Multiple interaction networks reveal that Lepidoptera larvae and adults prefer various host plants for diet and pollination
Abstract
Plant–Lepidoptera interactions are often studied using the pollination or herbivore networks only. Lepidoptera species are involved in two types of plant–insect interactions because they are herbivores as larvae and pollinators as adults. The study of entangled networks is critical, since the interaction of different networks can affect the overall network and community stability. Here, we studied the interaction of plants and Lepidoptera on the Yongxing Island, South China Sea. A plant–lepidopteran pollination network and a plant–lepidopteran herbivore network were built by using data from flower–pollinator and leaf–herbivore interactions. We then combined the two networks into a single network. We measured plant composition similarity within each sub-network and across sub-networks for Lepidoptera species. Our findings indicate that the plant–Lepidoptera pollination network and the herbivory network share significant proportions of Lepidoptera but small proportions of plant assemblages. The pollination network had higher nestedness and connectance than the herbivore network. Agrius convolvuli was the most specialized species, while Zizina otis had the highest species strength in the pollination network. Most Lepidoptera species were highly specialized in the herbivore network and their importance positively correlated across the two networks. Furthermore, there was no dietary composition similarity between the two networks for most Lepidoptera species. Our findings highlight the visible structural difference between the pollination and the herbivore networks. Adult Lepidoptera selects different plants for oviposition and feeding, a strategy that may benefit their reproduction and survival by sustaining adequate resources for their two life stages and the diversity of both plants and insects in oceanic island communities.
INTRODUCTION
Networks are effective methods for characterizing the complexity of ecological community (Landi et al. 2018). Over the last two decades, network approaches have been widely used to study a variety of community-level interactions, including mutualistic, parasitic, and facilitative interactions (Bascompte et al. 2003; Verdú & Valiente-Banuet 2008; Fang & Huang 2016; Delmas et al. 2019). By quantifying many structural metrics and demonstrating the implications of theses metrics for the maintenance and stability of community, the network approach has improved our understanding of ecological dynamics and symbiotic mutualisms (Yoshikawa & Isagi 2013; Fang & Huang 2016). However, most network studies focus on a single type of biological interaction, such as competitive, mutualistic, or antagonistic, while those involving multiple types of interactions are still relatively rare (Sauve et al. 2016; García-Callejas et al. 2018; Revilla et al. 2021; Timóteo et al. 2022; Yan 2022; Yelenik et al. 2022).
In natural communities, most species simultaneously participate in several types of interactions with other species, resulting in the interconnection of various networks (Ings et al. 2009; Melián et al. 2009; Yoshikawa & Isagi 2013; Hackett et al. 2019). A plant species' flowers, for example, can be pollinated by visitors, its leaves consumed by herbivores, and its seeds dispersed by birds, implying that pollination, herbivory, and seed dispersal networks all operate concurrently. Furthermore, due to ontogenetic shifts, the species' role may change throughout its life cycle. Lepidoptera (e.g. butterflies and moths) are herbivores that eat leaves as larvae and pollinate flowers as adults (Altermatt & Pearse 2011; Johnson et al. 2017); for example, the hawkmoth Cephonodes hylas larvae fed on the leaf of Guettarda speciosa and their adults pollinated on the flowers of Morinda citrifolia, Scaevola taccada, and Stachytarpheta jamaicensis (this study, Fig. 1). As a result, the plant–Lepidoptera adults' pollination network and the plant–Lepidoptera larvae herbivory network communicate with one another via shared Lepidoptera and plants. At various life stages, Lepidoptera species are involved in pollination and herbivory networks, producing different types of positive mutualistic interactions and negative antagonist interactions in the overall network of plant–Lepidoptera interaction. Although parasitic insects may benefit from specialization, Lepidoptera only live as larvae on plants and their adults may visit multiple plants. It was reported that plant–herbivore interactions may favor specialization, whereas plant–pollinator interactions may favor generalization (Thompson 2005; Fontaine et al. 2009; Fontaine & Thébault 2015). Plant–animal networks may be disturbed; thus exploring the relationship between adult and larval food sources may be critical in the face of significant biodiversity loss (Altermatt & Pearse 2011). The integration of different network types has the potential to change the network's stability (Fontaine et al. 2011; Mougi & Kondoh 2012; Sauve et al. 2014). Studies of plant–Lepidoptera interactions and networks combining plant–Lepidoptera pollination and herbivory networks are still scarce (Martins & Johnson 2013), which will have potentially significant but largely unexplored implications. Entangled interactions, such as plant–Lepidoptera pollination and herbivory networks, may evolve between plant and Lepidoptera species assemblages. It is critical to integrate interaction types for assessing the ecological and evolutionary implications of complex networks.

Pollination interactions benefit plant reproductive success (Ollerton et al. 2011; Grass et al. 2018), whereas herbivore foliar damage lowers fitness and has a strong impact on plant growth and reproduction (Strauss et al. 2002; McCall & Irwin 2006). Herbivores that eating leaf potentially influencing the resource availability of surviving flower especially in herbaceous plant. Leaf herbivory, for example, has been shown to affect floral characters (Strauss et al. 1996), flowering phenology and number (Mothershead & Marquis 2000; Hanley & Fegan 2007), floral odor (Schiestl et al. 2014), and nectar or pollen production (Bruinsma et al. 2014). These changes may have an impact on the behavior of flower visitors (Poveda et al. 2003; Lucas-Barbosa et al. 2013; Bruinsma et al. 2014), resulting in decreased reproductive success (Kessler et al. 2011). Pollinators prefer the morphological and olfactory cues of undamaged plants to those of herbivore-damaged plants (Kessler et al. 2011; Schiestl et al. 2014). As a result, we propose our first hypothesis that adult Lepidoptera choose different plant species for oviposition and pollination, that is, adults pollinate more plant species than larval host plant species.
As plant ontogeny develops, herbivores feeding on leaves frequently turn into florivores on flowers, and this transformation may thus induce resistance in flowers (McCall et al. 2018). Leaf damage, for example, can cause petal changes which reduce the fitness of Spodoptera exigua larvae (McCall et al. 2018). In the period of relative small levels of initial leaf damage, plants may protect themselves from floral damage through induction of resistance (McCall et al. 2018). It might be a practical way for Lepidoptera species to choose the same plants for food since the flowers could be intact. Contrary to our first hypothesis, plants have been reported to be consumed and pollinated by different stages of the same Lepidoptera species (Irwin 2010; Kessler et al. 2010; Altermatt & Pearse 2011). For example, the hawkmoth Manduca sexta and the tobacco plant Nicotiana attenuata interact (Kessler et al. 2010). Furthermore, adults prefer to oviposit on plants that offer their food sources, so they will oviposit on plants which are the most suitable for their nectar productiveness (Scheirs et al. 2000; Scheirs & De Bruyn 2002). Adult M. sexta, for example, lays more eggs on Datura stramonium flowers that were supplemented with extra nectar than on un-supplemented plants or plants that flower nectar were removed (Adler & Bronstein 2004). Flower nectar has been reported to contain some important information for larvae about the appropriateness of leaves, that is, leaf defenses (Adler & Bronstein 2004; Adler et al. 2012). Danaus plexippus has also shown that the existence of poisonous cardenolides in nectar influences oviposition choices of adults, and flower nectar has a positive correlation with the level of leaf cardenolide in milkweeds (Manson et al. 2012; Jones & Agrawal 2016). As a result, our second hypothesis is that adult Lepidoptera prefers the same plant species for oviposition and pollination, that is, adults pollinate the same plants as larval host plant species.
Studies simultaneously considering species importance in multiple interaction types under a network framework have started to appear in recent years, while their conclusions are contradictory (Olesen et al. 2018; Mello et al. 2019; Timóteo et al. 2022). For instance, Olesen et al. (2018) found that the importance of birds in seed dispersal network and pollination network is positively correlated. However, the importance of bats in frugivory network and nectarivory network has no correlation (Mello et al. 2019). Recently, a meta-analysis suggested that species importance is positively associated across multiple ecological interaction networks (Timóteo et al. 2022). To fully understand the ecosystem structure and function, more experimental researches involving multiple interaction types in the same community and space-time are needed and important.
Here, we characterized the pattern of plant–Lepidoptera interactions in the Yongxing Island community, South China Sea, by constructing qualitative pollination and herbivory networks and integrating the two network types into the overall plant–Lepidoptera network. We hypothesized that the network-level structural metrics of these entangled sympatric networks would be different, as proposed by comparison of different network types (Thébault & Fontaine 2008; Fontaine et al. 2009; Olesen et al. 2018). We used two metrics (connectance and nestedness) to characterize the networks' structures and analyzed the species-level characteristics (specialization and species strength) of shared Lepidoptera species using pollination and herbivory networks. The importance of Lepidoptera species was presented by species-level index species strength, since it reflects the potential impact of species on the other species which it links (Bascompte et al. 2006; Timóteo et al. 2022). We examined the variation in plant-feeding breadth, including dietary specialization. Furthermore, to understand the relationship between Lepidoptera larval and adult food sources and to test which hypothesis this relationship supports, we investigated whether or not Lepidoptera larvae and adults choose the same plants for food plants.
MATERIALS AND METHODS
Study site and periods
The Yongxing Island (16°49′N, 112°20′E) is the largest islet of Xisha Islands with a total area of 2.6 km2, locating in South China Sea. We collected pollination and herbivore data on plants and Lepidoptera from 15 October to 30 December 2018 on this oceanic island. Field surveys of all plant species were conducted along the roads of the island. In the two continuous months, the set of plant species in bloom and active Lepidoptera species had not altered. We collected data at species rather than quadrats, to maximize the probability of identifying all floral pollinators and leaf herbivores. This system is well-suited for such a study because the moderate numbers of plant and Lepidoptera species on oceanic island are beneficial to collect relative complete data in the field.
Pollination data collection
Pollination observation was conducted on sunny days without wind across two consecutive months, from 9:30 AM to 7:30 PM. We recorded Lepidoptera visits to flowers of each plant species during field observations. We observed pollination on a different route in sunny days and walked the entire island once every 2 days. During our observation, one visit was recorded every time a Lepidoptera visitor made contact with the stigma and/or anther of a flower exceeding 1 s. One Lepidoptera species that visited one plant species more than 10 times in the entire observation period was confirmed as a potential pollinator (hereafter, named pollinator). All Lepidoptera that visited flowers were recorded, regardless of their visit efficacy. As some hawkmoth species are active in the night, we also conducted light-trapping surveys. To verify if the hawkmoths sampled in light traps were pollinators, we examined the pollen grain loads of each species' individuals for pollen analysis. We took off pollen grains deposited on the beak of hawkmoths using gelatin cubes and removed them to new slides. These slides were slowly heated to melt the jelly, and then covered by coverslip to fix the pollen sample. Pollen grains on slides were observed under electron microscope. We identified pollen grains by comparing with pollen that was collected from other co-flowering plants in the community. If the hawkmoth species carried pollen from the plants they visited, we assumed that this hawkmoth species was the pollinator, although we did not evaluate the visitation efficiency to host plant species. All Lepidoptera pollinators were first divided into butterflies and hawkmoths in the field and then captured for further identification to the species level later by an entomologist (see Acknowledgments). Specimens of plant species and Lepidoptera species are all deposited at the South China Botanical Garden, Chinese Academy of Sciences herbarium.
Herbivore data collection
In this study, herbivores were represented by Lepidoptera larvae, which feed on leaves and were leaf-chewing herbivores, leaving distinct bite marks easily obvious on the leaf surface. All externally feeding holometabolous Lepidoptera larvae were captured by tweezers from the leaves of plants. Most of the leaves from all plants that we could contact were carefully examined. We took damaged leaves and Lepidoptera larvae indoors and reared them in the insect feeding box. We offered them new fresh leaves that were collected from their host plant species until eclosion, which would be identified. The process of rearing larvae to adults helped us to construct the plant–Lepidoptera herbivore interactions correctly, because damaged leaves offered an accurate record of herbivory. We checked all damaged leaves and collected the Lepidoptera larvae from all the plant species in our study periods in Yongxing Island community. We reared a total of 22 Lepidoptera species, and host plants of another three butterflies (Borbo cinnara, Potanthus confucius, and Vanessa indica) were confirmed by searching relevant literature and books. Herbivore guiled was collected from 65 plant species in the community for 2 consecutive months. We sampled each plant species with equal effort to reduce biases and only retained records for Lepidoptera larvae that were successfully reared to adults.
Network-level indices
We constructed three qualitative plant–Lepidoptera interaction networks. The whole network was composed from all the interactions between plants and Lepidoptera insects. We constructed plant–Lepidoptera pollination network and plant–Lepidoptera herbivore network from the collected data of flower–pollinator and leaf–herbivore interactions, respectively. Pollination network was established by direct observation and pollen load analysis. Each network was bipartite, with links between plant species and Lepidoptera species. To evaluate the degree to which the two networks share species, the similarity of Lepidoptera and plant species between the pollination network and the herbivore network was first measured. We used the Sørensen index for measuring the similarity, as this index is resilient to under-sampling (Rempala & Seweryn 2013). This index, also known as Sørensen's similarity coefficient, is a statistic used for comparing the similarity of two samples. Its value ranges from 0 to 1, with larger values suggesting higher similarity. We calculated the cluster coefficient for each network, which showed the number of realized links divided by the number of possible links. We characterized the network-level structure of the plant–Lepidoptera interactions using two most appropriate indices based on a binary network: connectance and nestedness. Connectance measured the realized ratio of possible links, and it is corrected with network generalization (Dunne et al. 2002a). Networks that included more generalized species typically show high connectance and can enhance network robustness through increased redundancy of the species they interacted with (Winfree et al. 2014; Lara-Romero et al. 2019). Network robustness is a critical aspect of community stability, which reflects the tolerance of communities for local species extinction (Dunne et al. 2002b). Nestedness defines the extent to which specialist species interact with subsets of species that generalist species also interact with in the network (Almeida-Neto et al. 2008). Networks with high nestedness means high redundancy which can enhance network robustness against the random extinction of specialist species, since the generalist core of the network is still stable (Blüthgen et al. 2008; Sebastián-González et al. 2015). The number of interacting species and sampling effort can affect network metrics (Blüthgen et al. 2006; Vizentin-Bugoni et al. 2016). Therefore, the significance of metrics was assessed by comparison with null model networks. The significances of the two metric values were tested by comparing them with the values of 1000 randomly generated networks from the “r2dtable” null models. This model returns a list of randomized matrices and keeps the number of interactions and the number of links constant. The 95% confidence interval for each metric was estimated from the 1000 simulated values, and if a metric value did not overlap with the confidence interval, it would be considered significant. Models yielded by this null model hold the same degree of distribution and connectivity with the focal network. Network plots and network-level indices calculation were carried out in the “bipartite” package (Dormann et al. 2008) in R3.4.4. We also drew the force-directed layout of three plant–Lepidoptera interaction networks in the “sna” package in R with the argument mod “fruchtermanreingold” and default parameters used for this layout (Pocock et al. 2016).
Species-level indices of Lepidoptera species
We characterized the behaviors of the shared Lepidoptera species between pollination network and herbivore network in relation to plant species. Sørensen index was used to measure the similarity in plant composition between the Lepidoptera species within each interaction network and between these two networks. Two species-level indices were calculated, which presented distinct network metrics of Lepidoptera: (1) species-level specialization (d′), which measures each species' specialization according to its discrimination from random selection of interacting species, and its values are between 0 (no specialization) and 1 (perfect specialists) (Blüthgen et al. 2006); and (2) species strength, which measures the sum of dependencies of each species and it aims at quantifying a species’ relevance across all its partners. Larger values of species strength indicate that more pollinators or plants depend on a specific plant or pollinator species (Bascompte et al. 2006). These two species-level indices of each Lepidoptera species were calculated for three networks, respectively. The species strength represented the importance of each Lepidoptera species for the two subnetworks and two species strength values of each Lepidoptera species, respectively, reflecting its importance as a pollinator and as an herbivore for the community. To examine the relationship of the Lepidoptera species' importance between herbivore network and pollination network, we performed Pearson correlation analysis between two values of species strength for the two subnetworks in SPSS 19.0. We also estimated the sampling completeness for each constructed network, following Chacoff et al. (2012), and computed the Chao 1 estimator of species richness using the iNEXT package (Hsieh et al. 2014) in R. All network-related indices calculation was carried out in the “bipartite” package (Dormann et al. 2008) in R3.4.4.
RESULTS
Properties of the three network types
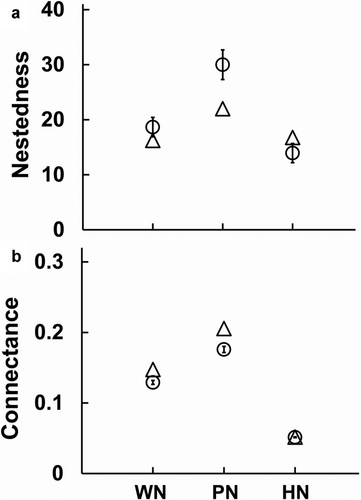
A total of 188 links between 25 Lepidoptera species and 51 plant species distributed in 21 plant families were recorded in our study periods (Fig. 2a). We recorded 164 links between 42 plant species and 19 Lepidoptera species in the pollination network (Fig. 2b) and 31 links between 24 plant species and 25 Lepidoptera species in the herbivore network (Fig. 2c). Only 15 plant species were shared between the pollination and the herbivore networks. Mean numbers of links per species and cluster coefficient were 2.5/0.14 in the whole network, 2.7/0.19 in the pollination network, and 0.6/0.04 in the herbivore network, respectively. The pollination network and herbivore network shared 15 plant species and 19 Lepidoptera species. Among the 188 links, only seven links occurred in both the pollination network and the herbivore network. The Sørensen indices present species composition similarity between the pollination network and the herbivore network in our study. The values of the Sørensen indices in Lepidoptera and plant species were 0.864 and 0.455, respectively. Sampling completeness (%) in the whole network, pollination network, and herbivore network is 82.5%, 95.2%, and 35.7%, respectively (Table S1, Supporting Information). Network-level indices nestedness and connectance were significantly different among the three interaction network types (Fig. 3; Fig S1, Supporting Information). The values of nestedness and connectance were significantly different compared with the null model in all the three networks (t test, all P < 0.0001). Pollination network had the highest nestedness (22.00), followed by the herbivore network (16.78) and the whole network (16.24). The herbivore network had higher nestedness than null models, while whole network and pollination network both had lower nestedness than null models. Pollination network had the highest connectance (0.21), followed by the whole network (0.15) and the herbivore network (0.05). For connectance, all the three networks showed significantly higher values compared with the null models.
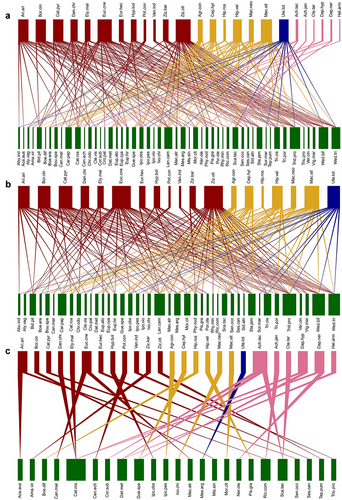
Species-level indices of the shared Lepidoptera species
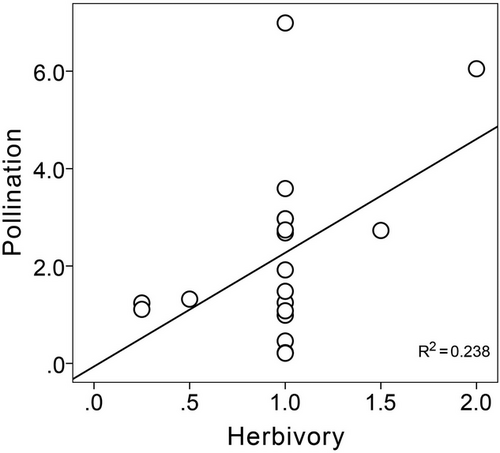
In both the whole network and the pollination network, the species with the most links was the Euchrysops cnejus, followed by Zizina otis and Macroglossum sitiene (Fig. 2). These three species accounted for 27.1% and 30.5% of all links in the whole network and the pollination network, respectively. In the herbivore network, only Acherontia lachesis had three links, and most Lepidoptera species had only one link (Fig. 2; Fig. S1, Supporting Information). In the whole network, Daphnis nerii was the most specialized species (d′ = 0.61) while Z. otis presented the highest value of species strength (7.69). In the pollination network, Agrius convolvuli was the most specialization species (d′ = 0.60) while Z. otis presented the highest value of species strength (6.99). In the herbivore network, most species had the same values of specialization (d′ = 1) and species strength (Tables S2,S3, Supporting Information). In the pollination network, the mean similarity between the Lepidoptera species pairs was 0.27 (±0.01), and 16.4% of all the vales were zero (Table S4, Supporting Information). However, in the herbivore network, there was no similarity (0) between the Lepidoptera species pairs except Danaus chrysippus and Elymnias malelas (Table S4, Supporting Information). 68.4% Lepidoptera species showed no dietary composition similarity (0), and the rest of the species showed very low similarity (0.13–0.29) for dietary composition between the pollination network and the herbivore network (Fig. 3; Table S3, Supporting Information). There was a positive significant correlation of Lepidoptera's species strength between the pollination network and the herbivore network (r = 0.488, P = 0.034) (Fig. 4).
DISCUSSION
According to our findings, the plant–Lepidoptera pollination network and herbivore network share significant proportions of Lepidoptera but low proportions of plant assemblages. The network-level structures of pollination network and herbivore network were vastly different. Herbivore network had a significantly smaller network size than pollination network. The structural differences between the network types have been studied (Genini et al. 2010; Fontaine et al. 2011; Lima et al. 2020). Our community's pollination network and herbivore network also had different network-level structural properties. Nestedness was higher in the pollination network than in the herbivore network. Nested networks are distinguished by highly asymmetrical interactions and a highly interconnected species in the center, as observed more frequently in mutualistic interaction networks (Bascompte et al. 2003). The nested structure of pollination networks implies that there are many generalist species interacting with each other and forming a core to which specialized species bind. The structure of antagonistic networks, such as herbivory bipartite networks, is characterized by unified groups of interacting species but with few interactions among groups (Lewinsohn et al. 2006). This structure implies that generalist species are less common. The pollination network also had significantly higher connectance than the herbivore network. The relative generalization of most pollinators resulted in high network connectance in the pollination network. A previous meta-analysis on the structure of 34 pollination networks and 23 herbivory networks also found higher connectance and nestedness in pollination networks than in herbivory networks (Thébault & Fontaine 2010). Studies have shown that generalization was more prevalent in pollination networks, whereas specialization was more prevalent in herbivory networks (Memmott et al. 2004; Fortuna & Bascompte 2006; Fontaine et al. 2009). Our findings support the hypothesis that mutualistic networks have more generalist species than antagonistic networks, even when entangled sympatric networks share species assemblages.
In our study community, the pollination network has a high level of generalization, which corresponds with previous studies reporting generalist pollinator species being easier to establish on oceanic islands (Kaiser-Bunbury et al. 2009; Traveset et al. 2016; Wang et al. 2020a,b, 2021), and the herbivore network have a high level of specialization, which corresponds with other studies about herbivory (Forister et al. 2015). This is the first time compartments in the plant–Lepidoptera adult pollination network and plant–Lepidoptera larvae herbivore network have been reported. Only six of the 19 shared Lepidoptera species between the pollination network and the herbivore network have a few overlap of plants for herbivory and pollination. For each pair of Lepidoptera species, the plant assemblages differ significantly between the pollination network and the herbivore network. In the Yongxing Island community, Lepidoptera serves as an important pollinator for numerous plant species, especially for plants that bloom at night while acting as an herbivore for a limited number of plants.
The mobility of the two stages of Lepidoptera differs. Larvae frequently grow on the same plant on which they were born. For larvae diets, it would be increase the cost to be generalist if they travel because of the limitation of space. Adults, on the other hand, are more mobile and are likely to encounter a greater variety of plants (Picot et al. 2019). Pollination and herbivore generalization may be affected by environmental factors. If herbivores specialize on one plant species which is not visited by their predators, it may provide them a safe space, because their predators usually have searching patterns related to specific plant species (Jeffries & Lawton 1984). Pollinators may face less ecological pressure to specialize because their flower-visiting time is shorter than that of herbivores, which spend part of their development on plants (Fontaine et al. 2009). Therefore, difference ecological process between pollinators and herbivores may be one of the explanations for the differences of Lepidoptera species' specialization and species strength between pollination networks and herbivore networks. Herbivore specialization is also driven by plant chemistry. The variety of likely interactions in plant–herbivore interaction networks is partly determined by the adequacy of toxin composition in plants and herbivore detoxifying strategies (Wittstock & Gershenzon 2002). The potential benefits of being a generalist may be limited due to an evolutionary balance between their ability to detoxify many toxic compounds and their effectiveness of the detoxification processes (Fontaine et al. 2009). Specialization is the cost of being able to feed on increasingly toxic hosts, and generalist herbivores are more vulnerable to a given toxin than specialist herbivores (Cornell & Hawkins 2003). Highly specialized plant–herbivore networks are primarily the result of the evolution of chemical defenses in the plants, as well as the herbivore adaptation to these defenses (Becerra 2015; Forister et al. 2015).
Competition for flower rewards among pollinators is a vital character of interactions between plants and pollinators (Fontaine et al. 2009; Fründ et al. 2013), and it may cause the generalization of Lepidoptera pollination as a means of avoiding the competition among long-proboscis Lepidoptera species. Most Lepidoptera species in our community did not display dietary similarities for food–plant composition between the pollination network and the herbivore network, implying that Lepidoptera adults choose different plants for oviposition and pollination, that is, Lepidoptera adults do not choose their larval host plant for nectar resources. This pattern contradicted previous research (Altermatt & Pearse 2011), which found that Lepidoptera adults use nectar resources from their larval host plants as their diet more frequently than expected. Several Lepidoptera species in our area fed exclusively as larvae on wind-pollinated plants that did not secrete nectar.
Adult Lepidoptera are often searching for mates as well as larval host plants for oviposition in the same community. This complex behavior that combines feeding and reproduction may result in excessively using the same plant for the two activities (Forister et al. 2009; Altermatt & Pearse 2011). Using different plant species for the two behaviors may help to reduce the over-consumption of a single species. Our findings support our first hypothesis that adult Lepidoptera pollinated different plants than larval host plant species in the oceanic island community. Lepidoptera are more attracted to the morphology of undamaged plants than to plants on which they have laid their eggs. This pattern may be advantageous in maintaining their reproduction and survival in an isolated island community by sustaining adequate resources for their two life stages.
Our results showed that Lepidoptera species' importance was positively associated across the herbivore network and the pollination network. Our research provides new evidence and supports that the importance of species in a community is not only limited to a single one but also to multiple niche dimensions of their functional niche (Timóteo et al. 2022). Plant–animal interactions are not only vital to understand a single ecosystem function, such as pollination or herbivory, but also the multiple niche dimensions of interactions have important impacts on the structure of whole ecosystems and perhaps affect their tolerance to species extinctions or biological invasion (Fontaine et al. 2011). It is necessary to consider multiple species interactions for better understanding the complexity of ecosystems and the role of the same species in the different interaction networks. Studies combining multiple plant–animal interactions in the same community may deepen our understanding of how important species shape the structure and function of entire ecosystems.
There are three main limitations to our approach. First, we only analyzed binary networks, that is, the qualitative networks, since it is a big challenge to collect the quantitative data of plant–Lepidoptera pollination and herbivore networks in the Yongxing Island community. Weighted networks are much better for assessing a species’ local, realized niche, while binary data are better for looking into regional, fundamental niches (Corso et al. 2015; Fründ et al. 2016; Miranda et al. 2019). Second, the purpose of our study was to test our two hypotheses which could be realized by using monolayer networks. Meanwhile, whether a multilayer approach is more appropriate than a monolayer one depends on the specific research question (Pilosof et al. 2017). Thus, we still analyzed the tripartite networks formed by lepidopterans and plants as two separate bipartite networks. However, multilayer networks provide a versatile and powerful framework for investigations of community ecology, which allows us to address questions that are not feasible using monolayer networks. For example, multilayer networks can be used to study genetic relatedness between individuals as a function of both dispersal and intra-group social behavior, and reciprocal effects between ecological and nonecological systems (e.g. Baggio et al. 2016; Pilosof et al. 2017). Finally, we actually assessed the subnetworks and could not represent complete plant–Lepidoptera pollination or herbivore network. Our study might have some meanings for comparing the difference of interaction network structure among different interaction types in the same community at the same time from a functional perspective, although they may not represent complete local networks. The structure of networks changes across different scales, from the entire system to its layers, communities, motifs, dyads, and nodes (Minoarivelo & Hui 2016; Pinheiro et al. 2019).
This is the first study to examine the variation in interaction types in a plant–Lepidoptera network. We found complex structures of plant–Lepidoptera interaction networks in the Yongxing Island habitat. Our findings propose that studies about plant–animal networks need a cautious evaluation of each species pair's interaction type. In the future, studies on the integration of different types of interaction networks made up of a large range of taxa to clarify entire community dynamics will be required. In the Yongxing island community, Lepidoptera larvae and adults prefer various host plants for diet and pollination. This pattern might be important for preserving the diversity of plants and insects in the oceanic island community.
ACKNOWLEDGMENTS
We thank Meihong Wen for her help in the field. We are grateful to Prof. Min Wang, South China Agricultural University, for identifying Lepidoptera specimens. We highly appreciate the logistical support for field work of Xisha Ocean observation and research station, South China Sea Institute of Oceanology, Chinese Academy of Sciences, Guangzhou, China. This research was supported by the Natural Science Foundation of Guangzhou (grant number 202201010218), the National Natural Science Foundation of China (grant numbers 32271613 and 32170232), the Guangdong Forestry Bureau Fund (grant number 890-2020-XMZC-2758-01-0001), and the Science and Technology Basic Works Program of Ministry of Science and Technology of China (grant number 2019FY202100).
CONFLICT OF INTEREST
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.




