Matrilinear hierarchy in the American black bear (Ursus americanus)
Abstract
The American black bear (Ursus americanus) was long thought to be solitary and its social organization has not been well described. Here, we present new data on black bear social structure. The objectives of the study were to make detailed observations of the behavior of wild black bears to determine their social interactions and structure. We tested whether black bears interacted socially beyond mating and competing for resources, if black bears tracked relationships and interacted regularly even when resources were not limited, and whether the social structure of a population of black bears was based on a matrilinear hierarchy. We collected data by direct observation of bears from 1993 to 2014. Observations of 1210 social interactions at a provisioning site indicated that females compete and form matrilinear hierarchies. Dominant bears established a hierarchy for food, control of space, and control of younger bears. Post interaction scent marking took place, which suggested that dominant females were conditioning subordinates to their scent marks. Affiliative behavior occurred between related and unrelated bears and helped to establish the social structure of the bear community. Based on our data, human–bear conflicts can be reduced by behavioral modifications by humans when they encounter bears. Knowledge of bear behavior and the matrilinear hierarchy provide a basis for non-lethal management of bears that find themselves in a bear–human conflict situation.
INTRODUCTION
The social structure of many mammalian populations is dependent upon the degree of social interactions among members of a group. Mammals that live in stable social groups usually have dominance hierarchies (Smale et al. 1993; Frank 1996), but the nature of these hierarchies varies among species. Some wild mammalian carnivores such as gray wolves (Canis lupus) have well-developed social structures, which in wolves are based on a family group of a mated pair and surviving young of 2 seasons (Murie 1944; Mech 1966, 1970). Spotted hyenas (Crocuta crocuta) have a complex group structure (Kruuk 1972) within which a mother's social rank influences her cub's access to food, survival, and reproductive success (Frank 1996; Holekamp et al. 1996). Juvenile spotted hyenas and some primates acquire ranks immediately below those of their mothers (Holekamp & Smale 1991) through associative learning mechanisms (Engh et al. 2000). Other groups of living carnivores, however, like the African lion (Panthera leo), do not have a hierarchical system and there is no leader in a lion pride (Schaller 1972). Elephant seals (Mirounga angustirostris) establish social hierarchies in which the males of the highest rank remain near the breeding females but do not defend specific sites (LeBoeuf & Peterson 1969). Some monkeys and apes live in complex social groups with linear dominance rankings (Bekoff et al. 2002).
There is a high degree of behavioral variability among carnivores because some are group-living and others solitary (Gompper & Wayne 1996). Behaviors involve decision making based on potential costs and benefits of a particular action or choice. Solitary mammals probably learn behaviors through inheritance and asocial learning (Galef & Laland 2005). Social learning takes place through interaction with other animals, often a parent, and allows animals to quickly develop adaptive behaviors (Heyes 1994; Galef & Whiskin 2001). Such learning fosters establishment of social structures in populations.
Even for asocial animals, there is social learning and interaction among conspecifics. Bears are generally considered to be highly intelligent but relatively asocial animals (Morehouse et al. 2016). Information on their life history is reported by Rogers (1987), DeBruyn (1999), Kilham and Gray (2002), Noyce and Garshelis (2011), and Kilham (2013). Bears have a large brain, well-developed memory, behavioral plasticity, and curiosity (Gittleman 1986; Gilbert 1999; Mazur & Seher 2008; Morehouse et al. 2016). Knowledge about their social interactions would be useful in testing hypotheses about how their complex sociality and foraging behavior shape their cognition. Likewise, orangutans (Pongo spp.) have also been described as solitary. Nevertheless, long-term studies in nature indicate that female orangutans exhibit social hierarchies when territories overlap and that there is observational social learning and socially induced practice of routine skills in immature animals that contribute to high offspring survival (Schuppli et al. 2016; van Noordwijk et al. 2018).
Many species of bears feed communally where there are high concentrations of food. At those sites, clear hierarchies are established among brown bears (Ursus arctos), black bears (Ursus americanus), and polar bears (Ursus maritimus) (Storonov & Stokes 1972; Meslow 1983; Derocher & Stirling 1990; Craighead et al. 1995). Most social learning in bears probably occurs during the long period of mother–cub association, but even minimal parental care selects for social learning (Gilbert 1999; Goth & Evans 2005). Grizzly bears (U. arctos) learn behaviors from their mothers that can lead to conflict behaviors with people (Morehouse et al. 2016). Foraging behavior is learned from mothers in wild black bears in Sequoia and Yosemite National Parks (Mazur & Seher 2008). Hopkins III (2013) reports that mother–offspring social learning is the primary mechanism responsible for black bears foraging on human food in Yosemite, although some bears learn to forage on human food independently. These data suggest that bears have the ability to establish relationships among individuals and a social structure in a population. The question remains, however: What kind of social structure exists in populations of wild bears? The goals of this study were to make detailed observations of the behavior of wild black bears (U. americanus) to determine their social interactions and social structure. These data are important because information on their social structure will provide new insights into the cognitive ability of this species and will have important management implications for resolving bear–human interactions.
Brown bears have a social system in which matrilinear assemblages exist and related females use a common area that is largely exclusive (Støen et al. 2005). Among brown bears in Sweden and Norway, molecular genetics indicated that home ranges of related bears overlapped more than those of non-related bears (Støen et al. 2005). However, there were no data on the behavioral interactions among the bears that established and maintained the matrilinear hierarchies. Here, we describe a matrilinear hierarchy in a population of black bears (U. americanus) in New Hampshire, USA, based on 23 years of studies of bear behavior and 8 years of studies of behavioral interactions between related female bears at a communal feeding site. We tested the following hypotheses: (1) Black bears interact socially beyond mating and competing for resources. (2) Black bears track relationships and interact regularly even when resources are not limited. (3) The social structure of a population of black bears is based on a matrilinear hierarchy.
The data from our study provide a better understanding of the social structure of these bears and indicate that black bears have complex interpersonal relationships that are based on communication between bears and that support an organized society. Knowledge of the complex social behaviors of black bears can provide information for the behavioral management of the species and insights into alternative methods of managing “problem bears” in urban settings.
MATERIALS AND METHODS
Study area
The study area was roughly 132 km2 of northern hardwood forest comprising red oak (Quercus rubra), beech (Fagus grandifolia), sugar maple (Acer saccharum), red maple (Acer rubrum), white ash (Fraxinus americana), white birch (Betula papyrifera), and yellow birch (Betula alleghanienses) with patches of softwoods, including red spruce (Picea rubens), eastern white pine (Pinus strobus), hemlock (Tsuga canadensis), and balsam fir (Abies balsamea). It was located in west central New Hampshire in the eastern half of Lyme and the western half of Dorchester townships. Smarts Mountain, at 987 m above sea level, was the highest peak surrounded by a number of smaller hills and ridges. There were 4 major ponds and numerous wetlands in the study area that provided fruit from various shrubs, including blueberry (Vaccinium spp.), huckleberry (Vaccinium spp.), mountain holly (Ilex montana), winterberry (Ilex verticillata), nannyberry (Viburnum lentago), and dogwood (Cornus spp.). Logging throughout the area created openings with raspberry (Rubus spp.) and blackberry (Rubus spp.). The decaying wood and sunlight from logging increased production of ants (Formicidae), wasps (Vespidae), and grubs (Coleoptera). Acorns, beech nuts, fruit from the shrubs, ants, and other insects provided food for the bears (personal observation).
Methods
The data presented here were collected as part of a long-term study of bear social behavior beginning in 1993 when the senior author began studying the behavior of orphaned black bear cubs. We organized this study to test 3 hypotheses: (1) Black bears interact socially beyond mating and competing for resources. (2) Black bears track relationships and interact regularly even when resources are not limited. (3) The social structure of a population of black bears is based on a matrilinear hierarchy.
In the first component of the study, bear cubs were brought to him by the New Hampshire Fish and Game Department to be raised, rehabilitated, and returned to the wild. They were very young, orphaned at ages 7–12 weeks, when still in their natal dens, so they had no experience with their biological mothers outside the winter den. We bottle fed the cubs until they were able to eat natural foods and live in a natural enclosure (2 ha). While the bear cubs were living in the natural enclosure, the senior author documented the behavior of cubs while he walked them untethered in the forest several times a week to give them experience in their natural environment and expose them to wild bears (Kilham & Gray 2002; Kilham 2013). Most of those bears were then released into the wild at about 18 months of age and were not a part of our later studies. They were no longer dependent upon the investigator and lived independent lives. We knew that the orphaned bears became wild like any other bear because over 300 of them were released into the wild throughout New Hampshire and were observed by Fish and Game and other biologists and the senior author to successfully lead normal bear lives. Some were outfitted with radio telemetry collars so that their movements were recorded (Smith et al. 2016). This portion of the study prepared the senior investigator to carry out the second and third portions of the study by familiarizing him with bear behavior under natural conditions.
The behaviors described in this manuscript were carried out by wild bears. Most of the bears in our study were born and raised in the wild. One female cub, SQ, born in 1996, raised by the senior author in the first component of the study and released in 1997, remained in the study area after her release. At the conclusion of the data collection in 2014, she was 18 years old and raising her ninth litter of cubs in the wild. The second and third components of the study focused on her, her descendants, and the unrelated bears with which they interacted. It was the habituation of bear SQ to the senior author that gave him access to the wild bears that were her descendants.
In the second component of the study, the senior author fitted SQ with adjustable VHF collars in 1998 that could be taken on and off without sedating her (Kilham & Gray 2002; Kilham 2013). Because SQ was habituated to his presence, the senior author was able to observe her behavior at close range in the wild for up to 5 h at a time, including intraspecific social interactions, foraging, scent marking, and tracking of other bears. The senior author also fitted other bears with VHF collars to be able to follow them. Additional methodological details are described in Kilham and Gray (2002) and Kilham (2013). The methods used in this component of the study were similar to those employed by Rogers and Wilker (1990), DeBruyn (1999), Stringham (2002), and Fredriksson (2005).
The third component of the study centered on a provisioning site established in 2004. Here, the senior author observed and documented social interactions among wild bears from 2007 until 2014. Most of the data presented here were from this component of the study. The provisioning site was protected on private property approximately 2 km from the nearest road and 1 km from the nearest human residence. The senior author drove his truck to the site in the evening and provided corn (approximately 1.5 kg/bear) that was available to any bear that arrived. The provisioning site was organized so that he could observe bears from his truck. The senior author distributed corn between 5 to 10 different piles, 3–40 m from the truck and 5–60 m apart (Fig. 1). The goal was to get bears to come while he was there to observe them, not to make them dependent on the food provided. The corn was not an essential part of their diet. The bears were wild and could come and go freely. The senior author distributed the food so as to minimize competition and conflict over food. In general, between 1 and 12 bears, including cubs, were at the site on a given night with an average of 5. Bears fed on different piles of corn and there were usually unused piles available to the bears. The senior author made observations most evenings from May 1st to November 1st for periods averaging 1.5 h per evening for a total of 1472 days from 2007 to 2014. Provisioning areas and other concentrated food sources (like salmon rivers or garbage dumps for bears) have been used for behavioral studies of bears (Herrero 1983; Craighead et al. 1995), dolphins (Mann & Smuts 1999), and chimpanzees (Goodall 1986; Wilson & Wrangham 2003).

The senior author identified bears using distinguishing features and characteristics, including their facial markings, head shape, body shapes, body length, color pattern on muzzle, behaviors, scars, tears in ears, white chest patches, ear tags, limps, and radio collars (Storonov & Stokes 1972; Herrero 1983). Pedigree was determined using personal knowledge of cubs and mothers from birth and micro-satellite DNA using 11 polymorphic markers (Coster & Kovach, unpublished data). DNA was extracted from hair samples using a QIAGEN QIamp DNeasy Blood and Tissue® kit (Valencia, CA) with the slight procedural modification of adding dithiothreitol to the lysis buffer to break down the di-sulfide bonds found in hair proteins. Genotyping was performed with the following 11 highly variable microsatellite markers: G1A, G10B, G10C, G10L, G1D, G10X, G10M, Mu10, Mu23, Mu59, and Mu50 (Paetkau et al. 1995; Bellemain & Taberlet 2004). Sex identification was performed with a Y chromosome marker (SRY gene fragment) that amplified only in males. The personal knowledge and genetic analysis gave a consistent pedigree.
Agonistic interactions between pairs of bears were categorized as Level 1, 2, or 3, based on the intensity of the dominance enforcing behavior (DEB) that took place. Level 1—Rank was apparently already established and the subordinate bear walked away in the presence of the dominant bear. Level 2—One dominant bear established rank over the other bear without challenge from the subordinate by chasing on the ground or up a tree. Level 3—Both bears stood up to each other, or increased action and intensity were required in establishing rank (e.g. face-to-face encounters, swatting, and biting). Behaviors typical of each level of interaction are described in Table 1. To examine the structure of the dominance hierarchy, the senior author used only interactions between pairs of bears he could identify. For each interaction, he recorded the identity of the “winner” and the “loser” (the subordinate bear). The winner, or the dominant, was the bear that caused the other to lose its position. Rank was then assigned based on the number of individuals each bear was dominant over.
| Behavior or Interaction | Description |
|---|---|
| Level 1 | |
| Bump | Subordinate leaves food on sight of dominant bear. |
| Taking Food From | Dominant walks toward subordinate to take food. |
| False Charge | A quick lunge, double paw swat on ground while expelling loud blast of air. Can be offensive or defensive. |
| Level 2 | |
| Hunting | Dominant bear hunts down or tracks subordinate to chase it. |
| Chase | Dominant bear chases subordinate on short (<50 m) or long (>50 m) chases. |
| Treeing | Dominant bear forces subordinate bear to climb tree to escape dominant. |
| Level 3 | |
| Chase Up Tree | Dominant bear climbs tree after subordinate. |
| Huh, Huh, Huh (HHH) | A reverberation of air in the chest and throat that always has a negative connotation. |
| Face-To-Face encounter | Dominant and subordinate face-off, sometimes exchanging swats and usually “Huh, Huh, Huh” vocalizations. |
There would be limitations in the interpretation of the data from these studies if the bears were reared and dependent upon the investigator throughout the study. In the first component, the bear cubs were dependent upon the senior investigator and he served as a surrogate mother. However, the behaviors observed when the bears were walking in the forest were not dependent upon the author and were those of a natural bear. In the second and third components of the study, the behavior of SQ, her descendants, and other unrelated bears were not dependent upon the investigator. Because SQ was habituated to the investigator, she acted toward him as if he was another bear and the other bears generally ignored his presence.
We made observations of affiliative behavior during the course of all 3 components of the study. The data were not from planned experiments, but rather were the result of long-term observations of the behavior of bears that interacted with SQ. We did not develop an ethogram to describe these behaviors.
Data handling and statistical analysis
We presented our data with descriptive statistics. Data on bear behavior were collected by direct observations of bear cubs walking with the investigator in the forest, by direct observations of the behavior of radio telemetered bears, and by direct observations of bear interactions at the provisioning site. We considered doing a linear analysis using indices of linearity such as Landau's h and Kendall's K as well as a network analysis as suggested by Shizuka and McDonald (2012). However, we had an advantage in our study. We knew every individual in the third component of the study, only recorded interactions between those individuals, had no missing data, and were able to assess the interactions of those individuals. We believe that our descriptive statistics as shown in the tables are clear in indicating the interactions and the dominance relationships in the matrilinear hierarchy. We were able to determine the social organization and social structure as well as information on the mating system- following the framework of Kappeler (2019).
Animal care
The New Hampshire Fish and Game Department provided Scientific Research and Rehabilitation permits, as well as the cubs for research, rehabilitation, and release to the wild. Permits covered all aspects of the study over the entire study period and were renewed annually. All handling of animals was conducted within the guidelines of handling research animals (Sikes et al. 2011) and was approved by the New Hampshire Fish and Game Department. The senior author conducted this research before he was enrolled as a doctoral student at Drexel University, so we did not request post hoc approval from the Drexel University Institutional Animal Care and Use Committee.
RESULTS
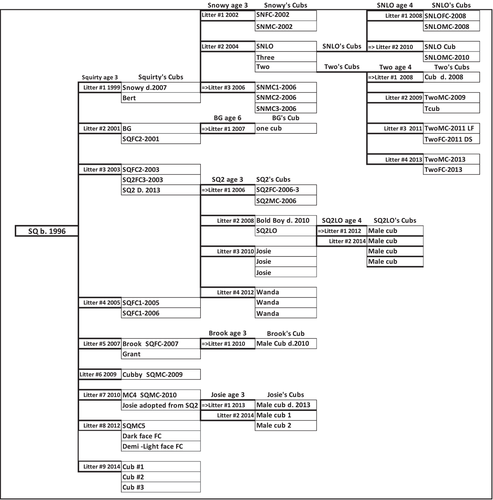
The bear, SQ, was born in 1996, raised by the senior investigator, and released onto the study site in 1997. She had her first litter of cubs when she was 3 years old and produced 9 litters by 2014 resulting in 20 cubs in the first generation (Fig. 2). Of those cubs, 12 left the study area, 5 females remained and produced 23 offsprings, and 3 cubs remained with SQ in 2014. Three of SQ2's female cubs remained in the research area and produced 15 offspring of their own over the course of the study.
The senior author observed a total of 1210 dominance interactions at the provisioning site among females in SQ's direct lineage during 2007–2014 (n = 93–239 per year). The number of related bears at the site each year is indicated in Table 2. These data supported hypothesis 1, black bears interact socially beyond mating and competing for resources. These interactions, which all took place at the provisioning site, revealed a clear matrilinear hierarchy of dominant bears enforcing dominance over subordinate related females (Table 2). These data supported hypotheses 2 and 3. Black bears track relationships and interact regularly even when resources are not limited and the social structure of a population of black bears is based on a matrilinear hierarchy. The bear, SQ, who established the hierarchy, was dominant over all of her descendants and in turn her descendants variously dominated one another. They all invariably dominated all of their own direct descendants. The matriarch, SQ, showed dominance enforcing behaviors (DEB) listed in Table 1 toward all of her female descendants, which served to establish and maintain her dominance over them. This pattern was consistently demonstrated by each bear, which indicated a linear dominance structure. Each bear showed DEB toward all her lower ranking female kin in a descending fashion; that is, the second ranking bear would show DEB toward every bear below her in the hierarchy, as would the third ranking bear, and so forth down the hierarchy. The lowest ranking female did not show DEB toward any other bears but was treated with DEB by all other bears in the study area. Females of equal rank had face-offs that included swatting and Huh, Huh, Huh (HHH) vocalization (level 3 interactions). There were 2 face-offs in 2010 between SQ and SQ2 and 5 face-offs between different pairs of bears in 2011. The hierarchy changed from year to year reflecting the loss of family members due to hunting. SQ, 18 years old in 2014, remained the matriarch and the dominant female throughout the course of this study.
| a. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Dominance 2007 | |||||||||
| Bear ID | SQ | SN | SQ2 | BG | SNLO | Two | Three | SQ2FC1 | Total |
| SQ(COY) 11 yrs. | 11 | 20 | 1 | 6 | 8 | 46 | |||
| SN(Y) 9 yrs. | 7 | 1 | 1 | 9 | |||||
| SQ2(Y) 5 yrs. | 5 | 7 | 11 | 3 | 3 | 29 | |||
| BG(COY) 7 yrs. | 1 | 1 | 1 | 3 | |||||
| SNLO(B) 5 yrs. | 1 | 3 | 2 | 6 | |||||
| Two(B) 5 yrs. | 2 | 2 | |||||||
| Three(B) 5 yrs. | 1 | 1 | |||||||
| SQ2FCl 2 yrs.(NBA) | 0 | ||||||||
| Subtotal | 1 | 16 | 27 | 1 | 15 | 22 | 8 | 6 | 96 |
| b. | |||||||
|---|---|---|---|---|---|---|---|
| Dominance 2008 | |||||||
| Bear ID | SQ | SQ2 | SNLO | Two | SQ2FC1 | Brooke | Total |
| SQ(Y) 12 yrs. | 5 | 3 | 9 | 14 | 29 | 60 | |
| SQ2(COY) 6 yrs. | 2 | 2 | 4 | 8 | |||
| SNLO(COY) 6 yrs. | 1 | 1 | 1 | 3 | |||
| Two(LC, B) 6 yrs. | 6 | 4 | 10 | ||||
| SQ2FC1(U) 3 yrs. | 10 | 10 | |||||
| Brooke(B) 2 yrs.(NBA) | 1 | 1 | 2 | ||||
| Subtotal | 0 | 5 | 5 | 13 | 26 | 44 | 93 |
| c. | |||||||
|---|---|---|---|---|---|---|---|
| Dominance 2009 | |||||||
| Bear ID | SQ | SQ2 | SNLO | Two | SQ2LO | Brooke | Total |
| SQ(COY) 13 yrs. | 35 | 14 | 3 | 14 | 3 | 69 | |
| SQ2(Y) 7 yrs. | 1 | 8 | 5 | 14 | 28 | ||
| SNLO(Y) 7 yrs. | 1 | 7 | 3 | 11 | |||
| Two(COY) 7 yrs. | 2 | 5 | 7 | ||||
| SQ2LO 2 yrs.(NBA) | 2 | 1 | 3 | ||||
| Brooke(LC)(B) 3 yrs. | 1 | 2 | 3 | ||||
| Subtotal | 1 | 37 | 24 | 10 | 37 | 12 | 121 |
| d. | ||||||||
|---|---|---|---|---|---|---|---|---|
| Dominance 2010 | ||||||||
| Bear ID | SQ | SQ2 | SNLO | Two | SQ2LO | Brooke | Tcub | Total |
| SQ(Y) 14 yrs. | 20 | 5 | 20 | 19 | 64 | |||
| SQ2(COY) 8 yrs. | 1 | 8 | 11 | 1 | 1 | 22 | ||
| SNLO(COY) 8 yrs. | 1 | 3 | 5 | 9 | ||||
| Two(Y) 8 yrs. | 1 | 1 | 29 | 1 | 32 | |||
| SQ2LO 3 yrs.(U) | 1 | 1 | 1 | 13 | 16 | |||
| Brooke (U) 4 yrs. | 3 | 3 | ||||||
| Tcub 2 yrs. | 0 | |||||||
| Subtotal | 1 | 21 | 7 | 33 | 62 | 3 | 19 | 146 |
| e. | ||||||||
|---|---|---|---|---|---|---|---|---|
| Dominance 2011 | ||||||||
| Bear ID | SQ | SQ2 | SNLO | Two | SQ2LO | Josie | Tcub | Total |
| SQ(COY) 15 yrs. | 21 | 9 | 8 | 14 | 19 | 11 | 82 | |
| SQ2(Y) 9 yrs. | 14 | 3 | 6 | 13 | 1 | 37 | ||
| SNLO(Y) 9 yrs. | 4 | 5 | 3 | 12 | ||||
| Two(COY) 9 yrs. | 1 | 1 | 1 | 3 | ||||
| SQ2LO(B) 4 yrs. | 4 | 1 | 5 | 2 | 12 | |||
| Josie 2 yrs.(NBA) | 3 | 3 | ||||||
| Tcub 3 yrs.(U) | 0 | |||||||
| Subtotal | 0 | 21 | 28 | 12 | 25 | 42 | 21 | 149 |
| f. | |||||||
|---|---|---|---|---|---|---|---|
| Dominance 2012 | |||||||
| Bear ID | SQ | SQ2 | SQ2LO | Two | Josie | Tcub | Total |
| SQ(Y) 16 yrs. | 10 | 6 | 4 | 41 | 3 | 64 | |
| SQ2(COY) 10 yrs. | 1 | 1 | 2 | ||||
| SQ2LO(COY) 5 yrs. | 11 | 4 | 15 | ||||
| Two(Y) 10 yrs. | 5 | 5 | 11 | 21 | |||
| Josie(B) 3 yrs. | 7 | 5 | 12 | ||||
| Subtotal | 0 | 10 | 11 | 23 | 50 | 20 | 114 |
| g. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dominance 2013 | ||||||||||
| Bear ID | SQ | SQ2 | SQ2LO | Josie | Wanda | Tcub | Two | Demi | SQ-LF | Total |
| SQ(COY) 17 yrs. | 27 | 6 | 23 | 12 | 1 | 21 | 7 | 97 | ||
| SQ2(Y) 11 yrs. | 7 | 5 | 11 | 7 | 2 | 1 | 4 | 13 | 50 | |
| SQ2LO(Y) 6 yrs. | 16 | 4 | 5 | 4 | 1 | 1 | 31 | |||
| Josie(COY) 4 yrs. | 2 | 5 | 13 | 2 | 3 | 2 | 27 | |||
| Wanda 2 yrs.(NBA) | 2 | 1 | 8 | 2 | 13 | |||||
| Tcub(COY) 5 yrs. | 1 | 5 | 2 | 2 | 10 | |||||
| Two(COY) 11 yrs. | 1 | 4 | 2 | 1 | 8 | |||||
| Demi 2 yrs.(NBA) | 1 | 1 | 2 | |||||||
| SQ-LF 2 yrs.(NBA) | 1 | 1 | ||||||||
| Subtotal | 7 | 30 | 16 | 53 | 46 | 12 | 6 | 41 | 28 | 239 |
| h. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dominance 2014 | |||||||||||
| Bear ID | SQ | Josie | Wanda | Two | SQ2LO | Tcub | Demi | LFF | SNLOC | SQ2LOC | Total |
| SQ(Y) 18 yrs. | 32 | 43 | 1 | 2 | 21 | 8 | 107 | ||||
| Josie (COY) 5 yrs. | 3 | 21 | 4 | 1 | 6 | 12 | 47 | ||||
| Wanda(B) 3 yrs. | 1 | 3 | 3 | 5 | 2 | 43 | 21 | 3 | 81 | ||
| Two 12 yrs.(B) | 4 | 3 | 3 | 10 | |||||||
| SQ2LO 7 yrs.(B) | 2 | 1 | 3 | ||||||||
| Tcub 6 yrs.(B) | 1 | 1 | |||||||||
| Demi 3 yrs.(B) | 2 | 2 | |||||||||
| LFF 3 yrs.(B) | 1 | 1 | |||||||||
| Subtotal | 4 | 36 | 70 | 9 | 8 | 2 | 73 | 47 | 3 | 0 | 252 |
| Total dominance interactions | 1210 | ||||||||||
- The data in this table reflect agonistic social interactions between dominant and subordinate related bears. All of the bears in this table were direct descendants of the matriarch SQ. Winners are listed along vertical axis; losers are listed along horizontal axis. Rank is in descending order from top to bottom and from left to right. The most dominant bear is at the top and left. Numbers signify the quantity of interactions between the bears. The winners are listed by name and age at time of observation. Abbreviations indicate status of animal when it entered the study: COY, cubs of the year; Y, yearlings; B, bred; LC, lost cub; NC, no cubs; NBA, not of breeding age; U, unknown status.
The greatest number of interactions took place between bears that were closest in rank and challenged the hierarchy. For example, in 2007, SQ interacted 11 times with her oldest daughter SN who was second in rank. She interacted 20 times with SQ2 who was 4 years younger and was establishing her rank. SQ clearly indicated her dominance over SQ2 by face-to-face encounters, chasing, false charging, and taking her food (Table 1). SQ interacted with SN by chasing and false charging. SQ2 interacted with SN 12 times, losing 7 times to the older bear, but winning 5 times. The long-term outcome of these interactions was not established, however, because SN was killed during the hunting season of 2007. In 2008, SQ2 became the number one subordinate in the hierarchy and she held that position until she was killed in the hunting season of 2013. There continued to be interactions between SQ and SQ2, but they became less frequent in later years.
Agonistic behaviors relating to assertion of rank were typically of short duration, with the longest chases usually lasting less than 10 min. They appeared to be expressions of dominance rather than attempts to drive off the subordinate bear. Agonistic behavior by the dominant bear continued until resolution occurred, occasionally as a result of contact or close encounter. The subordinate bear usually returned to finish eating with no further action taken against her. We quantified this result in 2014 and found that subordinate bears returned to feed after agonistic encounters with higher ranking bears 98.8% of the time (i.e. on 242 of 245 occasions). Other agonistic behaviors that took place at the field site included: defense of cubs of the year (COY) (e.g. 2 times in 2011), weaning of yearlings (e.g. 4 times in 2011), aggression toward males (e.g. 26 times in 2011), and aggression toward unrelated females (e.g. 18 times in 2011). It was not uncommon for bears to target and drive off specific bears.
Based on the above data, it appeared that SQ behaved as if she had “Rules” (Table 4) by which she managed her matriarchal society. If a bear broke one of those “rules”, then SQ reacted by punishing that bear with chasing, swatting, and biting. The degree of punishment varied according to the seriousness of the infraction. She interacted with her granddaughters more than with other bears because the granddaughters did not recognize her dominance (Supplemental Information 1). SQ reacted if subordinate bears did not show respect, did not avoid her, did not make a wide arc around her when passing by, took her food, or did not stay an appropriate distance away.
The intensity of SQ's attention to other bears was reflected in the number of chases made after a given bear. Those chases did not subside until SQ made contact or compliance was achieved and the other bear acknowledged her dominance. For example, SQ chased SQ2 5 times, chased Two 7 times and 4 unrelated males once each in 2010. SQ chased Josie 15 times, chased SNLO 5 times, SQ2LO 12 times, Tcub 2 times and 3 unrelated males once, twice, and 4 times in 2011 (Supplemental Information 2).
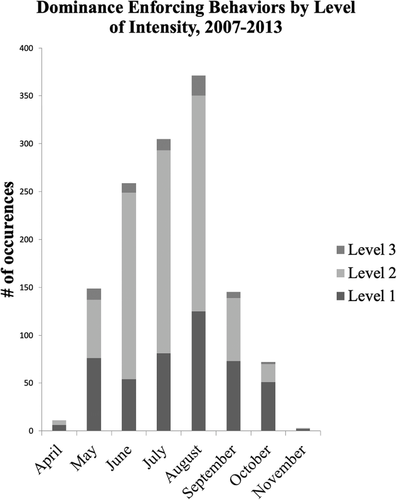
While the senior author did not observe the end of all agonistic behaviors, many of the ones he did see were followed with marking behavior by the dominant bear: stiff-legged walk (SLW), mark with urine (M/U), walk over sapling (WOS), or full back rub (FBR) (Table 3). Dominance enforcing behavior peaked in August and quickly declined in frequency during September and October (Fig. 3). Bear breeding season in that area was from the last week of May until the first week of July, but that did not affect the dominance enforcing behaviors because mating did not affect the interactions of the females at the provisioning site.
| Behavior | Description |
|---|---|
| Stiff-legged walk (SLW) | Bear walks stiffly sliding front paws forward disrupting ground beneath. |
| Mark with urine (M/U) | Bear uses urine to mark, in tiny drops or gushes. |
| Walk over sapling (WOS) | Bear walks over sapling transferring scent from belly, the sapling pops back up acting as olfactory antenna. |
| Full back rub (FBR) | Standing bipedal, the bear rubs back on tree or object by flexing knees or wiggling. |
| For bears below SQ in hierarchy: |
|---|
| Avoid her space |
| Do not approach |
| Do not take her food |
| Do not walk toward |
| Do not false charge |
| Do not challenge |
| Do not get too close to her cubs |
Affiliative behaviors
Affiliative behavior also occurred and strengthened the matrilinear hierarchy. Throughout the course of all 3 components of the study, we made observations of bear behavior that indicated that they had affiliative behavior. The data acquired were not from a series of planned experiments, but rather were the result of long-term observations of the behavior of the group of bears related to SQ. Bears acted cooperatively and supported one another. For example, one cub, Josie, birthed by SQ2 was adopted and raised by SQ her maternal grandmother. In April 2010, SQ2 had 3 cubs (2 males and a female) in a den about 4 miles from the provisioning site and SQ had one cub, a female that she brought to the provisioning site. In May, one of SQ2's cubs, the female, was smaller than the others and not growing well. On Wednesday May 12, SQ2 came to the provisioning site for the first time and approached the senior author with a plaintive look like that of a dog asking for something from its owner. The senior author did not understand what she wanted. Thursday night one of her cubs was heard by a neighbor crying in a large pine tree near the provisioning site but was gone a few hours later. On Saturday evening, when the senior author came to the clearing, SQ had 2 cubs. One was her original female and the other was the small, weakened female birthed by SQ2. GPS data from the VHF collars on SQ and SQ2 indicated that they were together on Thursday night. So the adoption was negotiated that night. SQ raised that cub (Josie) until she was weaned and entered the matrilinear hierarchy in 2011 (Table 2e).
Bears shared food with unrelated bears. Moose, a 23-year-old unrelated bear, had a home range in an area dominated with beech trees. The senior author put a VHF collar on her in 2004. In 2005, the beech crop failed while there was a large acorn crop in SQ's home range. In October, Moose came to the provisioning site and passed within 3 m of SQ to eat a pile of corn. SQ ignored Moose although she maintained her matrilinear hierarchy with her related bears. Moose later came to the site in other years, even bringing her cubs. So SQ shared her extra food. In many years, SQ shared food with more than 20 other bears, many of them strangers. In 2010, there were 10 related bears and cubs and 34 unrelated bears that came to the provisioning site. In 2011, there were 13 related bears and their cubs and 25 unrelated bears that came to the provisioning site. They all accepted SQ as the dominant bear. Data from SQ's VHF collar indicated that she fed in other bear's territories as well.
Another example of cooperation occurred with the bear Brooke, a female born in 2007. She entered the hierarchy in 2008 (Table 2c) as the lowest ranging female. In 2009, she gave birth to a single cub but failed to raise it. She could not establish a home range but was able to stay in the area by forming a friendship with a subadult male and in 2010 moved up one level in the hierarchy (Table 2d). They traveled together and fed together. She did not give birth again and in 2011 was killed by a hunter. The hunter reported that she was in the company of another bear when he shot her.
DISCUSSION
Female social hierarchies have been suspected in brown bears (Craighead et al. 1995) and family relationships have been described in black bears (Rogers 1987). Støen et al. (2005) inferred that brown bears had matrilineal hierarchies based on studies of the molecular genetics of populations and the finding that the home ranges of related bears overlapped more than the home ranges of unrelated bears. The data that we presented here demonstrate that black bears establish and maintain strong matrilinear dominance hierarchies that are maintained by clear “Rules” and are quite stable through time. Female hierarchies were maintained throughout their active year with social interactions peaking in August (Fig. 3). We also discovered that bears cooperate and that affiliative behavior helps to form the social structure of the bear community.
Rogers (1987) concluded that adult female black bears recognized their independent offspring and tolerated them in their territories. We found that adult females not only tolerated their female offspring but fostered them. Bear SQ maintained a matrilinear hierarchy that established clear boundaries for interactions among the group of bears in the study.
Black bears are forest animals, making observations difficult except at concentrated food sources; therefore, it has been difficult for previous studies to determine the reasons for movements and behaviors recorded via telemetry. For example, Rogers (1987) and Noyce and Garshelis (2011, 2014) both found that black bears in Minnesota have home ranges, establish territories, and migrate across the landscape. However, they were unable to discern the mechanisms by which those territories were established and maintained and could only infer the behavioral and social mechanisms by which bears followed similar migration pathways. Because the senior author was able to directly observe bears at his food site, he was able to document the underlying social hierarchy that determined many bear behaviors.
Agonistic behavior between female bear relatives changed through the active season. Agonistic behavior was low in April and May because all the females had cubs at this time, which were either small cubs of the year (COY) or larger yearlings (Fig. 3). Females with COY stayed near the den because the cubs were developing their ability to travel and climb. Females with yearlings, however, had access to a much greater abundance of food, such as emerging vegetation with freely available nutrients, which allowed them to meet their nutritional needs without having to forage widely. Mothers with yearlings were also subjected to little social competition for food, because succulent emerging growth was widely available in the spring of the year. Once the females with yearlings bred in June, they drove off their yearlings and started reasserting their position in the hierarchy and securing a high quality home range (unpublished data) to raise their next litter of cubs Therefore, interactions increased sharply in June and July before peaking in August. Agonistic behavior quickly diminished in September and October as the females concentrated on acquiring food to store energy for hibernation
Chasing and marking behavior established rank (Table 2) in black bears and may have conditioned subordinates to associate scent marks of dominant bears with their claiming of territory. When subordinate bears were treed, post-chase marking by the dominant bear occurred (SLW, M/U, FBR, WOS) (Table 3) suggesting that the dominant bear was conditioning the subordinate bear to its scent. Bears marked at feeding sites, which allowed incoming bears to encounter a dominant bear's scent, know who it was, and respond appropriately. For example, a male cub that the senior author was rehabilitating was bitten by a large male with a single puncture wound. On subsequent walks, when the cub came across the large male's scent from a marking area, he responded with a long fearful moan and walked away (Kilham & Gray 2002). de Waal (1986) links the aggression of dominance interactions to social tolerance based on a study with a large colony of semi-captive chimpanzees. He states that, “ritualized submission is imposed on losers of dominance struggles by winners; losers are offered a ‘choice’ between continued hostility or a tolerant relationship with a clearly signaled difference in status” (de Waal 1986). Black bears also exhibit ritualized submission and dominance behavior continues until the loser accepts its lower status in the relationship.
Aggressive behavior exists in many species and social mechanisms like dominance have evolved such that they mitigate its harmful/deleterious effects. These mechanisms are so powerful that they allow the use of restrained aggression to define relationships without disrupting them (Bernstein et al. 1974). In an experiment with rhesus monkeys where unfamiliar pairs were put together, Maxim (1976) found that after initial fighting, there was a decrease in aggression by the dominant individual and a decline in the fear responses by the subordinate until they fell into a “friendly” zone. Similarly, agonistic behavior in black bears, such as chasing and marking, decreased aggressive behavior over time, while establishing and maintaining the matrilinear hierarchy among our study bears (Table 2).
The greatest number of interactions took place between bears that were closest in rank and challenged the hierarchy. These data show that bears have an understanding of rank. Hobson et al. (2021) report that aggression heuristics underlie animal dominance and provide support for group-level social information. In 172 social groups across 85 species in 23 orders, the main patterns of rank-dependent social dominance are downward heuristic (aggress uniformly against lower-ranked opponents), close competitors (aggress against opponents ranked slightly below self), and bullying (aggress against opponents ranked much lower than self). Our data indicated that black bears demonstrated complex social dominance patterns indicating that they had higher levels of social information use. Schuppli and van Schaik (2019) report that wild orangutans have a clear cultural repertoire and clear ecological correlates for most cultural elements. They conclude that social learning and therefore culture is widespread among animals and is an important avenue to local adaptation. Because we have documented that black bears socially learn skills in a matrilinear hierarchy, our data support the conclusion that black bears have social learning and culture. We support the conclusion of Schuppli and van Schaik (2019) that culture is more widespread and pervasive than commonly thought in animals.
Although SQ and other dominants down the hierarchy asserted dominance over female relatives through agonistic encounters, serious aggression rarely occurred, and the dominant bear tolerated subordinate females. The matrilinear hierarchy that we measured was a longitudinal hierarchy (Strauss & Holekamp 2017) with a downward heuristic along with close competitors (Hobson et al. 2021). There would be a benefit to the matriarch and all of her female relatives in the maintenance of the matrilinear hierarchy due to the increased survival and fitness of offspring and perpetuation of the matriarch's genes (Fig. 2).
Matrilinear hierarchies occur in other species of non-human animals including bottlenose dolphins (Tursiops aduncus), (Frère et al. 2010), African elephants (Loxodonta africana), (Whittemyer & Getz 2007; Archie et al. 2006), and spotted hyenas (Crocuta crocuta), (Smale et al. 1993); however, all of these animals live in typical social family groups. Association patterns of bottlenose dolphins in Australia depended upon home range overlap, matrilineal kinship, and bi-parental kinship. Associations take place most often between females that are bi-parentally related and that share the same matriline. Elephants have linear age and size ordered hierarchies in which older, larger females consistently dominate smaller, younger females in a multilevel social structure (Archie et al. 2006). The size of family units is affected by the age of matriarchs, and units led by grandmothers are larger than units led by younger matriarchs. In hyenas, juveniles obtain social rank based upon the rank of their mothers (Holekamp & Smale 1991). Although black bears do not live in social family groups, they show some of the same matrilineal relationships, characteristic of other mammalian species that are more obviously social.
Demographic processes help to shape the patterns of social relationships among individuals in a population. Shizuka and Johnson (2020) argue that the integration of demographic processes and social processes is needed to better understand the causes and consequences of variation in social structure among species and populations. Because we knew every individual in the third component of our study, we were able to observe how the dominance hierarchy changed from year to year when animals were removed due to hunting (Table 2). The dominance hierarchy remained stable when a bear was removed by hunting. The remaining bears maintained the same relative position in the hierarchy and the bears below the one that was removed simply moved up one place in the hierarchy.
We observed instances of affiliative behavior ranging from the adoption of one bear cub by its grandmother when the mother could not feed it to sharing of food with unrelated bears, and cooperative behavior of a female and male bear. These behaviors increased the selective fitness of individual bears and helped to shape the social structure of the bear community. Natural adoption of cubs of one mother by another bear mother has been suggested in the past for brown and black bears (Erickson & Miller 1963; Alt 1984) but has not been previously verified.
Management implications
Black bear research has been dominated by management concerns, and therefore, tends to be focused on understanding dynamics at the population level. Very little research has been conducted on behavior, especially among individually known animals. Our study of individually known black bears revealed an abundance of complex social behaviors that are helpful in guiding behavioral management of the species. For example, better knowledge of behavior and social structure provides insights into alternative and potentially less invasive techniques for managing “problem bears” in urban settings other than routine management methods, such as capture, removal, and euthanasia, which can result in psychological and physical harm to the bear and may not adequately address the root cause of the problem in a behaviorally informed manner. Perhaps other members of the bear family, including giant pandas, also thought to be solitary (Schaller et al. 1985), will turn out to be social in similar ways. Studying these animals in greater depth may reveal more complex social dynamics and strategies than previously thought to exist.
Up to 900 000 black bears live in North America and many humans have moved to live in close proximity to those bears. Based on our data, human–bear conflicts can be reduced by behavioral modifications. Bear interactions can be reduced by eliminating food sources. Since bears share food sources with other bears, they assume people are inviting them to share their food as well when they place food around their houses and barns. Removing bird feeders, not feeding pets outside, keeping chicken coups behind electric fences, cleaning outdoor grills all remove the attraction of food. Remove the food and the bear goes away.
If you meet a bear, you can defuse the situation by realizing that the bear is also afraid of the human and seeks to reduce the conflict. It usually attacks in self-defense only if the person shows weakness. The bear may false charge in combination with chomping, huffing, and snorting. It is seeking to avoid direct confrontation and determine if there is a sign of weakness or submission. Running will encourage chasing by the bear. Yelling, breaking sticks, making yourself big by waving hands can escalate the situation into an attack, especially if the bear is very close. By standing erect, staying calm, looking at the bear, and speaking softly you show dominance, keep the bear honest, and reduce its urge to advance. Speaking softly also calms the person down and conveys a message of appeasement that the bear understands. Does the bear have a pleasant face or is it scared? Have you done something to provoke it? Stay calm and talk softly as if the bear is a friend or your pet dog. Deescalating a conflict allows the bear time to assess you as a threat and to leave. Be alert in the woods. If you see a bear, make a noise, create some movement, and talk to it so that it knows you are there. It will probably avoid you. There are very few predacious attacks. They can best be avoided by being alert and staying erect so that you do not seem to be an easy meal. More detailed information on human–bear interactions can be found in Kilham and Gray (2002) and Kilham (2013).
SUMMARY
The data that we presented here demonstrated that black bears established and maintained strong matrilinear dominance hierarchies that were maintained by clear “Rules” and were quite stable through time. Female hierarchies existed and were maintained throughout their active year. Agonistic behavior between female relatives was low in April and May and peaked in August. Chasing and marking behavior established rank and may have conditioned subordinates to associate scent marks of dominant bears with their claiming of territory. Although SQ and other dominants down the hierarchy asserted dominance over female relatives through agonistic encounters, serious aggression rarely occurred, and the dominant bear tolerated subordinate females. The matrilinear hierarchy changed from year to year as animals were removed from the system by hunting. Affiliative behavior occurred between related and unrelated bears and helped to establish the social structure of the bear community. Knowledge of bear behavior and the matrilinear hierarchy provides a basis for non-lethal management of bears that find themselves in a bear–human conflict situation.
ACKNOWLEDGMENTS
We thank the New Hampshire Fish and Game Department, especially Andrew Timmins, for assistance throughout this project. Debbie Kilham and Phoebe Kilham assisted in raising bear cubs during the project. Vicki Smith assisted in compiling data and preparing figures. Support for this research came from the Kilham Bear Center, Lyme, New Hampshire, the Global Cause Foundation, Fairfax Virginia, and the Betz Chair of Environmental Science endowment at Drexel University.




