Diet suitability through biological parameters in Ostrinia furnacalis (Lepidoptera: Crambidae) clades
Abstract
The Asian corn borer (ACB), Ostrinia furnacalis, (Lepidoptera: Crambidae), a significant threat to corn crops, necessitates comprehensive research on its populations, across multiple clades. This study addresses the efficacy of various diets, such as an antibiotic mixed diet of amoxicillin mixed with artificial diet, wheat germ flour artificial diet and fresh corn leaves, for the successful mass rearing of three clades of O. furnacalis, and investigates variations among the insects reared under laboratory conditions. We validate these findings for O. furnacalis clades using biological attributes. Notably, clade III demonstrated enhanced biological characteristics with an antibiotic mixed diet, in contrast to clades I and II, with higher growth indexes observed during mass rearing. Through revealing a suitable diet for the clade, this research advances our understanding of O. furnacalis dynamics and offers valuable insights for practical applications in an integrated pest management program.
Introduction
Maize (Zea mays L.) is one of the most important food crops in the world, providing at least 30% of the dietary calories to more than 4.5 billion people in 94 developing countries. In parts of Africa and Mesoamerica, maize alone contributes over 20% of dietary calories (Erenstein et al., 2022; Lu et al., 2024).
Numerous lepidopteran pests consume maize crops, leading to substantial shortfalls in production. Among them, Ostrinia furnacalis (Lepidoptera: Crambidae) is considered one of the most injurious insect pests of the maize crop (Abbas et al., 2024; Alam et al., 2023; Hiruy & Getu, 2018). It has a wide distribution from eastern Asia to the western Pacific islands and causes a yield loss of 6–9 million tons annually in China (Li et al., 2022). The adults are active from early May to late October each year (Han et al., 2020). The adults of O. furnacalis are categorized into three clades (I, II and III), and confirmed by their unique cytochrome c oxidase subunit I (COI) gene sequences, morphological and biological features. The COI gene sequences play a role in the expression of specific morphological and biological features of O. furnacalis. The COI sequences for clade I span from MN720652 to MN720668, the COI sequences for clade II span from MN720669 to MN720686 and the COI sequences for clade III span from MN720687 to MN720703. As a result of differentiation in the COI sequences, the three clades show differences in wing postmedial line, male sacculus construction, lifespan, male dynamics and host preferences. These differences create substantial difficulties in pest forecasting and control (Han et al., 2020). So, a more efficient approach for maize growers is to manage this species at the clade level rather than addressing the entire species. The clades of O. furnacalis provide a remarkable model for studying their life history through the quantification of biological characters and fitness parameters under various diets, as a means for controlling this pest.
Mass rearing is a technique employed to produce large numbers of insects fed on natural or artificial diets (Guo et al., 2019) for multifaceted study purposes, including insect biology, insecticide testing and the production of biological control agents (Rahayu & Trisyono, 2018). Artificial diets are widely used to rear several insect and biological control agents efficiently, yielding insects with superior fitness over those reared on natural diets (Rahayu & Trisyono, 2018; Yang et al., 2024). Several studies have been carried out on the benefit of using artificial diets in lepidopterans such as O. furnacalis (Rahayu & Trisyono, 2018), Helicoverpa armigera (da Silva et al., 2021), Plodia interpunctella (Jung et al., 2021) and Spodoptera frugiperda (Abbas et al., 2023; Ramos et al., 2022). In contrast, using a natural diet in the laboratory seems to be more laborious because it requires daily changes of the diet and produces a limited number of insects (He et al., 2023; Zhao et al., 2023). Various infections and diseases arise in insects during laboratory rearing. More recently, different antibiotics have been added to artificial diets to treat diseases in insects, contributing to the success of large-scale culturing (Thakur et al., 2016; Zahra, 2021). The existing literature suggests that insects possess a limited amount of energy. When insects are affected by disease, they use a significant portion of their energy in fighting the disease. This, in turn, leaves the insects with less energy for growth and development (Gao et al., 2020). So, when an antibiotic is added to an artificial diet, it prevents the insects from becoming diseased and preserves their energy for growth (Elnahal et al., 2022; Van Boeckel et al., 2015). For example, silkworms (Bombyx mori) are larger, with heavier cocoons, when reared on a diet with antibiotic added (Savithri, 2007), rifampicin and aureomycin has been added to an artificial diet to treat aphids (Lin et al., 2015) and penicillin is used to treat disease in Spodoptera exigua (Thakur et al., 2016). The growth of gut bacteria in Plutella xylostella increased in insects reared on an artificial diet supplemented with rifampicin (Gao et al., 2021). Another antibiotic, chloramphenicol, has also been added to artificial diet for the optimal growth of insects (Li et al., 2020). However, there is limited knowledge on the growth of O. furnacalis clades regarding the effects of antibiotics.
Therefore, this article aimed to investigate whether these three clades of O. furnacalis respond similarly or differently in terms of survival across all life stages when reared on a natural diet or on various artificial diets. Our hypothesis focused on analyzing the impact of artificial diet with antibiotic added, artificial diet alone and natural diet on the three clades of O. furnacalis during mass rearing. To address this hypothesis, we conducted a comprehensive analysis of the insects by considering various biological characteristics, including developmental time, weight of larvae, pupae and adults, larval head capsule width, pupation percentage, adult emergence rate and sex ratio. Our research aims to contribute knowledge by providing a comprehensive evaluation of biological characteristics throughout all life stages under the influence of different diets in laboratory settings. This investigation lays the foundation for more refined rearing techniques.
Materials and methods
Adult collection and clade separation
From early April 2023 to late October 2023, adult females of O. furnacalis were captured manually using an aerial net and adult males were captured using funnel traps baited with the sex pheromone of O. furnacalis (Chen et al., 2016) at Jilin Agricultural University, Changchun, China (43.815°N, 125.398°E). The captured male and female adults were then transported to the laboratory. These adults were separated into clades by identifying the postmedial line pattern on their forewings (Han et al., 2020). Afterwards, adults (males and females) of each clade were paired into three separate insect breeding mesh cages (30 × 30 × 30 cm) and kept within a growth chamber, held at 25.7 ± 1.6°C and 57.7 ± 3.8%, with a photoperiod of 12 h light : 12 h dark, for mating. The adults were provided with a 10% aqueous honey solution with distilled water as their diet on a moist cotton swab, which was replaced daily. Tissue papers were placed inside the cages for egg laying, and these were also replaced daily. Eggs laid by females until their death, were obtained from the populations of each clade. The laid eggs were collected and transferred to round plastic cups (4 cm × 4 cm) for hatching.
Preparation of diets
Wheat germ flour artificial diet was used in this study. Amoxicillin antibiotic mixed with artificial diet was used as the antibiotic diet in this study. For the natural diet, we cultivated non-Bt maize seeds in pots inside the greenhouse. The planting medium of pots consisted of soil mixed with manure in a ratio of 2:1, providing a nutrient-rich substrate. Fresh and healthy leaves were selected and given to the O. furnacalis larvae (see Table 1).
| Sr no. | Ingredients of Antibiotic diet Percentage (%) | Ingredients of Artificial diet Percentage (%) | Ingredients of Natural diet Percentage (%) | |||
|---|---|---|---|---|---|---|
| 1. | Wheat germ flour | 150 | Wheat germ flour | 150 | Fresh maize Leaves | 100 |
| 2. | Brewer yeast powder | 50 | Brewer yeast powder | 50 | ||
| 3. | Paraben | 4 | Paraben | 4 | ||
| 4. | Sorbic acid | 4 | Sorbic acid | 4 | ||
| 5. | Agar | 16 | Agar | 16 | ||
| 6. | Sucrose | 15 | Sucrose | 15 | ||
| 7. | Ascorbic acid | 4 | Ascorbic acid | 4 | ||
| 8. | Amoxicillin | 0.25 | ||||
Insect rearing and treatments
We reared the eggs obtained from the three clades for six generations on the artificial diet in the laboratory within a growth chamber (Smart Light Incubator; Ningbo Southeast Instrument Co., Ltd, Ningbo, Zhejiang, China), maintaining an average temperature of 25.7 ± 1.6°C and a relative humidity of 57.7 ± 3.8%, under a photoperiod of L12:D12. The seventh generation was used for the life table studies. Upon larval emergence from eggs of the seventh generation, each larva was individually placed in a transparent plastic cup (5 cm × 5 cm × 5 cm).
The study included three diets: the artificial diet mixed with antibiotic, the artificial diet and the natural diet. Each clade comprised 50 repetitions, and each repetition involved a single larva. Specific diets were introduced into each cup and data were collected for each parameter.
Biology and fitness parameters
The experiments were categorized into two groups to mitigate the physiological stress caused by observations and handling of O. furnacalis.
In the first group we measured the developmental time (days) of larvae to progress from the first to the fifth instar, larval weight and head capsule width (mm in logarithm). Twenty larvae were randomly selected, confirming their activity and vitality using a delicate brush. We measured their weight with the help of an analytical balance (AW120; Shimadzu, Kyoto, Japan) to determine their average weight at this early stage. From the group of 20 larvae, 16 larvae were randomly chosen to measure the width of their head capsule.
In the second group we measured the weight of pupae and adults, the pupation percentage/adult emergence (from larvae to adults) and the sex ratio (male or female). We selected 20 pupae for weight measurement after 1 day of pupal molting. These pupae were placed in plastic round cups until adult emergence. The number of adults and sex ratio (male/female) that emerged were counted, as well as the developmental time and weight of the adults.
Data analysis
Two-way analysis of variance (ANOVA) was employed by considering two factors (clade and diet) in each life table parameter, such as developmental time (life history from newly hatched larvae to adults), size of larvae and pupae (larval and pupal weight, and larval head capsule width), pupation (number of pupae) and adult emergence and compared the means using Tukey test. The relationship of head capsule widths (transformed to logarithms) with larval instars and weight of O. furnacalis was assessed using regression and correlation analysis at the clade level for each diet. The ratio of the head capsule width was calculated based on Dyar’s rule (Gullan & Cranston, 2014) at the clade level for each diet. Sex ratio (male and female adults) was analyzed using Chi-Square Test (Gomez & Gomez, 1984).
Results
Developmental time of O. furnacalis
Regarding the developmental time parameter, we found significant differences among the three clades for each diet. Clade III demonstrated shorter developmental times for larval and pupal stages and longer adult lifespans on the antibiotic diet (3.37, 7.49 and 5.20 days, respectively; df = 49, F = 282.430, P <˂ 0.0001), followed by the artificial diet (3.49, 8.24 and 4.46 days, respectively; df = 49, F = 3.945, P <˂ 0.0400) and the natural diet (3.85, 8.90 and 4.10 days; df = 49, F = 3.216, P <˂ 0.0908). In the same way, clade II had shorter developmental times for larval and pupal stages and shorter adult lifespans on the antibiotic diet (3.51, 8.01 and 5.11 days, respectively; df = 49, F = 146.614, P <˂ 0.0010), followed by the artificial diet (3.63, 8.30 and 4.38 days, respectively; df = 49, F = 65.950, P <˂ 0.0030) and the natural diet (3.92, 9.30 and 4.01 days, respectively; df = 49, F = 3.240, P <˂ 0.1697). Clade I also performed well on the antibiotic diet (df = 49, F = 123.133, P <˂ 0.0010), compared with the artificial diet (df = 49, F = 37.500, P <˂ 0.0500) and the natural diet (df = 6, F = 1.074, P <˂ 0.7861) (see Table 2).
| Development time (24 h) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Stages | Natural Diet | Artificial Diet | Antibiotic Diet | ||||||
| Clade 1 | Clade 2 | Clade 3 | Clade 1 | Clade 2 | Clade 3 | Clade 1 | Clade 2 | Clade 3 | |
| I1 | 2.93 ± 0.005c | 2.94 ± 0.005b | 2.98 ± 0.005a | 2.87 ± 0.005b | 2.9 ± 0.005b | 2.96 ± 0.005a | 2.62 ± 0.01a | 2.68 ± 0.01ab | 2.76 ± 0.005b |
| I2 | 2.74 ± 0.005c | 2.76 ± 0.005b | 2.80 ± 0.005a | 2.24 ± 0.005c | 2.28 ± 0.005b | 2.32 ± 0.005a | 2.08 ± 0.008a | 2.11 ± 0.01a | 2.15 ± 0.005a |
| I3 | 3.18 ± 0.005c | 3.24 ± 0.005b | 3.31 ± 0.005a | 2.31 ± 0.003c | 2.4 ± 0.005b | 2.5 ± 0.005a | 2.19 ± 0.005a | 2.23 ± 0.01b | 2.34 ± 0.005b |
| I4 | 3.49 ± 0.005c | 3.60 ± 0.005b | 3.70 ± 0.005a | 3.55 ± 0.005c | 3.68 ± 0.005b | 3.8 ± 0.005a | 3.52 ± 0.01a | 3.67 ± 0.01b | 3.90 ± 0.005c |
| I5 | 6.55 ± 0.005c | 7.09 ± 0.005b | 7.81 ± 0.005a | 6.50 ± 0.005c | 6.91 ± 0.005b | 7.09 ± 0.005a | 6.42 ± 0.01a | 6.88 ± 0.01b | 7.07 ± 0.01c |
| Pupa | 9.50 ± 0.003a | 9.30 ± 0.005b | 8.90 ± 0.005b | 8.42 ± 0.003a | 8.30 ± 0.005b | 8.24 ± 0.005c | 8.10 ± 0.02a | 8.01 ± 0.01a | 7.49 ± 0.03a |
| Adult | 3.89 ± 0.005a | 4.01 ± 0.01b | 4.10 ± 0.005c | 4.13 ± 0.02b | 4.38 ± 0.01a | 4.46 ± 0.01a | 5.02 ± 0.005a | 5.11 ± 0.005b | 5.20 ± 0.005c |
- Values (Means ± S.E), followed by same letter for each row were not significantly different. (Natural diet = Clades: df = 49 (I: f = 1.074,p <˂ 0.7861, II: f = 3.240,p <˂ 0.1697, III: f = 3.216,p <˂ 0.0908); Artificial diet = Clades: df = 49 (I: f = 37.500,p <˂ 0.0500 II: f = 65.950,p <˂ 0.0030 III: f = 3.945,p <˂ 0.0400); Antibiotic diet = Clades: df = 49 (I: f = 123.133,p <˂ 0.0010 II: f = 146.614,p <˂ 0.0010 III: f = 282.430,p <˂ 0.0001).
Weight of O. furnacalis
There were significant differences found in the weights of the three clades on each diet. Clade III displayed greater weights for larvae, pupae and adults reared on the antibiotic diet (11.65, 68.27 and 69.05 mg, respectively; df = 49, F = 55.456, P <˂ 0.0001), followed by the artificial diet (10.63, 66.27 and 67.97 mg, respectively; df = 49, F = 32.345, P <˂ 0.0600) and then the natural diet (9.92, 65.85 and 66.10 mg, respectively; df = 49, F = 2.92, P <˂ 0.7865). Clade II also showed higher weights of larvae, pupae and adults on the antibiotic diet (11.26 mg, 67.97 mg, 68.68 mg, respectively; df = 49, f = 46.615, p <˂ 0.0010), followed by similar weights on the artificial diet (9.75, 64.43 and 65.85 mg, respectively; df = 49, F = 35.67, P <˂ 0.066) and the natural diet (9.75, 64.43 and 65.85 mg, respectively; df = 49, F = 1.99, P <˂ 0.7984). Similarly, larvae, pupae and adults in clade I were heavier on the antibiotic diet (11.00, 67.88 and 68.38 mg, respectively; df = 49, F = 42.348, P <˂ 0.0003), followed by the artificial diet (10.25, 65.86 and 66.10 mg, respectively; df = 49, F = 31.12, P <˂ 0.0700) and the natural diet (9.66, 63.90 and 65.20 mg, respectively; df = 49, F = 2.11, P <˂ 0.0911) (see Table 3).
| Weight (mg) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stages | n | Natural Diet | Antibiotic Diet | Artificial Diet | ||||||
| Clade 1 | Clade 2 | Clade 3 | Clade 1 | Clade 2 | Clade 3 | Clade 1 | Clade 2 | Clade 3 | ||
| I1 | 100 | 0.07 ± 0.005a | 0.09 ± 0.005a | 0.11 ± 0.005a | 0.07 ± 0.005a | 0.09 ± 0.005a | 0.11 ± 0.005a | 0.09 ± 9.81a | 0.11 ± 9.81a | 0.25 ± 9.81a |
| I2 | 16 | 0.28 ± 0.005c | 0.33 ± 0.005b | 0.45 ± 0.005a | 0.38 ± 0.005c | 0.44 ± 0.005b | 0.58 ± 0.005a | 0.58 ± 0.005c | 0.68 ± 0.005b | 0.90 ± 0.005a |
| I3 | 16 | 2.10 ± 0.005c | 2.25 ± 0.005b | 2.39 ± 0.008a | 2.61 ± 0.005c | 2.80 ± 0.005b | 2.98 ± 0.01a | 2.92 ± 0.005c | 3.24 ± 0.005b | 3.74 ± 0.005a |
| I4 | 16 | 9.99 ± 0.008c | 10.20 ± 0.005b | 10.40 ± 0.005a | 12.10 ± 0.005c | 12.23 ± 0.005b | 12.82 ± 0.005a | 14.20 ± 0.005c | 14.40 ± 0.005b | 14.86 ± 0.005a |
| I5 | 16 | 35.88 ± 0.005c | 35.90 ± 0.01b | 36.26 ± 0.005a | 36.10 ± 0.005c | 36.26 ± 0.005b | 36.66 ± 0.005a | 37.23 ± 0.005c | 37.89 ± 0.005b | 38.50 ± 0.005a |
| Pupa | 16 | 63.90 ± 0.008c | 64.43 ± 0.005b | 65.85 ± 0.005a | 65.86 ± 0.005c | 66.03 ± 0.005b | 66.27 ± 0.005a | 67.88 ± 0.005c | 67.97 ± 0.01b | 68.27 ± 0.005a |
| Adult | 16 | 65.20 ± 0.005c | 65.85 ± 0.005b | 66.10 ± 0.005a | 66.10 ± 0.005c | 67.06 ± 0.005b | 67.97 ± 0.005a | 68.38 ± 0.005c | 68.68 ± 0.005b | 69.05 ± 0.005a |
- Values (Means ± (S.E), followed by same letter for each row were not significantly different. (Natural diet = Clades: df = 49 (I: f = 2.11,p <˂ 0.0911, II: f = 1.99,p <˂ 0.7984, III: f = 2.92,p <˂ 0.7865); Artificial diet = Clades: df = 49 (I: f = 31.12,p <˂ 0.0700 II: f = 35.67,p <˂ 0.066 III: f = 32.345,p <˂ 0.0600); Antibiotic diet = Clades: df = 49 (I: f = 42.348,p <˂ 0.0003 II: f = 46.615,p <˂ 0.0010 III: f = 55.456,p <˂ 0.0001).
Head capsule width relative to each larval instar stage and weight among the clades
The regression and correlation analysis revealed that the three clades were very similar in terms of head capsule size relative to instars and weight of O. furnacalis larvae, with correlation values close to 1 for these parameters. Thus, there was a solid relationship between larval head capsule width, instar and weight of O. furnacalis larvae. Interestingly, for the relationship between head capsule width and larval instar, the third and fourth instars of clade III had significantly wider head capsule widths for all diets (1,4 mm in logarithm, y = 0,1996x + 0,2,855, R2 = 0,9,934). Whereas clades II and I had fewer wide head capsule widths (clade II, 1,3 mm in logarithm, y = 0,1984x + 0,2,886, R2 = 0.9979; 1,2 mm in logarithm, y = 0,1956x + 0,2,887, R2 = 0,9,974) with all diets (Fig. 1). In terms of the relationship between head capsule width and the weight of O. furnacalis larvae, clade III had wider head capsule widths correlated with heavier weights (y = 676608 × 64326, R2 = 0,9,963, P < 0.0001), compared with clade II (y = 65608 × 62233, R2 = 0,9,948, P < 0.0001) and clade I (y = 66890 × 62233, R2 = 0,9,936, P < 0.0001) (Figure 1 and 2).


Pupation and sex ratio of O. furnacalis (%)
At the clade level, clade III had a higher number of pupae proceed to the adult stage on natural, artificial and antibiotic diets (74.8%, 81.1% and 89.5%, respectively; df = 49, F = 48.451, P <˂ 0.005) than clade I (64.2%, 71.3% and 78.3%, respectively; df = 49, F = 3.21, P <˂ 0.3173) or clade II (72.5%, 79.7% and 81.7%, respectively; df = 49, F = 0.053, P <˂ 0.8132) (Fig. 3). Also, clade III had a higher sex ratio (df = 49, =0.65,0.64,0.63, x2 <˂ 0.0010) compared with clade I (df = 49, =0.55,0.54.0.53, x2 <˂ 0.0603) or clade II (df = 49, =0.51,0.50,0.49, x2 <˂ 0.0040) on natural, artificial and antibiotic diets. Clade III showed a higher pupation rate and sex ratio on the antibiotic diet (Table 4).
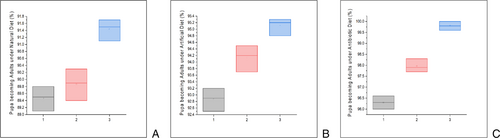
| Adult emergence (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Natural Diet | Artificial Diet | Antibiotic Diet | |||||||
| Clade 1 | Clade 2 | Clade 3 | Clade 1 | Clade 2 | Clade 3 | Clade 1 | Clade 2 | Clade 3 | |
| Larvae to Adult (number) | 64.2 ± 0.11a | 72.5 ± 0.11b | 74.8 ± 0.08c | 71.3 ± 0.11a | 79.7 ± 0.11b | 81.1 ± 0.11c | 78.3 ± 0.11a | 81.7 ± 0.11b | 89.5 ± 0.05c |
| Sex Ratio (%) | |||||||||
| Male | 43.2 ± 0.20a | 44.2 ± 0.14b | 49.8 ± 0.14c | 48.3 ± 0.14a | 52.2 ± 0.17b | 56.6 ± 0.14c | 34.9 ± 0.08a | 39.5 ± 0.05b | 42.1 ± 0.05c |
| Female | 41.7 ± 0.20a | 43.7 ± 0.14b | 44.1 ± 0.14c | 42.4 ± 0.14a | 42.7 ± 0.17b | 43.3 ± 0.14c | 59.0 ± 0.08a | 58.5 ± 0.05b | 57.9 ± 0.05c |
| Clades (Average) | 149.1 | 160.4 | 168.7 | 162 | 174.6 | 181 | 172.2 | 179.7 | 189.5 |
| Diet (Average) | 159.4 | 172.5 | 180.46 | ||||||
- Values (Means ± SE, followed by same letter for each row were not significantly different.
Suitability of diets in three clades of O. furnacalis
The LGI fitness parameters displayed significant improvement in clade III, as compared with clades I and II, on an antibiotic diet, compared with both artificial and natural diets, with values of 21.15, 19.35 and 17.66, respectively. The PGI also exhibited notable growth for clade III, compared with clades I and II, with significant results on an antibiotic diet (9.07), compared with artificial (8.725) and natural (8.03) diets. The TDI also indicated significant results for clade III, compared with clades I and II, on an antibiotic diet, surpassing the performance on artificial and natural diets (33.72, 33.57 and 30.53, respectively). The SGI revealed that the insects experienced better growth in clade III, compared with clades I and II, on an antibiotic diet (4.63), in comparison with the artificial (4.36) and natural (4.25) diets. Likewise, the FI demonstrated superior results for clade III, compared with clades I and II, on an antibiotic diet (396.25), compared with artificial (388.71) and natural (370.09) diets (Table 5).
| Fitness of O. furnacalis | Clades | Larval Growth Index (LGI) | Average (total) | Pupal Growth Index (PGI) | Average (total) | Total Developmental Index (TDI) | Average (total) | Standardized Growth Index (SGI) | Average (total) | Fitness Index (Amir-Maafi et al.) | Average (total) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Natural diet | 1 | 13.91 ± 0.008c | 17.66 | 4.05 ± 0.02a | 8.03 | 21.91 ± 0.03a | 30.53 | 1.50 ± 0.005b | 4.25 | 236.01 ± 0.24c | 370.09 |
| 2 | 15.91 ± 0.03b | 5.02 ± 0.01a | 240.58 ± 0.05b | 2.09 ± 0.01a | 250.37 ± 0.35c | ||||||
| 3 | 18.89 ± 0.04b | 7.04 ± 0.02a | 27.10 ± 0.09b | 2.16 ± 0.006a | 312.91 ± 0.16c | ||||||
| Artificial Diet | 1 | 17.09 ± 0.01a | 19.25 | 6.04 ± 0.005a | 8.72 | 29.76 ± 0.03b | 33.57 | 3.10 ± 0.005a | 4.36 | 354.82 ± 0.09c | 388.71 |
| 2 | 18.29 ± 0.01b | 7.89 ± 0.005a | 32.54 ± 0.03b | 3.28 ± 0.005a | 366.31 ± 0.83c | ||||||
| 3 | 20.37 ± 0.005a | 9.24 ± 0.005a | 34.41 ± 0.07b | 4.72 ± 0.005a | 387 ± 1.11c | ||||||
| Antibiotic Diet | 1 | 18.02 ± 0.02b | 21.25 | 7.10 ± 0.003a | 9.07 | 31.77 ± 0.05b | 33.72 | 3.50 ± 0.01a | 4.63 | 382.92 ± 3.07c | 396.25 |
| 2 | 21.84 ± 0.03b | 8.06 ± 0.005a | 33.49 ± 0.06b | 4.63 ± 0.005a | 350.01 ± 0.45c | ||||||
| 3 | 23.91 ± 0.008a | 10.05 ± 0.02b | 35.91 ± 0.03b | 5.78 ± 0.006a | 455.82 ± 0.09c |
- Values (Means ± SE, followed by same letter for each row were not significantly different.
Discussion
Biological performance studies on clades of O. furnacalis under different diets are crucial for a comprehensive understanding of their survival and dispersal. This knowledge is particularly relevant to agroecosystems, where understanding variations in the developmental periods and population dynamics of the clades of O. furnacalis is essential for effective management. Although many previous studies have evaluated the development of O. furnacalis on different host plants and artificial diets (Jie et al., 2022; Rahayu & Trisyono, 2018; Xin-Hua et al., 2023), there is limited information on the biological performance of the clades of O. furnacalis (Han et al., 2020).
However, the results reveal that a natural diet of fresh maize leaves significantly affects the biological parameters of developmental time and weight for each clade of O. furnacalis, leading to prolonged developmental times and lower weights of larvae. This suggests that these leaves are less suitable for the insects of each clade. These variations are associated with low food quality, the nutritional constitution of different plant tissues and the relationship between protein and carbohydrates that are unavailable to the insects (Bisht et al., 2018; Wang et al., 2019). For some lepidopteran species, a prolongation in developmental time indicates compensatory action, where larvae subjected to a poorer nutritional source tend to compensate for food stress by lengthening the immature stage and not reaching the ideal stage for proper weight gain (Silva et al., 2018). Previous research has shown that an antibiotic diet outperforms then other diets in terms of development because it is a high-caliber diet that requires less time for growth (Mohd Yusof et al., 2021).
The antibiotic diet used in this study led to heavier weights in all life stages of O. furnacalis, whereas the artificial and natural diets did not yield weights comparable with the antibiotic diet. Numerous scientists have said that the use of antibiotics inhibits the growth of invading microorganisms, leading to favorable conditions for insect development and weight gain (Kim et al., 2022; Morales-Ramos et al., 2022). It is possible to gradually eliminate a few microbes in some insects when feeding the insects with antibiotics over several generations. Clade III outperformed the other clades with a shorter development time and higher weight than Clades I and II. Previous studies have indicated that the inclusion of antibiotics in artificial diets can suppress the growth of invading microorganisms, contributing to favorable insect development (Li et al., 2020).
We established strong relationships of head capsule width with each instars stage and their weights. Our results tell that clade III as compared to clade I and II O. furnacalis had most wider head capsule width ranged between (1.4–1.7 mm in logarithm) with significant increase in 4th–5th instar in antibiotic diet. Because it saves the energy level of insects, that helps them to grow well. These results aligning with previous research on different species such as Lobesia botrana (Delbac et al., 2010) and S. frugiperda (Tiwari et al., 2023). Over results also align with the another findings specific on O. furnacalis, that also indicated a correlation of larval instars with larval head capsule widths and weight (Rahayu & Trisyono, 2018; Tiwari et al., 2023). Our findings also matched with another research that showed that size increment ratio of larval head capsule width in each molt generally constant from 1.3 to 1.7 mm in logarithm (Gullan & Cranston, 2014).
The number of pupations, adult emergence and sex ratio produced from the larvae fed with the antibiotic, artificial and natural diets were not closely related, indicating that these diets did not affect O. furnacalis Clades in the same way. In our findings, natural and artificial diets supported the larvae in developing into larger female adults, while the antibiotic diet supported the development of more male adults and exhibited a better sex ratio than artificial and natural diets. Similar results were found in this study (Gopalakrishnan & Kalia, 2022). Based on our findings, the biological and fitness parameters of different Clades of O. furnacalis reared on the antibiotic diet were rated as good, while the artificial diet exhibited moderate fitness. In contrast, the natural diet proved to be a poor diet. Regarding Clades, Clade III exhibited higher fitness as compared with Clade I and II.
The significance of incorporating antibiotics into artificial diets act as a growth booster for the mass rearing of three Clades of O. furnacalis in the laboratory (Galarza et al., 2021; Phuong et al., 2016). Antibiotics function as growth promoters by addressing bacterial infections (Wang et al., 2021) and potentially amplifying the overall growth and development (Galarza et al., 2021). This research holds the potential to significantly enhance the successful mass-rearing of three Clades of O. furnacalis in the laboratory. Despite the success presented in this study, further improvement of the existing antibiotic diet is needed.
Conclusion
The antibiotic diet used in this study showed a notable effectiveness in comparison to both artificial and the natural diets, by producing large number of O. furnacalis healthy insects. Besides diet, rearing conditions also played a crucial role in successful insect rearing. Consequently, the antibiotic diet, along with rearing conditions described in this study, can be employed for culture maintenance of all three O. furnacalis Clades. These investigations highlighted the reliability and effectiveness of the antibiotic diet for mass rearing of O. furnacalis. In relation to Clades, the biological parameters of all three Clades exhibited most significant results when subjected to the antibiotic diet. Notably, Clade III displayed markedly enhanced growth and development compared to Clade I and II. However, it remains crucial to ascertain whether these O. furnacalis Clades, reared in laboratory conditions, respond similarly to the natural conditions. Nevertheless, further comprehensive studies are essential to enhance and refine this antibiotic diet for future applications.
Author contribution
Aleena Alam: conceptualization, methodology, and writing—original draft preparation. Sohail Abbas, Wang Liangzhu, Qin Weibo, Feng Xiao: Conducted the experiment. Wu Haichao, Arzlan Abbas, Liu Jiali: statistical analysis and making table and figures. Muhammad Shakeel, Farman Ullah, Jamin Ali, Khalid Ali Khan, Hamed A. Ghramh: polishing and revising the manuscript. Chen Ri Zhao and Xie Zhiming: Funding the manuscript. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
We would like to thank Julio C Rojas for their comments on the earlier version of this paper that significantly improved the manuscript. The authors extend their appreciation to the deanship of scientific research (RGP2/491/44) at King Khalid University, Saudi Arabia.
Funding information
We thank Jilin Province Major Science and Technology Project Mission Statement (20230302005NC), Jilin Province Science and Technology Development Plan Project (20190303075SF) and Program for Innovative Research Team of Baicheng Normal university (IRTBCNU) for their financial support towards this research.




