Diamond 2000; Nespolo et al. 2002), such as those involved in nutrient metabolism (liver), transport (gastrointestinal tract, heart), and excretion (kidneys). A reversible reduction of these organs’ size and function can therefore reduce energetic demands. Organ size and function flexibility occur across taxa in the face of various environmental changes (Piersma & Lindström 1997). For example, the viscera of pythons undergo extensive atrophy and structural modifications during (non-brumation) fasting (Hansen et al. 2013), which is likely to have direct effects on their metabolic rate (Secor & Diamond 1997; McCue et al. 2005). The vertebrae of marine iguanas shrink following El Niño events in preparation for the upcoming harsh conditions and food shortage (Wikelski & Thom 2000). The mass of the liver is known to vary between seasons in several lizard species (Goldberg 1972; Taylor 1986; Ramírez-Bautista et al. 2006). This has been linked to its role in energy storage in the form of glycogen and lipids (but see Taylor 1986). The gonads, whose function is often seasonal, can grow and develop during the breeding season and shrink during brumation (van Wyk 1990; Ramírez-Bautista et al. 2006). In mammals, seasonal organ plasticity is known from hibernating ground squirrels, which undergo atrophy of the skeletal muscles and intestinal mucosa, accompanied by hypertrophy of cardiac and adipose tissues (Carey 1990; Wickler et al. 1991). A unique case of seasonal size changes is the Dehnel phenomenon occurring in several shrew (Dehnel 1949; Lázaro et al. 2017, 2018) and weasel species (LaPoint et al. 2017), whereby the brain and braincase shrink during winter, a strategy shown to efficiently save energy (Schaeffer et al. 2020). We documented seasonal changes in activity and energy use of a reptile species and tested whether they are concurrent with size changes of several energetically costly organs. We hypothesized that organ size would decrease in winter. These changes could possibly follow a pattern of “programmed dormancy” (Wilsterman et al. 2021) whereby changes occur in preparation for winter and its associated reduction in energetic intake. In this scenario, the size of costly organs would decrease sharply at the onset of brumation and remain low throughout winter, thus reducing the overall energy needed to sustain the organism until spring (hypothesis #1, Fig. 1). Alternatively, in the case of a “responsive dormancy” (Wilsterman et al. 2021), the size of costly organs would decrease gradually throughout winter as brumation progresses. Organs would atrophy due to disuse or be actively degraded as an accessory source of energy, protein, and water (hypothesis #2, Fig. 1). In either case, the need for peak function in spring, immediately after the return to activity, may require maintenance of some organs throughout winter (e.g. the reproductive system; Gavaud 1983). These could remain unchanged or even grow in size during winter (hypothesis #3, Fig. 1).
MATERIALS AND METHODS
Study species
We tested our hypotheses in Tarentola annularis (Fig. 2), a large phyllodactylid gecko common across North Africa and northern sub-Saharan Africa (Jirků et al. 2010). T. annularis is a nocturnal, sit-and-wait predator, mostly found on walls and fences (Ibrahim 2004). In its native habitat in Egypt, it was reported to be active from spring to autumn but mostly inactive in winter (Saber et al. 1995; Ibrahim 2004). In recent years, after first being observed in 2014, it established an invasive population in Kibbutz Ein Gedi by the Dead Sea in Israel (Jamison et al. 2017). The population is assumed to have originated from pets that escaped or were released in the kibbutz. T. annularis is aggressive and much larger than the native geckos (up to 75 g; Bar et al. 2021) and thus quickly excluded the native species from its invaded range (Bar et al. 2021; SD, personal observation). In the winter and spring of 2017, the Israeli Nature and Parks Authority (INPA) attempted to eradicate this species. Culled specimens were deposited in the Steinhardt Museum of Natural History and preserved in ethanol. These specimens can serve as an excellent system for a semi-natural seasonal acclimation study (Huey & Buckley 2022), since they were naturally exposed to the seasonal changes in environmental conditions and were collected at random and in large numbers over several months. Isolating the effects of seasonality can be well accomplished in these specimens since they originate from a small source population and are spatially restricted to a single small settlement (Jamison et al. 2017). These factors reduce the morphological, physiological, genetic, and behavioral variability that would otherwise exist.

Activity surveys
We determined T. annularis activity by observing gecko density for 1 min at each of four artificially lit house walls along a 200-m line (each wall is ∼50 m2 in area). Surveys were conducted in Kibbutz Ein Gedi (31.45°N, 35.38°E) in the first hour after dark. We counted the number of geckos on each wall 21 times (roughly once a week) between October 26, 2022 and May 12, 2023. No specimen collection was conducted at or near the sites we surveyed for activity. Air temperatures at the site during the survey were obtained from the nearby Israeli Meteorological Service, Ein Gedi station (10-min resolution).
Respirometric trials
Metabolic measurements were conducted under Ethics Permit #18616 from the TAU Ethics Committee. We collected five adult males in winter (January 18, 2023), four in late spring (May 9, 2023), and five in summer (August 9, 2023). Geckos were kept in sealed containers from the moment of capture to prevent escape and potential introduction of this invasive species. During winter, we assumed that individuals were not feeding and thus postabsorptive. During the activity season, we deprived animals of food 5 days prior to measurements to avoid bias by the higher metabolic costs of digestion (Secor 2009). Water was given every 2 days. We weighed each individual, then placed it in a dark metabolic chamber inside a Peltier control incubator (to avoid vibrations, Thermo Scientific™, USA) maintained at 20°C and connected to a constant flow of CO2-free dry air. The flow rate was set to 100 mL min−1 (FB8, Sable Systems) and the chamber size was 750 mL. Air exiting the metabolic cell flowed through an Ascarite-Mg(ClO4)2 column (CO2 and H2O scrubbers) into an Oxzilla O2 analyzer (Sable Systems, USA). All individuals were measured in parallel for 18–20 h, with each cell being measured in turn for 1 out of every 5 hours. An empty, but otherwise identical, measurement chamber was used as a reference baseline. It was measured for 10 min between every two gecko measurements (all switches between cells were done automatically using an RM-8 multiplexer). We measured standard metabolic rates (SMR) calculated as the mean of oxygen consumption during the one continuous hour of measurement with the lowest values and compared log-transformed SMR between seasons using a fixed-slope linear model correcting for log-transformed body mass. For recording the data and for analysis, we used a UI-3 data acquisition interface and Expedata version 1.9.20 (Sable Systems). Values of were calculated according to equation (4.15) in Lighton (2018).
Organ size throughout the yearly cycle
We dissected 50 ethanol-preserved specimens from the Steinhardt Museum of Natural History collections that were killed as part of an Israel Nature and Parks Authority failed eradication program (see above). We randomly selected (from only adult specimens in good condition) specimens to represent all months in which eradication efforts were maintained (winter: December 2015, January and February 2017; spring: March and May 2017; summer: September 2017). We removed the heart, liver (with gall bladder), stomach (aboral and pyloric fundic regions), gonads (or gonads and eggs in gravid females), lungs (with bronchi, without trachea), and kidneys. If an organ was damaged or had been dissected by the museum for other purposes, it was excluded (stomach: seven individuals from May, liver: one individual from October). We dried the organs for 3 days using a drying oven at 60°C and weighed their dry mass using an analytic scale within 0.01 mg precision (Sartorius, Germany). Dissection and weighing were randomized and blinded (the researcher did not know which season each individual was from). To identify seasonal changes in the dry mass of each organ, we used generalized additive models (GAMs; Hastie & Tibshirani 1995) in the “mgcv” package (Wood & Wood 2015) in R version 4.2.2 (R Core Team 2022). The response factor of the GAM was organ dry mass (in grams) and the fixed factors were sex, snout–vent length (SVL), and the smoothed term of the day in which the specimen was collected, with five knots (k = 5) and a cyclic cubic regression spline to account for the yearly cycle (bs = “cr”; see LaPoint et al. 2017), fitted with a log-link function. Effects sizes (i.e. the degree of change in organ size between seasons) were estimated by comparing the dry mass of the same organs between the 2 months with minimal and maximal values, using generalized linear models (GLMs) in the “lme4” package (Bates et al. 2015), with date, sex, and SVL as fixed factors.
Organ size pre-brumation
To better understand changes in the period leading up to brumation (autumn, which was not represented in the museum specimens), we dissected 30 additional specimens that we collected under INPA permit #2022/43 032 throughout the autumn (10 specimens on each of the autumn months: October 6, November 3, and December 1 2022). Specimens were frozen but not fixed. We compared the mean dry masses between the three collection dates using GLMs with date, sex, and SVL as fixed factors, fitted with a log link function. R2 was approximated for all GLMs according to maximum likelihood (R2lik) using the “rr2” package (Ives & Li 2018).
RESULTS
Activity surveys
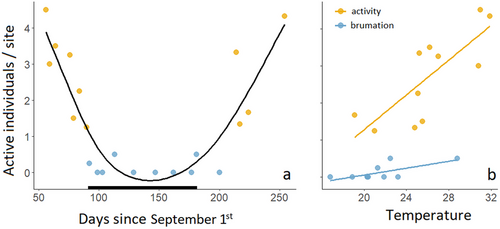
Many active individuals were observed from the start of the survey period, from October 2022 to November 29, 2022 (autumn), and between April 2, 2023 and the end of the survey period in May 2023 (spring). In these surveys, constituting the activity season, the mean number of active individuals per site (averaged across all surveyed walls for each survey evening) ranged between 1 and 4.5 (mean and median = 2.6). We observed almost no active individuals in the 10 surveys from December 1, 2022 to March 19, 2023, during which the mean number of active individuals, averaged between the sites for each survey, ranged from 0 to 0.5 (mean = 0.125, median = 0; Fig 3a; Table S1, Supporting Information). We thus consider early December to early March as the brumation period. Indeed, we found no food in the stomachs of specimens collected between December and February, but the stomachs of most individuals collected from March to November did contain food (SD, personal observation). The number of individuals per site in the activity season was linearly correlated with air temperature in the temperature range examined in (slope = 0.23 ± 0.04 and y = 0 individuals at 14.5°C, P < 0.001) but not during brumation (slope = 0.05 ± 0.06 and y = 0 individuals at 18.9°C; P = 0.451), and the difference between seasons was significant (P = 0.023, R2 = 0.89, Fig. 3b).
Respirometric trials
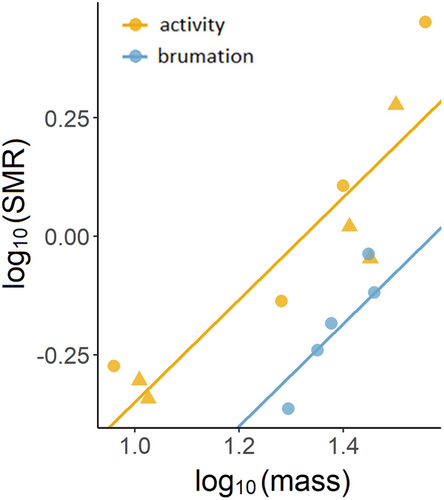
Specific SMR at 20°C (metabolic scaling was not significantly different from isometric: SMR ∝ M1.08±0.16) was 1.85 times higher in the active season (n = 9; 0.050 ± 0.005 mL O2/g/h) than during brumation (n = 5; 0.027 ± 0.002 mL O2/g/h; t = 4.36, P = 0.001, R2 = 0.83; Fig. 4). See Table S2, Supporting Information, for the raw data.
Organ size throughout the yearly cycle
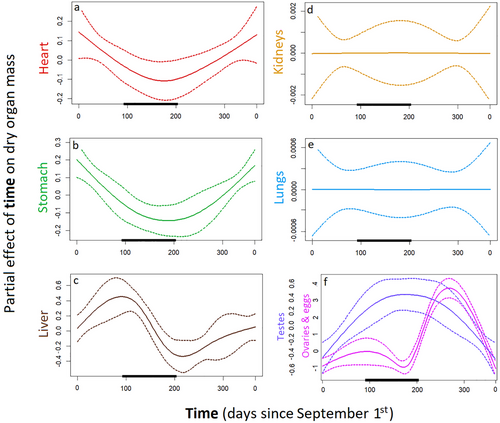
Museum specimens showed seasonal changes in the mean dry mass of the heart, stomach, liver, and gonads. Heart mass was 36.9% lower during brumation (Table 1 and Fig. 5a) and was correlated with SVL (P < 0.001) but not sex (P = 0.120). Stomach mass was 24.9% lower during brumation (Table 1 and Fig. 5b) and was correlated with SVL (P < 0.001) but not sex (P = 0.070). Liver mass decreased by 58.8% throughout brumation and increased throughout the activity season (Table 1 and Fig. 5c), and was correlated with both SVL (P < 0.001) and sex (livers were smaller in males by 46%; P = 0.021). Kidney mass was correlated with SVL (P < 0.001) but not sex (P = 0.829) and was not affected by the day of the year (Table 1 and Fig. 5d). Lung mass was correlated with SVL (P < 0.001) but not sex (P = 0.342) and was not affected by the day of the year (Table 1 and Fig. 5e). Mean testes mass was independent of SVL (P = 0.224) and increased by 100.4% in late autumn before decreasing through early summer (Table 1 and Fig. 5f). Mean ovary and egg mass increased sharply immediately following brumation and then decreased from late spring to early summer (Table 1 and Fig. 5f). See Table S3, Supporting Information, for the raw data.
| GAM | Organ dry mass | GLM | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Organ | n | DE | EDF | P | Highest | Lowest | n | Effect | P | R2lik |
| Heart | 50 | 68.1% | 1.148 | 0.016 | Late summer | Early spring | 19 | −36.9% | 0.035 | 0.77 |
| Stomach | 43 | 75.3% | 1.572 | 0.002 | Late summer | Winter | 14 | −24.9% | 0.034 | 0.61 |
| Liver | 50 | 72.6% | 3.074 | 0.000 | Late autumn | Early spring | 17 | −58.8% | 0.000 | 0.82 |
| Kidneys | 50 | 62.5% | 0.000 | 0.570 | — | — | — | — | — | — |
| Lungs | 50 | 71.9% | 0.000 | 0.443 | — | — | — | — | — | — |
| Testes | 33 | 30.3% | 1.677 | 0.009 | Winter | Late summer | 12 | +100.4% | 0.029 | 0.29 |
| Ovaries | 17 | 96.8% | 2.500 | 0.000 | Early spring | Winter | — | — | — | — |
- Generalized additive model (GAM) results include all specimens and test the significance (P, at α = 0.05, significant values in bold) of cyclical changes in organ mass across the year. The magnitude of changes (when the GAM was significant) is shown as the effect of brumation in general linear models (GLM) comparing only the time of year with the highest and lowest dry mass (exact dates can be found in Supporting Information). Ovary samples on each date were too small for the GLM. DE, deviance explained; EDF, effective degrees of freedom.
Organ size pre-brumation
At the onset of brumation (i.e. between October 6 and December 1), only testes mass changed significantly. In this period, we found that heart dry mass was correlated with SVL (n = 30, P < 0.001) but not sex and did not differ between December 1 and either November 3 (P = 0.531) or October 6 (P = 0.391). Stomach mass was correlated with SVL (n = 30; P < 0.001) but not sex and did not differ between December and either November (P = 0.079) or October (P = 0.224). Liver mass was correlated with both SVL (n = 29, P = 0.023) and sex (livers were 61% smaller in males; P = 0.020) and did not differ between December and either November (P = 0.439) or October (P = 0.857). Ovary mass was uncorrelated with SVL and date (n = 11, P = 0.618). Testes mass in this time was 10.33 ± 0.88 mg on average, was correlated with SVL (n = 18, P = 0.006), and was higher in December than in October by 3.78 ± 1.45 mg (t = 2.610, P = 0.021).
DISCUSSION
We found that seasonal changes in activity and metabolic rates of Tarentola annularis were accompanied by reversible organ size changes. The patterns differ among organs: The heart and stomach get smaller in early winter and rebound in spring, the liver grows throughout the summer and autumn and its size diminishes in winter, whereas the testes grow in the autumn and shrink in summer. Our measurements of organ size fit well with the oxygen consumption measurements (which itself is a measure of metabolic rate) and show an almost twofold suppression during brumation. The low metabolic rates we measured in mid-winter coincide with liver, heart, and stomach shrinkage. We, therefore, hypothesize that organ plasticity serves as an energy-saving mechanism since internal organ size is a major component of whole-body metabolic rate (Daan et al. 1990; Konarzewski & Diamond 1995; Secor & Diamond 2000; Nespolo et al. 2002). It is probable that additional mechanisms also reduce metabolic rates at the cellular level (e.g. Capraro et al. 2019, 2020). The temporal patterns of organ size changes are more compatible with our hypothesis #2; that is, changes occur as a result of the decrease in temperatures and prolonged fasting, rather than in anticipation of them. This suggests a “responsive” dormancy in this desert species compared to the “programmed” dormancy described for some North American reptiles (Wilsterman et al. 2021). The organs may atrophy due to disuse (Taylor 1986; Wickler et al. 1991; Secor & Diamond 1997; Hansen et al. 2013) or be consumed for energy (Goldberg 1972; Ramírez-Bautista et al. 2006). Hypothesis #1, whereby organ shrinkage would precede brumation as a mechanism to cut energetic costs in anticipation of inactivity and fasting, was not supported by the results. Organ shrinkage may manifest a starvation response (McCue et al. 2005) or part of a reverse acclimation (Tocher 1992), whereby prolonged exposure to the cold activates this mechanism to lower metabolic rates. Shrinkage may play an important role in the observed metabolic depression but is probably not a programmed, obligatory change directly aimed at saving energy (Schaeffer et al. 2020). The gradual degradation of the liver and gastrointestinal tract may serve as an important source of protein. We hypothesize that the patterns of organ remodeling described here are not unique to T. annularis, but shared across many of the species known to depress metabolism during brumation (Hailey & Loveridge 1997; Christian et al. 1999; Dubiner et al. 2023).
Changes in organ morphology throughout the life of an individual reptile were previously hypothesized to conserve energy during periods of food shortage. Recorded changes, however, have been in the skeleton and connective tissues but not the viscera (Romero & Wikelski 2003), or focused on the energy supply contained in the organs without considering the effects of tissue mass on metabolic rate. For example, liver size changes documented in other lizard species were linked to depletion of glycogen and lipid stores (Goldberg 1972; Ramírez-Bautista et al. 2006). Changes in the relatively small mass of liver glycogen and lipids cannot account for the drastic changes observed in overall liver size (Taylor 1986). We therefore suggest that, beyond serving as an energy supply, the shrinkage of the liver, heart, and gastrointestinal tract reduces the body's total energetic demands. These organs’ continued degradation throughout winter can further serve as a source of amino acids, which not only yields energy (Petrović et al. 1985) but also produces metabolic water. An additional water source emerges through the degradation of protein-bound water within the tissues, given that body-resident protein and its accompanying water content typically maintain a proportionate ratio of 3:7 (Klaassen 1996). In desert reptiles, water is mostly obtained from the prey (Nagy & Peterson 1988), so protein catabolism may be favored to maintain water balance when not feeding. This occurs during long flights in migratory birds under dry conditions (Gerson & Guglielmo 2011). The recycling of amino acids, when none are available from the diet, is also crucial in hibernators for maintaining protein balance (Hochachka & Guppy 1987). In male T. annularis, recycling and mobilization of amino acids may support the observed growth of the testes throughout winter. The lack of change in lung size may be due to reptile lungs being composed of very little tissue (reptilian lungs are much simpler and less finely divided than those of endotherms; Perry 2013). We hypothesize that the lack of change in kidney size may stem from the need to catabolize protein at high rates during brumation because such catabolism produces much nitrous waste. Despite its toll on energy reserves (Gavaud 1983), investing in the gonads before and during brumation (which fits with hypothesis #3, preparation for spring) probably enables reproduction immediately following the return to activity, granting a major fitness benefit (Warner & Shine 2007). Our results, showing testes growth during brumation, are supported by similar findings in the native range of T. annularis in Egypt (Ibrahim 2004). While other lizard species exhibit comparable seasonal plasticity in testes size (e.g. van Wyk 1990; Ramírez-Bautista et al. 2006), testes growth is normally shown to coincide with the reproductive season, not with brumation. The realization that T. annularis prioritizes the maintenance of reproductive over cardiac and digestive tissues is important, given this species’ invasive success in Israel (Jamison et al. 2017) and elsewhere (the United States; Krysko & Daniels 2005).
CONCLUSIONS AND FUTURE DIRECTIONS
Tarentola annularis in Israel are mostly inactive in the three winter months during which their standard metabolic rates decrease to nearly half. At the same time, their hearts, livers, and stomachs shrink, which we hypothesize is a mechanism for decreasing metabolic rates during fasting. Meanwhile, the testes grow in preparation for breeding in spring. To support (or refute) our results, and test the many hypotheses that arise from them, targeted research on the links between organ size, activity, metabolism, and environmental conditions should be undertaken. Possible directions would be examining organ sizes in the same individual throughout the year (e.g. using imaging techniques; see Hansen et al. 2013), testing possible triggers for the observed changes under laboratory conditions (food and water availability, temperature, daylight duration), or isotopically labeling proteins to determine the fate of amino acids from degraded organs. Comparing introduced populations to their native counterparts (and to local native species) will inform us whether organ plasticity has a part in facilitating invasion. While these and other questions remain unanswered, the evidence of seasonal organ remodeling provides an important step toward a mechanistic understanding of reptilian brumation. It serves as a strong demonstration of how morphological adjustments can profoundly affect animal physiology, underlying its adaptations to environmental change.
ACKNOWLEDGMENTS
We wish to thank Eldad Golan for helping with the surveys, Yan Ronen Liberman and Shiri Shubaev for helping with the dissections, Simon Jamison and our lab members for helping with the fieldwork, Karin Tamar for managing the museum specimens, and Anna Zimin and Noa Halevy for helpful comments on the first draft of the manuscript. Shahar Dubiner was supported by the Azrieli Graduate Studies Fellowship.




