Aoria rufotestacea Faimaire (Coleoptera: Chrysomelidae) long been confused as Bromius obscurus (Linnaeus) in Korea
Abstract
Western grape rootworm, Bromius obscurus (Linnaeus) is a well-known pest of grape vines in Europe and North America. We found that all the Korean specimens that have long been known as B. obscurus turned out to be Aoria rufotestacea Faimaire, showing with only about 83% sequence similarity in COI barcodes. The small puncture patterned specimens of A. rufotestacea were initially confused with A. scutellaris Pic, but confirmed to be A. rufotestacea with genitalic characteristics as well as 100% matched COI barcode sequence. Here we describe and compare the morphologically confusing species, B. obscurus and A. rufotestacea, with description of scutellaris-like wing variation, in detail with adult images and COI sequences.
Introduction
Eumolpinae are a large subfamily of Chrysomelidae, considered as a monophyletic group (Gomez-Zurita et al. 2005), including more than 500 genera and 7000 species (Jolivet & Verma 2008). Eumolpinae are widely distributed, numerous in the tropics and subtropics, and more eumolpine species are being discovered and described every year. Going north, Eumolpinae become rarer, progressively diminishing towards the north (Jolivet & Verma 2008). However, it seems that Bromius obscurus L. is rather common: in the Northern points of Siberia there are four Eumolpinae species recorded and one of them is B. obscurus (Jolivet & Verma 2008 via pers. comm. with Silfverberg).
All the larvae of typical Eumolpinae develop in soil, and are root feeders. Several eumolpine species are attracted by Vitis L., but, like B. obscurus, many feed on fireweed, Epilobium angustifolium L. (family Onagraceae), also known as rosebay willowherb (Jolivet & Verma 2008). Bromius obscurus (ssp. villosulus) feeding on grapes in France has become almost extinct due to insecticides and introduction of American grapes for grafting (P. Jolivet, pers. comm., 2010). Therefore, they are rare on Vitis vinifera in France, and it feeds on Epilobium hirsutum in humid places.
There are two color forms of B. obscurus: one with blackish forewings (B. obscurus obscurus) and the other with reddish forewings (B. obscurus villosulus). For some time, the red wing form was considered as a subspecies but no strong argument or evidence has been suggested and they are often considered as two varieties within a species. This may need further investigation, especially with a molecular approach.
Since they have been reported from East Asia, the species found in Korea, which is superficially similar to B. obscurus villosulus, has been, with no question or suspicion, considered as B. obscurus (Lee & An 2001; Lee & Cho 2006). The life cycle of the Korean species, although not studied in detail, seems to be similar as that of B. obscurus since both are found on Vitis, too. The Korean specimens were all B. obscurus villosulus-like, which is a red wing form.
Here we compared the two morphologically similar species: B. obscurus which turn out to be an extremely rare species if occurring in Korea and A. rufotestacea and its variation with scutellaris-like puncture pattern on elytra. Species descriptions and adult images are provided for the species, with the median lobe image of A. rufotestacea as well as the COI barcode sequences compared between B. obscurus and A. rufotestacea.
Materials and methods
In addition to the collection of Korean specimens located mainly at the National Science Museum and Chungbuk National University, we obtained B. obscurus specimens collected from Canada, USA, France, Germany, Russia and Taiwan. The specimens examined are deposited in the Insect Collections of the Department of Plant Medicine at Chungbuk National University, Cheongju, and also in the National Science Museum, Daejeon.
Genomic DNA was extracted using DNeasy Tissue kit (Qiagen, Hilden, Germany) and PCR amplification was conducted using the primer set (LCO1490 and HCO2198) following Folmer et al. (1994) with 40 cycles (95°C for 2 min, 55°C for 30 s, and 72°C for 45 s). For each PCR amplification, pre-denaturation at 95°C for 2 min and final polymerization at 72°C for 5 min were added at the beginning and end, respectively, of the 40 cycles. The PCR product was purified using QIAquick Gel Extraction Kit (Qiagen). The COI barcode sequences were compared using MEGA5 (Tamura et al. 2011).
Systematic accounts
Genus Bromius Chevrolat, 1837 [po-do-ggob-chu-ip-beol-le-sok]
Bromius obscurus (Linnaeus, 1758) [po-do-ggob-chu-ip-beol-le] (Figs 1, 2, 4, 5)

Antennal comparison between Bromius obscurus (upper) and Aoria rufotestacea (lower).
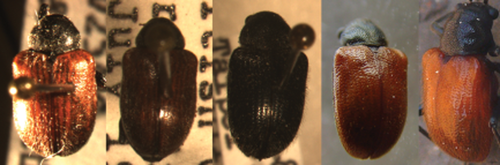
Adult images of Bromius obscurus (left three) and Aoria rufotestacea (right two). Far left is B. obscurus villosulus (red wing form), which is similar to A. rufotestacea in coloration.
Cheysomela obscurus Linnaeus, 1758:375.
Bromius obscurus: Gruev, 1979:114, 1992:3.
Length 5.0–6.0 mm. General color black or partly brown, legs black or with brownish tibiae, and 3 or 4 basal antennomeres reddish. Variations: elytra black covered by whitish hairs (typical form); elytra black, hairs yellowish, tibiae basally reddish brown (ab. weisei Heyden, 1883); elytra and tibiae brown, hairs whitish (ab. epilobii Weise, 1882); elytra brown, hairs yellowish (ab. villosulus Schrank, 1781) (Warchalowski 2010).
Head moderately punctured, nearly same size as compared with pronotal punctures. Antenna (Fig. 1) about 1/2 as long as body; 1st antennomere about 2.5 times as long as broad; 2nd about 2 times as long as broad; 3 somewhat shorter than 2; 4 slightly shorter than 2 + 3; 5 equal to 4, slightly longer than 6; 6–10 decreasing slightly in length; 11 slightly longer than 10, constricted apically. Thorax somewhat broader than long, broadest at before middle; pronotum subevenly convex and distinctly and strongly punctured. Scutellum nearly trapezoid and truncate apically. Elytron (Fig. 2) subparallel-sided in basal 2/3, narrowed apically; disc somewhat convex on the center, with wrinkles and very large, irregular punctures. Ventral surfaces weakly punctured. Legs slightly stout; hind tibia slightly arched, carinate at apex; hind tarsal segment 1 slightly shorter than 2 + 3.
Specimens examined. 2exs., Garibaldi High., British Columbia, Canada, 4. VII. 1988, CL& L Staines leg.; 1ex., Hwy 285 nr. Bailey N. Fork S. Platte R. Park Co., Colorado, USA, RW Baumann & WP Stark leg.; 1ex. near Hoosier Pass, Hwy. 9, Blue River, Summit Co., Colorado, USA, RW Baumann & WP Stark leg.; 2exs. 7–8,000ft., Brighton, Salt Lake Co., Utah, USA, 1. VII. 1990, R. Cordon leg.; 1ex., Mt. Summit, Whiteface, Essex Co., NY, USA, J. & B. Huether; 1ex. Hwy. 50, Tucker, Soldier Creek, Utah Co., USA, RW Baumann & WP Stark leg.; 2exs. nr. San Tze Ho, 8mi. N. Taipei, Taiwan, -.VI. 1962, L. Kunta leg.; 1ex., 44.05 N. 134.12 E, 650 m, Chuguvevka, Biol. Stat. 30 km SE, Kray. Siklnne-Alin., Primorskiy, Russia, 1. VI. 1993, L. Zerche et al., leg.; 1ex., Grãfenwarth, krs Schleiz, 5. VII. 1979, R. Sutter leg.; 1ex., Dübener Heide, b. Wöllkaû, Epilabiliw angusr, 19. VI. 1955, Dieckmann leg.
Distribution
Europe, Russia (The Urals, Siberia, The Kurils), Central Asia including Kazakhstan and Mongolia, China (Heilongjiang, Hebei, Henan, Shanxi, Gansu, Xinjiang, Hubei, Jiangsu, Hunan, Guizhou, Sichuan, Xizang, Jiangxi), Japan (Hokkaido, Honshu), Taiwan, North America.
Host plants
Cultivated Vitis vinifera L. and Onagraceae.
Remarks
The two genera are morphologically different: anterior margin of proepisternum is slightly convex and the marginal ridge of pronotum is thin and weak in Bromius, while the anterior margin of proepisternum is straight or concave in Aoria. When we examined and compared in detail the specimens recently collected from a grape vineyard in Chungbuk Province with the specimens of B. obscurus from other countries, we found the specimens we collected are not B. obscurus as they did not match in morphological details. We also found that all the images and specimens so far known as B. obscurus in Korea via the Internet as well as from scientific publications (Lee & An 2001; Lee & Cho 2006) turn out to be A. rufotestacea. This is probably due to two reasons: similar appearance between the two species (Figs 1, 2) and a preconceived notion based on wide distribution of B. obscurus including the adjacent countries such as Taiwan and Japan. We could not find any blackish wing form of B. obscurus, either. Our conclusion is that B. obscurus does not occur in Korea unless they are extremely rare. For a comparison in sequences, the COI barcode sequence of a New York sample of B. obscurus is compared with a Korean sample of Aoria rufotestacea (Fig. 5). We also compared 18S rDNA sequences (data not shown) between the two. We found they were distant enough to be considered as far distant species, if not different genera, i.e. sequence difference between the Korean specimen and the New York specimen (B. obscurus villosulus) was 17.1% in COI barcode.
Although we could not find B. obscurus villosulus collected in Taiwan when we examined the collection at the Smithsonian, both color forms of B. obscurus have been reported from China and Japan. Bromius obscurus obscurus has been reported from all over the China while B. obscurus villosulus has been reported from Hebei, Shanxi, Hubei, and Jiangxi (Hua 2002). In the case of Japan, there is a specific note on B. obscurus villosulus specimens collected in Japan and used as type specimens in the paper by Kimoto (1961), stating “those two forms are identical with a[n] European form villosulus.” The two subspecies of B. obscurus might need a thorough study in the future as their subspecific status is often disregarded, yet our preliminary molecular result indicated a possibility of subspecific discrepancy.
Genus Aoria Baly, 1863 [maep-si-deung-ggob-chu-ip-beol-le-sok]
Aoria (Aorria) rufotestacea Fairmaire, 1889 [maep-si-ggob-chu-ip-beol-le] (Figs 1-5)

Two types of puncture pattern in elytra of Aoria rufotestacea: left, large punctures; right, small punctures.
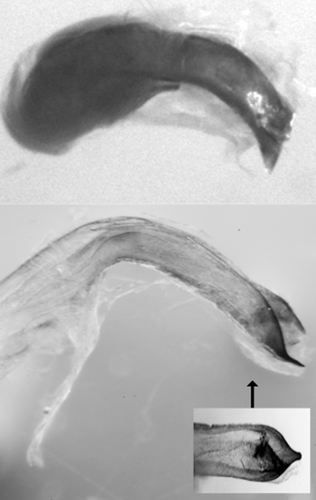
Lateral view of the median lobes of Bromius obscurus (upper; from USA) and Aoria rufotestacea (lower; from Korea).
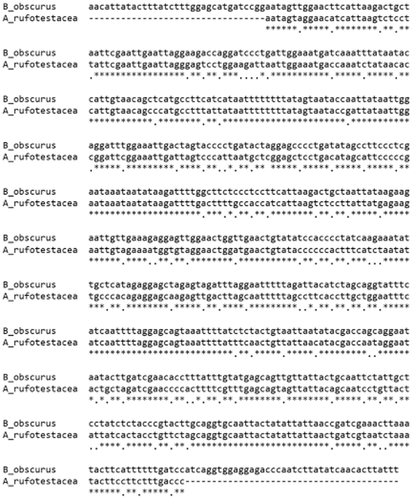
COI barcode sequences compared between Bromius obscurus (from New York, USA) and Aoria rufotestacea (from Korea).
Aoria rufotestaceas Fairmaire, 1889:70.
Bromius obscurus: Lee & An, 2001:80; Lee & Cho, 2006:25.
Length 5.5–7 mm. Black and reddish brown; head entirely black; antenna blackish, but reddish to pitchy on segments 1–4; prothorax entirely black; scutellum black; elytron reddish brown; ventral surfaces black with abdomen reddish brown; legs black, slightly pitchy reddish on tarsi. Body clothed yellowish white hairs above, ventral surfaces moderately clothed.
Head moderately punctured, nearly same size as compared with pronotal punctures. Antenna (Fig. 1) about 2/3 as long as body; 1st antennomere about 3 times as long as broad; 2nd 2 times as long as broad; 3 longer than 2; 4 as long as 2 + 3; 5 equal to 4, slightly longer than 6; 6–10 decreasing, slightly in length; 11 slightly longer than 10, constricted apically. Thorax very slightly broader than long, broadest at before middle; pronotum subevenly convex and distinctly and strongly punctured. Scutellum nearly triangular narrowed and rounded-truncate apically. Elytron (Figs 2, 3) subparallel-sided in basal 2/3, narrowed apically and broadly rounded apically; pronotum with nearly regular paired rows of large punctures which are nearly overlapped each other, or with two to three rows of irregularly arranged small punctures; the interstics impunctate. Ventral surfaces weakly punctured. Legs fairly prolonged; hind tibia slightly arched, and carinate at apex; hind tarsal segment 1 slightly shorter than 2 + 3.
Specimens examined. [large puncture pattern] 2exs., Mt. Taebak-san, Gangwon-do, Korea, 26. VII. 1976, S. M. Lee leg.; 1ex., Mt. Odae-san, Gangwon-do, Korea, 2. VIII. 1988, S. L. An leg.; 1ex., Mt. Soyo-san, Gyeonggi-do, Korea, 13. VII. 1986, S.M. Lee; 1ex. Hwacheon, Gangwon-do, Korea, 17. VI. 1987, K.Y. Park et U. Park; 1ex. Mt. Chiak-san, Gangwon-do, Korea, 13. VI. 1976, S.M. Lee; 1ex., Mt. Duwi-bong, Gangwon-do, Korea, 10. VI. 2000, S. L. An; 1ex., Mt. Seorak-san, Gangwon-do, Korea, 1. VII. 1993 Y.H. Kim; 2exs., Mt. Bomun-san, Chungcheongnam-do, Korea, 1. VII. 1992, S. L. An; 2exs., Mt. Juwang-san, Gyeongsangbuk-do, Korea, 28. VII. 1984, S.L. An; 1ex., Mt. Juwang-san, Gyeongsangbuk-do, Korea, 8. VI. 1991, S.L. An; 1ex., Mt. Juwang-san, Gyeongsangbuk-do, Korea, 14. VI. 1991, S.L. An; 1ex., Mt. Juwang-san, Gyeongsangbuk-do, Korea, 26. VII. 1984, Y.J. Kwon; 1ex., Mt. Bohyeon-san, Gyeongsangbuk-do, Korea, 29. V. 1998, Y.J. Kwon; 1ex., Mt. Ga-san, Gyeongsangbuk-do, Korea, 23. V. 1986, S.L. An; 1ex., Mt. Hagil-san, Gyeongsangbuk-do, Korea, 27. V. 1997, Y.J. Kwon; 1ex., Mt. Jiri-san, Gyeongsangnam-do, Korea, 21. VI. 2001, S.L. An; 1ex., Yongmunsa-Temple, Gyeongsangnam-do, Korea, 16. VI. 1980, Y.J. Kwon; 1ex., Mt. Hwangmae-san, Gyeongsangnam-do, Korea, 6. VI. 1998, S.J. Suh; 2exs., Seogwipo, Jeju-do, Korea, 20. VI. 1976, S.M. Lee; 1ex., Mt. Halla-san, Jeju-do, Korea, 11. VIII. 1984, Y.J. Kwon; 1ex., Mt. Halla-san, Jeju-do, Korea, 17. VI. 1994, S.L. An. [small puncture pattern] 1ex., Mt. Bukhan-san, Gyeonggi-do, Korea, 11. VII. 1976, S.M. Lee; 1ex., Gwangreung, Gyeonggi-do, korea, 21. V. 1982, K.J. Weon; 1ex., Mt. Odae-san, Gangwon-do, Korea, 2. VIII. 1976, Y.J. Kwon; 3exs., Mt. Duwi-bong, Gangwon-do, Korea, 10. VI. 2000, S.L. An; 3exs., Mt. Gariwang-san, Gangwon-do, Korea, 28. V. 1998, S.L. An; 1ex., Mt. Bomun-san, Chungcheongnam-do, Korea, 1. VII. 1992, S.L. An; 1ex., Mt. Gyeryeong-san, Chungcheongnam-do, Korea, 24. V. 1989, S.L. An; 1ex., Mt. Seodae-san, Chungcheongnam-do, Korea, 9. VI. 2002, S.L. An; 1ex., Mt. Songni-san, Chungcheongbuk-do, Korea, 10. VI. 1977, S.M. Lee; 1ex., Mt. Baegun-san, Jeollanam-do, Korea, 5. VI. 1999, Y.J. Kwon; 3exs., Mt. Seonam-san, Gyeongsangbuk-do, Korea, 9. V. 1998, S.L. An; 1ex., Mt. Sobaeg-san, Gyeongsangbuk-do, Korea, 5. VI. 1981, S.M. Lee; 1ex., Mt. Juwang-san, Gyeongsangbuk-do, Korea, 16. V. 1999, S.L. An; 1ex., Mt. Juwang-san, Gyeongsangbuk-do, Korea, 14. VI. 1991, S.L. An; 1ex., Mt. Palgong-san, Gyeongsangbuk-do, Korea, 28. V. 1985, Y.J. Kwon; 1ex., Mt. Palgong-san, Gyeongsangbuk-do, Korea, 2. VII. 1982, Y.J. Kwon; 1ex., Mt. Unmun-san, Gyeongsangbuk-do, Korea, 21. V. 1991, S.L. An; 1ex., Mt. Undal-san, Gyeongsangbuk-do, Korea, 26. V. 2000, S.L. An; 1ex., Mt. Hwaak-san, Gyeongsangbuk-do, Korea, 14. V. 1998, S.L. An; 1ex., Mt. Jiri-san, Gyeongsangnam-do, Korea, 29. V. 1979, Y.J. Kwon; 1ex., Mt. Jiri-san, Gyeongsangnam-do, Korea, 14. VI. 1999, S.L. An; 1ex., Mt. Halla-san, Jeju-do, Korea, 18. VI. 1984, S.L. An, 10 exs., Gangcheongnongwon, 491-58, Ewon-ri, Ewon-myeon, Okcheon-gun, CB, N36°18′06.07″ E127°23′34.17″, 18. V. 2009, G.H. Kim & S. Cho.
Distribution. Korea, S. China.
Host plants. Vitis vinifera L.
Remarks
As in the case of antennae (Fig. 1), the median lobe of B. obscurus is also thicker than that of A. rufotestacea (Fig. 4). The median lobe of A. rufotestacea is rather slender and long, with narrowed and, as noted by Medvedev (2012), pointed tip while that of B. obscurus is thick and short, with angled, blunt tip. Although the main specific confusion was between B. obscurus and A. rufotestacea, A. rufotestacea is in fact very similar to A. scutellaris Pic, 1923 in appearance. The main distinction was traditionally done based on the puncture pattern of elytra, and we first identified A. rufotestacea and A. scutellaris, which showed large puncture type and small puncture type, respectively. However, when we examined the genitalic characteristics and the COI barcode sequences, we found both types showed the same genitalic characteristics as A. rufotestacea. We confirmed they are all A. rufotestacea as we found both types (Fig. 3) showed 100% matching in COI barcode sequences. This could mean that the puncture pattern may not be a good character for distinguishing similar species, at least in this case. Accordingly, it may be necessary to reexamine the specific description of Aoria scutellaris in detail and compare it with other Aoria species in the future.
Discussion
Bromius obscurus is known to be geographically parthenogenetic with a single polyploid clonal form (3n) (Suomalainen et al. 1987). North American populations are diploid whilst the European counterparts are apomictic triploids. However, the beetle originated in Europe where it was originally bisexual (P. Jolivet, pers. comm., 2010). As asexuality is important in speciation and ecological adaptation, geographical parthenogenesis shown by B. obscurus may be a reason for its pandemically wide distribution (Lundmark & Saura 2006). Probably due to the rarity of males we could find only one male specimen among the five specimens we dissected for genitalia.
A recent interesting discovery on B. obscurus is a stridulatory apparatus found on the forewing and hind wing. The stridulatory apparata are found as a microsculpture spot near the end of each wing between RS and Cu veins and this apparatus is found also in other eumolpine species examined (Medvedev & Muravitzsky 2009). This hypothesis may be right, but the spot, named as taches medio-cubitales (Jolivet 1957–1959), may also be just a binding patch on the wings (Hammond 1979) as it is located where hind wing is angled and folded inwardly. When we examined, such a dark spot was also found in the Korean specimens, that is A. rufotastecea.
After examining many more specimens from various sites, we found that virtually all the specimens known as B. obscurus in Korea turned out to be either A. rufotestacea or A. scutellaris, an undescribed species to Korea. We also found that almost all the beetle-related websites in Korea incorrectly showed Aoria species as B. obscurus. One of the main distinctions between A. rufotestacea and A. scutellaris was traditionally done by the puncture pattern on elytra. However, when we examined our samples of both large and small puncture patterns, we found that their genitalia as well as the COI sequences (sequence similarity of 100% match) revealed the two types found in Korea are in fact the same species, A. rufotestacea. This obviously means the traditional way of distinguishing eumolpine species based on the elytra puncture pattern should be applied more carefully.
Acknowledgments
We thank Dr H. Hoch (Museum für Naturkunde, Humboldt Universität zu Berlin) for her help in obtaining specimens from Europe, Dr P. Jolivet (National Museum of Natural History, Paris) who not only sent us some specimens but made valuable comments on B. obscurus, and Dr A. S. Konstantinov (USDA Systematic Entomology, Smithsonian National Museum of Natural History) who allowed us to take and examine some specimens of B. obscurus collected from various countries. This work was supported by the research grant of Chungbuk National University in 2012.




