Livestock grazing is associated with the gut microbiota and antibiotic resistance genes in sympatric plateau pika (Ochotona curzoniae)
Abstract
With the overuse of antibiotics in health care and animal husbandry, antibiotic resistance becomes a serious threat to public health. Antibiotic residues from veterinary medicine have increased the dissemination of antibiotic resistance genes (ARGs) by horizontal gene transfer globally, leading to the enrichment of ARGs in wildlife. Plateau pika (Ochotona curzoniae) is a small herbivore endemic to the Qinghai–Tibetan Plateau. Previous studies reveal that pika evolves a coprophagy behavior toward cohabitated yak, which makes the pika population a potential reservoir of ARGs. Yet, little is known about the resistome of pika under different grazing intensities. Here, we sampled the cecum content of pika from three different grazing intensity areas in the Qinghai–Tibetan Plateau to evaluate the effect of grazing on its gut microbiota and resistome. By using the 16S full-length amplicon and metagenomic sequencing, our study revealed that livestock grazing significantly altered the gut microbial community of plateau pika as compared to prohibited grazing areas. We found bacterial lineage Prevotellaceae, Lachnospirales, and RF39 increased in grazing areas. Analysis of the resistome revealed that pika from continuous grazing areas enriched a higher abundance of colistin (MCR) and streptogramin (vat) resistance genes. Moreover, we observed significant correlations between the gut microbial community, ARGs, and mobile genetic element profiles, hinting that pika gut microbiota was an important shaping force of the resistome. In future studies, the continuous monitoring of wildlife gut resistome and environmental antibiotic residues is imperative for a better understanding and for tackling the horizontal gene transfer of ARGs across the wildlife–livestock interface.
INTRODUCTION
Antibiotic resistance happens when microorganisms develop the ability to survive exposure to antibiotics. Antibiotic resistance genes (ARGs) are evolutionarily ancient in bacteria or fungi (Aminov & Mackie 2007), but the overuse of antibiotics globally as clinical agents changed the evolution of ARGs by providing extra selection pressure, making antibiotic resistance one of the most serious public health threats (Larsson & Flach 2022; Zhang et al. 2022).
The mammalian gut inhabits a massive number of microbes, which is now considered the “neglected endocrine organ” involved in mammalian physiology, contributing to immunomodulation, nutrient metabolism, protection against pathogens, and even affecting host behaviors (Lozupone et al. 2012; Lynch & Pedersen 2016; Zhao et al. 2020; Zhao et al. 2022). The interactions between different microbial communities or microbes and host cells are essential for maintaining the lumen homeostasis of hosts (Hemarajata & Versalovic 2013; Ma et al. 2022). Consequently, disturbance of the microbiome by antibiotic use could cause dysfunction of normal bacteria and their metabolites, with potential risks in various chronic diseases (Queen et al. 2020). The widespread use of antibiotics for medical purposes has rapidly altered the human microbiome globally and may affect long-term health (Barton 2000; Hemarajata & Versalovic 2013; Tompson et al. 2021). However, the side effects of antibiotic treatment not only directly influence the homeostasis of gut conditions, but also lead to the selection of antibiotic resistance. This selection also occurs in the gut microbiome of domestic or wild animals that directly feed on water contaminated with medical waste (Pärnänen et al. 2019; Laborda et al. 2022). Current evidence supports that the enrichment of ARGs in wildlife is a sign of antibiotic resistance pollution, more than an ARG selection in the natural condition (Laborda et al. 2022; Zhang et al. 2022). Therefore, wildlife gut microbiota is likely to be an important reservoir for ARG dissemination (Martinez & Olivares 2011; Plaza-Rodriguez et al. 2020; Skarzynska et al. 2021).
A shared environment can increase the similarity of the microbial community of animals by convergence of diet among different host species, contributing to the direct or indirect cross-species transmission of gut microbes and the resistance gene they harbor (Lee et al. 2022). Interspecific horizontal gene transfer (HGT) of ARGs has been proved in cockroaches (Anacarso et al. 2016), house fly (Akhtar et al. 2009), and flea (Hinnebusch et al. 2002), which are widely distributed in human habitations. Evidence from the gut microbiome of brown rat (Rattus norvegicus) has shown that animals inhabiting hospitals have a higher abundance of ARGs than other environments (Hansen et al. 2016). Especially in grazing areas, overuse of veterinary drugs may produce antibiotic residues, which might spread through livestock manure and lead to contamination of the agroecosystem (Checcucci et al. 2020). The transport of antibiotic residues with surface runoff or soil on grassland will locally transfer antibiotic resistance to sympatric wildlife and change their gut microbiome (Laborda et al. 2022; Lee et al. 2022). This transmission route may correlate with the intensity of livestock grazing; however, the relationship between grazing intensity and the gut resistome of wildlife remains relatively understudied.
Plateau pika is a small herbivorous species distributed on the Qinghai–Tibetan Plateau. It is recognized as a keystone species in maintaining the biodiversity of high-altitude meadows (Lai & Smith 2003). It has been proved that yak feces, which contain abundant digestible sources, could help pika survive during hard winter, (Speakman et al. 2021); this evidence adds to the possibility of interspecific horizontal transfer of microbes or resistome. Given the fact that high concentrations of veterinary antibiotic residues are detected in soil or livestock manure (Pan et al. 2011; Qiao et al. 2012; Zhao et al. 2021), livestock density may be positively associated with environmental antibiotics, raising the question of whether the higher grazing intensity extends the antibiotic resistance reservoir in the gut microbiome of wildlife. However, little is known about the relationship between grazing intensity and pika resistome. We hypothesized that pika may acquire more ARGs in grazing areas compared to ungrazed areas. In this study, we selected six sampling sites representing three different grazing ingredients to explore the effect of grazing on gut microbiota and resistome of sympatric plateau pika. All sampling sites were located in the high-altitude meadows of the Tibetan Plateau, with the Yangtze River source region serving as a control treatment (prohibited grazing areas). We characterized the gut microbiota diversity of plateau pika using 16S full-length sequencing; the gut resistome profiles were also examined by metagenomic sequencing.
METHODS AND MATERIALS
Study area and sample collection
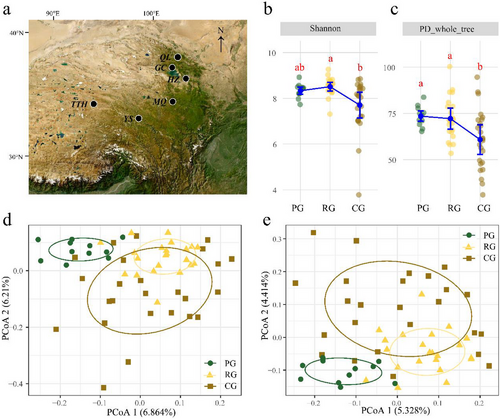
In this study, a total of 58 plateau pikas were captured at six sampling sites with different livestock grazing intensities selected in Qinghai Province, China, from July 5 to July 25, 2022. We selected six sampling sites (∼100 m × 100 m each) containing three different grazing intensities, that is, prohibited grazing (PG), rotational grazing (RG), and continuous grazing (CG) (Fig. 1a; sampling details are provided in Table S1, Supporting Information). The prohibited grazing area is located in the Tuotuo River basin at the source region of the Yangtze River. Rotational grazing areas are pastures that were grazed last year but not grazed in the sampling year; dry yak manure could be found in these areas. Sampling sites that have been grazing continuously this year are defined as continuous grazing areas. Pikas were live-captured using the nooses made of string that anchored in active burrows. Captured pikas were sacrificed with an abdominal injection of sodium pentobarbital, after which the cecum contents were immediately collected into 2-mL sterile tubes, labeled, and quickly stored in liquid nitrogen. Finally, all the samples were transported to the laboratory and stored at −80°C until further processing.
Amplicon sequencing and bioinformatic analysis
The total microbial DNA was extracted using the TGuide S96 Soil/Fecal DNA Kit (TIANGEN Biotech Beijing Co. Ltd., DP812) according to the manufacturer's protocol. DNA concentrations were monitored on 1.8% agarose gels. Full length of 16S rRNA genes were amplified used primer (27F: 5’-AGRGTTTGATYNTGGCTCAG-3’; 1492R: 5’-TASGGHTACCTTGTTASGACTT-3’) with barcode. All PCR reactions were carried out with 1.5 μL of genome DNA; 10.5 μL of nuclease-free water; 15 μL of KOD One TM PCR Master Mix, and 3 μL of barcode primer pairs. PCRs were conducted in 30-μL reactions with the following thermocycling conditions: an initial denaturation at 95°C for 120 s, followed by a 25-cycle (98°C, 10 s; 55°C, 30 s; 72°C, 90 s) and a final extension for 120 s at 72°C. Subsequently, the PacBio Binding kit was used to bind sequencing polymerase to SMRTbell® libraries in preparation for sequencing. The library quality was assessed on the Qubit@ 2.0 Fluorometer (Thermo Scientific). Finally, library preparations were sequenced on the PacBio platform.
Raw sequences were denoised and clustered using DADA2 (Callahan et al. 2016) under QIIME2 (Bolyen et al. 2019). A total of 3416 amplicon sequence variants (ASVs) were obtained. Taxonomy annotation was performed according to the Silva database (Quast et al. 2013), where reads that were annotated to chloroplasts and mitochondria were removed. Alpha diversity indices, including Shannon index, phylogenetic diversity, observed feature, Simpson index, and Chao1 index were calculated in QIIME2. Beta diversity was measured using Bray–Curtis and Jaccard distance matrices using the vegan (version 2.6-4) (Oksanen et al. 2013) package in R.
Metagenome sequencing and bioinformatic analysis
DNA extraction was conducted using the CTAB method (Lutz et al. 2011), and degradation degree, potential contamination, and DNA concentration were measured using Agilent 5400; samples that did not meet DNA quality requirements for library sequencing would be removed. DNA Library Prep Kit (NEBNext@ UltraTM, NEB, USA) was used to generate sequencing libraries following the manufacturer's recommendations. Sterile water was used as a negative control during DNA library construction. Index codes were then added to attribute sequences to each sample. DNA samples were fragmented by sonication to a size of 350 bp, and then DNA fragments were end-polished, A-tailed, and ligated with the full-length adaptor before further PCR amplification. PCR products were purified using the AMPure XP system, and then libraries were analyzed for size distribution by Agilent2100 Bioanalyzer and quantified using a real-time PCR procedure. Index-coded samples were clustered using a cBot Cluster Generation System based on the default methods of the manufacturer.
Raw sequence data of fungi, viruses, and bacteria were obtained by metagenome sequencing performed at Illumina Novaseq high-throughput platform. Sequence data were preprocessed using KneadData (0.7.4) (McIver et al. 2017) to remove the adapters, and then low-quality bases and host sequence were filtered using Trimmomatic (0.39) (Bolger et al. 2014) and Bowtie (2.3.5.1) (Langmead & Salzberg 2012). DIAMOND software (0.8.22) (Buchfink et al. 2015) was used to blast and annotate the clean reads with the antibiotic resistance gene database (CARDS) (Alcock et al. 2019) with an e-value cutoff of 1 × 10−5. Annotation of ARGs was completed in January 2023. Clean reads were also annotated for exploring horizontal gene transfer in microbial communities using the mobile genetic element database (MGE) (Pärnänen et al. 2018). Metagenome reads that failed to match the ARG and MGE database were filtered, and the gene abundance was obtained by counting the number of matches to each ARG and MGE database sequence for each sample based on the results of the matching.
Statistical analysis
Results of gut microbiota diversity and taxonomic abundance were obtained from 16 amplicon data. Five alpha diversity indices (Shannon, Chao1, Observed ASVs, phylogenetic diversity, and Simpson index) were calculated to evaluate the alpha diversity within samples. We used Kruskal–Wallis test to compare alpha diversity indices between different grazing areas. Comparison in microbial community structure was statistically conducted using analysis of similarity (ANOSIM) in the vegan package with 999 permutations and visualized by principal coordinates analysis (PCoA). We also compared the beta dispersion of samples within groups using the betadisper function in the vegan package. To deal with the unbalanced design in our study, PERMANOVA analysis (999 permutations) of ASV, ARG, and MGE composition was conducted using PRIMER software (version 7) with type III sums of squares (Anderson et al. 2008; Anderson 2017). All ASV, ARG, and MGE tables that present less than 20% of the total metagenomic sequencing samples were removed in statistical analysis. Differential analysis of bacterial taxa was performed using the LEfSe algorithm (Segata et al. 2011). DESeq2 (version 1.38.3) (Love et al. 2014) was used to identify differentially abundant genes of ARGs and MGEs between grazing areas. Procrustes analyses (Peres-Neto & Jackson 2001) with 999 permutations were conducted to assess the correlations between microbial profile (ASV level), ARGs, and MGEs, and visualized by PCA. The significance threshold was set as 0.05 in all analysis, and results from multi-comparison were corrected using the Benjamini–Hochberg procedure. Network analysis was conducted in R using package igraph (Csardi & Nepusz 2006) and psych (Revelle 2022), and visualization of the result was performed using the “Force Atlas” layout in the gephi software (version 0.9.2). Correlations analysis was performed using the Spearman method. A total of 695 ARGs, 108 MGE subtypes, and 345 bacterial species were used for network analysis after filtering out edges with adjusted P values less than 0.001 and absolute values of correlation coefficient less than 0.6.
Ethical statement
Our study conforms to the legal requirements of China, including those processes relating to animal welfare, conservation, and the journal's policy. The experimental procedures conform to the animal experimental protocol prescribed by the academic committee of the Shaanxi Institute of Zoology (ECSIZ-Protocol No. 18–20). The author who conducted the animal experiments has been trained by the Beijing Agency for Experimental Animals, China, with an authorized diploma.
RESULTS
Gut microbial diversity of pika
Mean values of alpha diversity indices were significantly lower in CG areas as compared to PG or RG areas (Fig. 1b,c; Table S2, Supporting Information). Specifically, the variance of Shannon and phylogenetic diversity indices increased in the RG and CG areas. There was also a significant effect of grazing intensity on the gut microbial community (Table 1, Fig. 1d,e). We found that samples from the PG area were clearly separated from the RG or CG area, while samples from the RG area and CG area overlapped more compared to the PG area. Additionally, the dispersion of samples was also significantly different between the grazing areas (Table 1).
| F | P-value | ||
|---|---|---|---|
| Beta dispersion | 4.98 | 0.01 | |
| R | P-value | ||
| ANOSIM | 0.17 | < 0.001 | |
| pseudo-F | P-value | R2 | |
| PERMANOVA | 2.41 | 0.001 | 0.08 |
- ANOSIM, analysis of similarity.
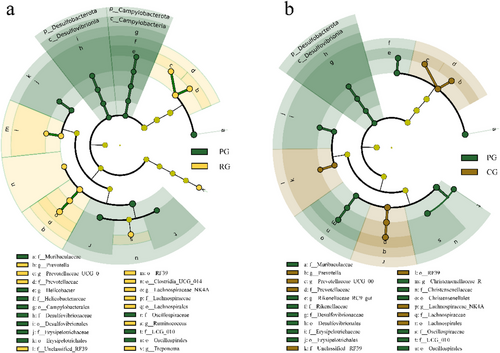
The predominant phyla in the cecum bacterial community were Firmicutes (mean ± SE: 56.18% ± 1.21%), Bacteriodota (31.87% ± 0.90%), Verrucomicrobiota (5.40% ± 0.52%), Spirochaetota (3.14% ± 0.48%), and Desulfobacterota (1.02% ± 0.14%). We compared the abundance of bacterial taxa. LEfSe results revealed that there were 26 differential taxa between PG and RG and 23 differential taxa between PG and CG. Samples from PG were characterized with enriched taxa in Desulfobacterota, Erysipelotrichales, Oscillospirales, and Muribaculaceae, as compared to RG or CG (Fig. 2). Clusters from the family Prevotellaceae and order Lachnospiraceae both enriched in RG and CG, as compared to PG.
Diversity of ARGs and MGEs
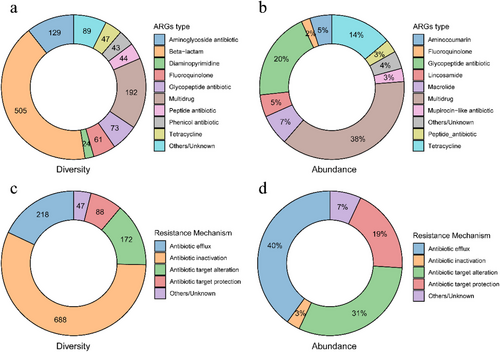
We found 1207 ARGs with a total of 24 types after filtering in the gut resistome of plateau pika. The top 10 ARGs were msbA (3.97% on average of abundance), rpoB2 (3.86%), Bado_rpoB_RIF (3.49%), novA (3.48%), macB (2.60%), efrB (2.49%), lmrD (2.19%), efrA (2.16%), teB46 (2.11%), and teb60 (1.98%). Beta-lactam contained 505 ARGs (Fig. 3a), while only accounted for 0.0016 of resistome abundance (Fig. 3b, included in the others). Multidrug (38%), glycopeptide antibiotic (20%), and tetracycline (14%) were the top three abundant ARG types with 192, 73, and 47 ARGs (Fig. 3). ARGs could be classified into six resistance mechanism groups, including antibiotic efflux, target alteration, inactivation, target replacement, reduced permeability to antibiotics, and target protection. More than 70% of the resistome abundance was contributed by antibiotic efflux and antibiotic target alteration mechanisms (Fig. 3d). We detected a total of 164 MGE subtypes consisting of 1148 genes. We found no significant effect of grazing intensity on the diversity (observed genes or elements) of ARGs (Fig. S1a, Supporting Information; χ2 = 0.24, P = 0.88) and MGEs (Fig. S1b, Supporting Information; χ2 = 0.85, P = 0.65). However, we found significant effects of grazing intensity on the composition of ARG (PERMANOVA: pseudo-F: 3.57, P = 0.007; ANOSIM: R = 0.24, P = 0.001) and MGE (PERMANOVA: pseudo-F: 2.22; P = 0.043) profiles in pika microbiome, while the ANOSIM of MGEs failed to reach a significant level (ANOSIM: R = 0.07, P = 0.13).
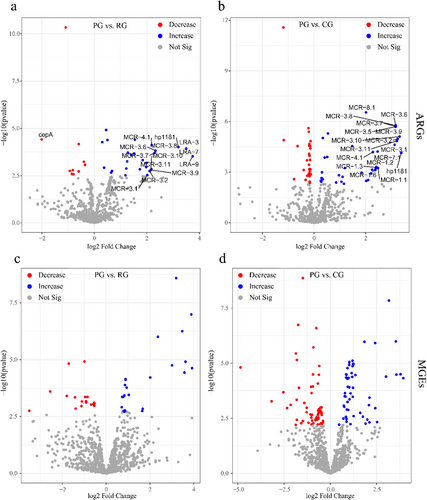
We used DESeq2 to analyze ARGs and MGEs that significantly differed between grazing areas. There were 31 and 41 (Table 2; Fig. 4b) ARGs enriched in RG and CG areas, respectively (Table 2; Tables S3,S4, Supporting Information). Most of the ARGs enriched in RG belonged to multidrug and peptide antibiotics, while those enriched in CG belonged to peptides, multidrug, and streptogramin. ARGs from mobilized colistin resistance (MCR) phosphoethanolamine transferase family accounted for the majority of the enriched genes. Similar to ARGs, we found that more differential MGEs were found between PG and CG (Fig. 4d), compared to a comparison of PG and RG (Fig. 4c). There are five types of MGEs enriched in grazing areas (RG, CG); they were insertion element-IS91, istB, istA11, plasmid, and transposase (Tables S5,S6, Supporting Information).
| Comparison | Drug class | Enriched in PG | Enriched in RG or CG |
|---|---|---|---|
| PG vs. RG | Aminoglycoside antibiotic | AAC6_Ie_APH2_Ia | |
| Beta-lactam | LRA-19, cepA, NmcR | LRA-7, LRA-9, LRA-3 | |
| Glycopeptide antibiotic | vanHO, vanXYE | vanRB, vanSB | |
| MLSB | ermQ | ||
| Multidrug | Sfra_tlrB_TYL, cfrA | MexA, AcrE, adeS, MexW, smeD, smeE, hp1181, Kpne_acrA, Eclo_acrA, Ecol_acrA | |
| Peptide antibiotic | eptA, ICR-Mc, MCR-3.1, MCR-3.2, MCR-3.5, MCR-3.6, MCR-3.7, MCR-3.8, MCR-3.9, MCR-3.10, MCR-3.11, MCR-4.1, MCR-7.1, MCR-8.1 | ||
| Tetracycline | tet(X) | ||
| Streptogramin | VatI | ||
| PG vs. CG | Aminocoumarin | novA | |
| Aminoglycoside antibiotic | AAC(6')-I30 | ||
| Beta-lactam | NmcR | ||
| Fluoroquinolone | patA | ||
| Fosfomycin | Ctra_murA_FOF | ||
| Glycopeptide antibiotic | vanHO, vanXYE | vanRB | |
| Lincosamide | lmrD, LlmA_23S_CLI, lmrC | ||
| Macrolide | macB | ||
| MLSB | erm(36) | ||
| Multidrug | efrA, efrB, msbA, Sfra_tlrB_TYL, chrB, rpoB2, Bado_rpoB_RIF | MexC, mtrC, smeD, hp1181 | |
| Peptide antibiotic | eptA, ICR-Mc, MCR-1.1, MCR-1.2, MCR-1.3, MCR-1.4, MCR-1.6, MCR-1.9, MCR-1.10, MCR-2.1, MCR-2.2, MCR-3.1, MCR-3.2, MCR-3.5, MCR-3.6, MCR-3.7, MCR-3.8, MCR-3.9, MCR-3.10, MCR-3.11, MCR-4.1, MCR-6.1, MCR-7.1, MCR-8.1 | ||
| Phenicol antibiotic | catB2, Paer_catB6 | ||
| Streptogramin | vatA, vatB, vatC, vatD, vatH, vatI | ||
| Tetracycline | tetB(46), tetA(60), tetB(60), tet(X), tet(M), tet(O), tet(Q), tet(S), tet(T), tet(W), tetB(P), tet(44), otr(A)S.rim, tet(W/N/W) | ||
| Tunicamycin | tmrB |
- CG, continuous grazing; PG, prohibited grazing; RG, rotational grazing.
Co-occurrence pattern of bacterial species, ARGs, and MGEs
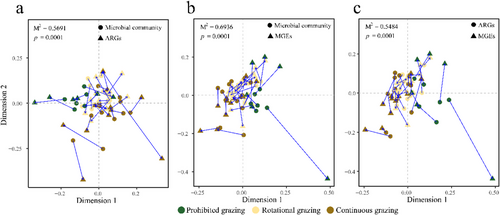
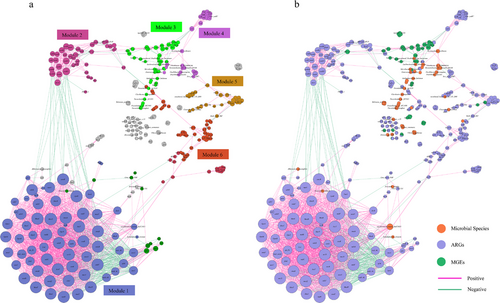
Procrustes analysis reveals an overall correlation between microbial community and ARG or MGE (Fig. 5). We found there was a significant congruence between microbiota and ARG (Fig. 5a, M2 = 0.5691, P = 0.0001), or ARG and MGE (Fig. 5c, M2 = 0.5484, P = 0.0001). Moreover, we observed a significant correlation between microbiota and MGE (Fig. 5c, M2 = 0.5484, P = 0.0001). Co-occurrence network analysis was conducted to explore the detailed relationship between microbial community and ARG/MGE. The network showed 387 nodes, 2187 edges, and 42 modules (Fig. 6; Fig. S2, Supporting Information). The positive correlations between these nodes accounted for 87.20% of the total edges, while 73.90% of the total nodes are ARGs. We identified six complex modules; among these modules, modules 1 and 2 were the most complex with 67 and 40 nodes, respectively (Fig. 6a). Module 1 consisted of 65 ARGs and 2 bacterial species. Species Uncultured bacterium BAC25G1 was shown to be positively associated with 21 ARGs. Species [Eubacterium] siraeum was negatively associated with cmlA. Module 2 consisted of 39 ARG nodes and one MGE node. Vancomycin resistance genes and their variant (vanR, vanS) were predominant nodes in module 2. There were 54 microbial nodes in our network, of which 37 edges were related to ARGs, 8 edges were related to MGEs, and 41 belonged to the association between microbes (Fig. 6b; Table S7, Supporting Information). We also observed a total of 47 MGE subtype nodes with 115 edges, among which 26 edges were negatively correlated to ARGs, like vanR variant, MCR, tet, van, and vat, and 69 edges were positive correlations between MGE subtypes. We found that most of the correlations between MGEs and ARGs were negative; only intI1, Xis_Tn916, and tnpA13 were positively correlated to the presence of tet(Q), Saur_mupB_MUP, and poxtA, respectively.
DISCUSSION
Here, we investigated the effect of grazing intensity on pika gut microbiota and resistome. We observed that the variance of microbial alpha diversity increased with the grazing intensity (Fig. 1b,c), and samples from continuous grazing areas were more dispersed than those from the prohibited areas, which altogether hinted that disturbance of pika gut microbiota by livestock grazing was context-specific. The livestock and environmental factors in different areas may play different roles in shaping the gut microbiota of plateau pika. Grazing intensity had no effect on the diversity of ARGs and subtypes of MGEs (Fig. S1, Supporting Information), but influenced the overall profile of ARGs and MGEs. Moreover, pika from CG areas enriched more ARGs than those from PG areas. Last, we found that microbial composition, ARGs, and MGEs in the gut microbiome of pika were closely correlated. This evidence is crucial for understanding the distribution of ARGs in wildlife under human disturbance.
Across-species studies reveal that species identity is more predominant in shaping the small mammal microbiota than habitat type (Kartzinel et al. 2019; Knowles et al. 2019), whereas within-species studies usually emphasize the host diet and environmental factors as important drivers in shaping the structure of mammalian gut microbiota (Carmody et al. 2015; Li et al. 2020; Li et al. 2021; Zhao et al. 2022). Previous studies have found that grazing exerts an indirect influence on wildlife gut microbiota. Li et al. (Li et al. 2019a; Li et al. 2021) found that vole's gut microbiota could be altered by plant diversity and coverage caused by continuous sheep grazing. Specifically, dietary species richness is positively correlated to the alpha diversity of gut microbiota in voles (Li et al. 2021). In this study, we found grazing intensity is negatively associated with the alpha diversity of pika gut microbiota. Grazing by livestock may decrease the dietary plant richness of the sympatric area, which indirectly alters the pika gut microbiota. However, the plant community in the pika habitat did not always predict their dietary richness (Li et al. 2016). Future research should assess pika's diet to quantify the contribution of plant species, which overlapped with livestock diet, in modulating pika gut microbiota. According to beta dispersion analysis, we observed that samples from the grazing areas exhibited increased interindividual variation compared to prohibited grazing areas (Table 1; Fig. 1d,c). Evidence from enclosure experiments reveals that livestock grazing could increase the pika population density compared to pika in ungrazed areas, which indicates that the aboveground biomass of plants is unlikely to be a limiting factor for pika fitness (Li et al. 2019b). Selective forage of livestock could change the diet composition of pika (Li et al. 2019b), and this modification may differ across pika burrows. Additionally, pika was demonstrated to display interspecific coprophagy on yak feces in the area where yaks were abundant (Speakman et al. 2021). Heterogeneity of habitat in grazing areas might contribute to interindividual variations of pika gut microbiota.
We found that bacterial lineage Prevotellaceae and Lachnospirales were significantly enriched in pika gut microbiota under grazing areas, as compared to ungrazed areas. Previous studies in pika argued that increased Prevotella in gut microbiota may be associated with the degradation of cellulose (Dai et al. 2015; Li et al. 2016). Genus Prevotella is abundant in yak stomach (Xin et al. 2019); the coprophagy behavior of pika in yak grazing areas may increase the abundance of Prevotellaceae lineage. In another study, researchers found that successive sheep grazing could significantly increase the abundance of lineage Prevotellaceae in the feces of Brandt's vole (Lasiopodomys brandtii) (Li et al. 2019a). Similarly, Lachnospiraceae is demonstrated to produce cellulose and hemicellulose-digesting enzymes in cow stomach (Ziemer 2014). We found lineage Desulfobacterota, Erysipelotrichales, and Oscillospirales were enriched in the prohibited grazing group (Fig. 2). These microbes are also found to co-occurr with Oscillospirales spp., which are recognized as next-generation probiotics (Chen et al. 2021). Chen et al. (Chen et al. 2021) also reported that the abundance of Desulfovibrio was positively correlated to the alpha diversity of gut microbiota. Desulfovibrio spp. could utilize organic nitrogen compounds in the human intestine (Willis et al. 1997). Biomass of legume species in the pika diet may influence the abundance of lineage Desulfobacterota. Several studies have documented the potential roles of Erysipelotrichaceae in host lipid metabolism and inflammation-related colonic disease (Kaakoush 2015).
Antibiotic resistance could be transferred at the human–wildlife interfaces, which poses major threats to both human health and wildlife conservation (Zhu et al. 2013; Bornbusch & Drea 2021; Laborda et al. 2022; Lee et al. 2022). China is one of the largest antibiotics consumers in the world, with more than 40% of the antibiotic products used in animal husbandry (Zhu et al. 2013; Van Boeckel et al. 2015). The growing husbandry industry and human disturbance are accelerating the dissemination of ARGs between humans and wildlife. For example, evidence from the colistin resistance gene MCR indicates that ARGs could be transferred from domestic animals to wildlife and humans (Kozak et al. 2009).
By assessing ARGs in the cecum content of plateau pika populations, we pictured the presence and diversity profile of wildlife ARGs across human disturbance gradients. Multidrug was the predominant ARG type in the pika gut microbiome with 38% relative abundance of the total genes (Fig. 3), which was consistent with other findings in mammalian species (Wang et al. 2021; Begmatov et al. 2022). We observed that pika from RG and CG groups enriched many ARGs belonging to multidrug and peptide antibiotic types (Table 2; Tables S3,S4, Supporting Information). Specifically, we found many MCR variants were enriched in grazing areas. MCR genes confer resistance to colistin in many pathogenic microbes, like Escherichia coli, Salmonella enterica, and Aeromonas spp. The overuse and misuse of colistin as a growth promoter in animal husbandry contribute to the global spread of MCR. In response to this, the use of colistin in the animal food chain has been banned in many countries; however, the global prevalence of MCR-1 is reported substantially in many clinical cases (Gharaibeh & Shatnawi 2019; Shen et al. 2021). Although colistin was banned in China as a feed additive in 2017, it may take years of regulatory implementation in remote areas, like Qinghai Province, the sampling site of our study. We suspect that the presence of MCR-1 and their variants in our study may be caused by the antibiotic residues in manure or soil. In addition, we also found streptogramin (A) antibiotic gene (vatA, vatB, vatC, vatD, vatH, and vatI) enriched in grazing areas. The potential bacterial hosts of these ARGs were species from Escherichia, Aeromonas, Staphylococcus, Enterococcus, and Paenibacillus. This is also alarming given that the genera mentioned above are common human opportunistic pathogens.
Most remarkably, several ARGs conferring resistance to tetracycline(tet), beta-lactam (LRA), and MLSB (erm) were found to be higher in the PG area (Table 2; Fig. 4a,b). Although many studies indicate that livestock manure application could increase the abundance of ARGs (Pan et al. 2011; Qiao et al. 2012; Fahrenfeld et al. 2014), it is difficult to conclude that the enrichment of ARGs is contributed by livestock grazing. Studies from soil resistomes revealed that various ARGs were detected in an undisturbed environment (Yang et al. 2019). For example, Heather et al. found that remote Alaska soil is a reservoir for beta-lactamase resistance genes (LRA) (Allen et al. 2009). Similarly, vancomycin resistance genes were detected from 30 000-year-old permafrost soil (D'Costa et al. 2011). Lee et al. (Lee et al. 2022) found that wild boar (Sus scrofa) possessed a higher abundance of antibiotic resistance bacteria than grazing cattle. In relation to our study, pikas in grazing areas have diverse habitats compared to those from ungrazed areas and may acquire ARGs directly from yaks via feces, or indirectly from human wastes in the environment. The high prevalence of MCR and vat variants in the pika population poses a potential threat to both livestock and Tibetan herdsmen. The interrelationship among the microbial community, ARGs, and MGEs suggests the possibility of horizontal gene transfer between pika and the environment via microbes. We used co-occurrence network analysis to explore whether MGEs and certain bacteria species contribute to the enrichment of ARGs. In our study, most co-occurrence cases happened within ARGs and their variants, which is similar to the network conducted in wild tick resistome (Wei et al. 2022). We noted that class 1 integron-integrase gene (intI1) was negatively correlated to ARGs, like MCR (total variants), vanRB, and vatD, while positively correlated to tet(Q). A recent study reveals that the intI1 is the main contributor driving the transfer of ARGs in a water-sediment system (Deng et al. 2020). We also found that the Uncultured bacterium BAC25G1 was positively associated with many ARGs, and more attention should be paid to this bacterium in the future.
In summary, we provided new insight regarding the profiles of gut microbiota and resistome of plateau pika under different grazing intensities by using 16S full-length and metagenomic sequencing. Our data demonstrated that grazing practices in the Qinghai–Tibetan Plateau may play an underestimated role in shaping wildlife gut resistome. Given that intensive livestock grazing could increase the risk of population outbreak of sympatric plateau pika (Li et al. 2019b), the accumulation of yak manure may be a vital factor for the prevalence of ARGs in pika population via interspecific coprophagy behaviors (Speakman et al. 2021). It is still difficult to conclude whether antibiotic residues in grazing areas are responsible for the high levels of ARGs in pika populations because climatic factors and global migratory birds may also be involved in the dissemination of ARGs across different regions. Therefore, continuous monitoring should be undertaken in the future. There were several limitations in this study. The plant community around pika burrows should be measured in future studies, which may help us to disentangle the role of dietary species richness on pika gut microbiota. Besides, the comparison of different grazing intensities at the same altitude is also vital to diminish the factors like oxygen concentration or plant diversity patterns that may potentially impact the pika microbiome. We also need to detect environmental antibiotic residues and conduct manipulated experiments to further elucidate the effect of yak feces on pika gut microbiota.
ACKNOWLEDGMENTS
This work was supported by the Second Tibetan Plateau Scientific Expedition and Research Program (STEP, 2019QZKK0501), the National Natural Science Foundation of China (32172436), and the Science and Technology Program of Shaanxi Academy of Science (2022K-16). We appreciate the help of Wekemo Tech Group Co., Ltd., Shenzhen, China in data analysis. [Correction added on 30 October, after first online publication: Missing funding information has been added.]
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interests.
Open Research
DATA AVAILABILITY STATEMENT
The original sequence data used in the study is available at NCBI Sequence Read Archive under accession number: PRJNA977813. [Correction added on 10 June 2024, after first online publication: Data availability section and text has been added.]




