Molecular phylogeny of the higher taxa of Odonata (Insecta) inferred from COI, 16S rRNA, 28S rRNA, and EF1-α sequences
Abstract
In this study, we sequenced both two mitochondrial genes (COI and 16S rRNA) and nuclear genes (28S rRNA and elongation factor-1α) from 71 species of Odonata that represent 7 superfamilies in 3 suborders. Phylogenetic testing for each two concatenated gene sequences based on function (ribosomal vs protein-coding genes) and origin (mitochondrial vs nuclear genes) proved limited resolution. Thus, four concatenated sequences were utilized to test the previous phylogenetic hypotheses of higher taxa of Odonata via Bayesian inference (BI) and maximum likelihood (ML) algorithms, along with the data partition by the BI method. As a result, three slightly different topologies were obtained, but the BI tree without partition was slightly better supported by the topological test. This topology supported the suborders Anisoptera and Zygoptera each being a monophyly, and the close relationship of Anisozygoptera to Anisoptera. All the families represented by multiple taxa in both Anisoptera and Zygoptera were consistently revealed to each be a monophyly with the highest nodal support. Unlike consistent and robust familial relationships in Zygoptera those of Anisoptera were partially unresolved, presenting the following relationships: ((((Libellulidae + Corduliidae) + Macromiidae) + Gomphidae + Aeshnidae) + Anisozygoptera) + (((Coenagrionidae + Platycnemdidae) + Calopterygidae) + Lestidae). The subfamily Sympetrinae, represented by three genera in the anisopteran family Libellulidae, was not monophyletic, dividing Crocothemis and Deielia in one group together with other subfamilies and Sympetrum in another independent group.
Introduction
The insect order Odonata contains approximately 5500 species and is divided into three suborders: Anisoptera, Zygoptera, and Anisozygoptera (Tofilski 2004). Anisoptera is characterized by a broader hindwing, possession of the crossvein that divides the discoidal cell into a triangle and super triangle in both wings, and outstretched wings when at rest, whereas Zygoptera is characterized by a hindwing essentially similar to the forewing, wings parallel to the body when at rest, and widely separated eyes (Needham 1903; Tillyard 1923, 1928). The third suborder Anisozygoptera is composed of two living species, found in Japan and in the Himalayas, respectively. Although the Anisozygoptera is morphologically intermediate between Anisoptera and Zygoptera in adult body and wings, this suborder is often grouped together with Anisoptera, under the name Epiprocta (Bechly 1996; Lohmann 1996; Trueman 1996; Rehn 2003; Hasegawa & Kasuya 2006).
Along with the phylogenetic position of Anisozygoptera there have been many studies that have attempted to resolve the relationships within the Odonata, but conflicting relationships still exist. One of the unresolved relationships includes each monophyly of Anisoptera and Zygoptera. The monophyly of Anisoptera has been proposed by wing venation (Fraser 1957; Fig. 1A), flight apparatus and copulatory structures (Pfau 1991; Fig. 1B), and also from wing venation with the cladistic parsimony method (Trueman 1996; Fig. 1C), whereas the non-monophyly of Zygoptera was proposed in these studies (Figs 1A–C). On the other hand, the monophyly of both suborders have been supported by other several studies (Carle 1982; Bechly 1996; Rehn 2003; Figs 1D–F).
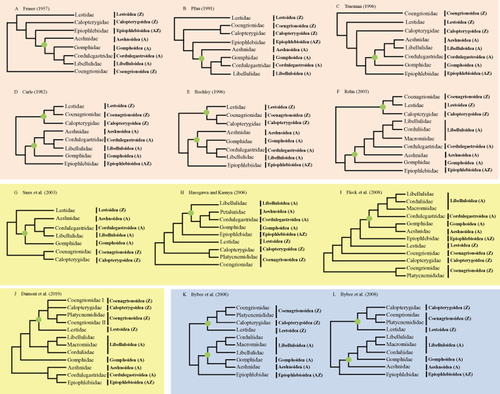
Representation of previous phylogenetic hypotheses of Odonata. (A)–(F) represent the hypotheses based on morphological characters, (G)–(J) represent the hypotheses based on molecular analysis, and (K) and (L) represents the hypotheses based on both molecular and morphological data. Within parentheses indicate subordinal names (A, Anisoptera; Z, Zygoptera; and AZ, Anisozygoptera). Dots on nodes indicate monophyly of suborders. We followed Dijkstra et al. (2013a) for classification of Odonata.
Monophylies of several superfamilies have also been ambiguous among morphological studies, providing conflict and unresolved relationships. For example, Fraser (1957) supported monophylies of superfamilies for a limited group such as the Libelluloidea for Anisoptera and the Coenagrionoidea and the Calopterygoidea for Zygoptera. Pfau (1991) supported monophylies for the Coenagrionoidea and Calopterygoidea, and Libelluloidea. Trueman (1996) supported monophyly only for Libelluloidea, Carle (1982) for Coenagrionoidea and Libelluloidea, Bechly (1996) for Calopterygoidea, Coenagrionoidea, and Libelluloidea, and Rehn (2003) only for Libelluloidea. Due to the lack of monophylies in most superfamilies, familial relationships are also mostly unresolved and inconsistent among studies. Nevertheless, the sister relationship of Corduliidae and Libellulidae within Libelluloidea was concordantly found in Trueman (1996), Bechly (1996), and Rehn (2003) (Figs 1C,E,F). Among superfamilial relationships in Anisoptera, either Aeshnoidea (Figs 1A,B,E) or Gomphoidea (Figs 1C,F) has been placed as the most basal lineage. Other superfamilial relationships are mostly obscured by non-monophyletic superfamilies in Anisoptera.
Molecular phylogenetic studies within Odonata provided somewhat more consistent relationships regarding higher taxonomic relationships among studies, but still relationships within several taxonomic levels are neither well resolved nor consistent (Figs 1G–L). Saux et al. (2003) sequenced a partial 12S ribosomal RNA (rRNA) from 26 species belonging to Anisoptera and Zygoptera (Fig. 1G). Also, Hasegawa and Kasuya (2006) sequenced both a partial 16S and 28S rRNAs from 32 species belonging to Anisoptera, Anisozygoptera, and Zygoptera (Fig. 1H). Both of these studies, that included limited taxonomic diversity, have shown paraphyly of Zygoptera, placing the damselfly family Lestidae, belonging to Lestoidea, as the sister to either the monophyletic Anisoptera (Fig. 1G) or the monophyletic Anisoptera + Anisozygoptera (Fig. 1H). Subsequent further extensive work was performed by Fleck et al. (2008) based on the 2026 aligned positions from 16S rRNA, tRNAVal, and 12S rRNA. With the inclusion of 121 species in 12 families and five superfamilies for Anisoptera, one species for Anisozygoptera, and 17 species in seven families and four superfamilies for Zygoptera, this study also supports paraphyly of Zygoptera, associating the damselfly family Lestidae to the monophyletic Anisoptera + Anisozygoptera (Fig. 1I). On the other hand, molecular data by Dumont et al. (2010) and combined data of morphological, molecular, and fossil record by Bybee et al. (2008) have shown monophyletic Zygoptera (Figs 1J–L).
Among the anisopteran families the Libellulidae is the largest group that is distributed world-wide containing ∼1000 species in 11–13 subfamilies, with one of the subfamilies, Sympetrinae, containing ∼200 species in 22 genera (Tillyard 1917; Fraser 1957; Bridges 1994; Steinmann 1997; Pilgrim & Von Dohlen 2008). The monophyly of Sympetrinae has been supported by morphological studies. For example, Fraser (1957) classified this subfamily based on four synapomorphic characters such as non-extended last antenodal crossvein (ACV) of the forewings beyond the subcosta, placement of the arculus between the first and second ACV, conspicuous radial and medial planates, and broad hindwing base with a highly visible anal loop. However, these characters are shared with other subfamilies, lacking synapomorphy for the subfamily Sympetrinae (Ware et al. 2007; Pilgrim & Von Dohlen 2008).
In order to expand our understanding for odonate phylogeny and enrich molecular markers, in this study we sequenced a total of ∼3.8 kb consisting of mitochondrial COI (1147 bp), mitochondrial 16S rRNA including tRNALeu (UUR) and tRNAVal (1368–1374 bp), nuclear 28S rRNA (830–842 bp), and nuclear elongation factor-1α (EF-1α) (541 bp). These sequences were utilized to infer evolutionary patterns of each gene, and to test the previous phylogenetic hypotheses of Odonata.
Materials and methods
Taxon sampling
The 71 odonate species included in this study (Table S1) is composed of 3 of the 3 suborders, 7 of the 8 superfamilies, and 10 of the 28 families in world-wide odonate diversity (Bridges 1994; Rehn 2003). At least one family was included in the analysis to represent the odonate superfamily, but Hemiphlebioidea, represented by a single species (Hemiphlebia mirabilis), was not included in this study. On the other hand, the Epiophlebia superstes (from Japan), which is one of the two species belonging to Anisozygoptera was included in this study. Voucher specimens were deposited in the National Institute of Biological Resources, Incheon, Korea.
DNA extraction, PCR, cloning, and sequencing
With the plan to increase the gene number and sequence length for the subsequent expanded study we used all fresh specimens that were newly collected, except for E. superstes. After collection in the field, the samples were frozen at –70°C until being used for molecular analysis. Total DNA was extracted from hind legs or partial thorax muscle using a Wizard Genomic DNA Purification Kit, in accordance with the manufacturer's instructions (Promega, Madison, WI, USA). To amplify 1147 bp of COI gene the primers, ODO-COI-F2 for the forward direction and both ODO-COI-R1 and ODO-COI-R2 for the reverse direction were designed from three full-length mitochondrial genomes of Odonata (Yamauchi et al. 2004; Zhang et al. 2008; Lee et al. 2009) and used selectively for better amplification. From the same full-length sequence information two sets of primers for 16S rRNA were designed from the 5’ end of ND1 and the 3’ end of tRNAVal to amplify ∼1370 bp encompassing the whole tRNALeu and 16S rRNA, and used selectively for better amplification. On the other hand, the primers for both ∼835 bp of 28S rRNA and 541 bp of elongation factor-1 alpha (EF-1α) were each adapted from pre-existing published ones (Hasegawa & Kasuya 2006; Pilgrim & Von Dohlen 2008). The primer information is listed in Table 1.
| Gene | Primer name | Sequences (from 5’ to 3’) | References |
|---|---|---|---|
| COI | ODO-COI-F2 | GGATCTCTAATTGGAGATGATCA | Designed in this study |
| ODO-COI-R1 | CGTCGTGGTATTCCTCTTAGT | Designed in this study | |
| ODO-COI-R2 | TCTGAATATCGTCGTGGTATTCC | Designed in this study | |
| ODO-COI-Inter-F4a | ATCAAATACCWYTATTTGTATGAGC | Designed in this study | |
| ODO-COI-Inter-R1a | CTTCDGGRTGTCCAAAGAATC | Designed in this study | |
| ODO-COI-Inter-R2a | GAAATTATWCCAAATCCTGG | Designed in this study | |
| 16S rRNA | ODO-16S-F1 | ATTGGGACCTTTACGAATTTGA | Designed in this study |
| ODO-16S-F2 | TTATTTGGCCCCTTACGAAT | Designed in this study | |
| ODO-16S-R1 | GCTCTAAAATATGCACACATCG | Designed in this study | |
| ODO-16S-R2 | TATGTCAGGTCAAGGTGCAA | Designed in this study | |
| ODO-16S-Inter-F2a | ATTATGCTACCTTTGCACGGTC | Designed in this study | |
| ODO-16S-Inter-R1a | GGCAAATATARTTCTCGCCTG | Designed in this study | |
| 28S rRNA | ODO-28S-F1 | AAGGTAGCCAAATGCCTCATC | Hasegawa and Kasuya (2006) |
| ODO-28S-R1 | AGTAGGGTAAAACTAACCT | Hasegawa and Kasuya (2006) | |
| EF1-α | Lib EF1Fa | GGAGAATTCGAAGCTGGTATCTC | Pilgrim and Von Dohlen (2008) |
| Lib EF1Ra | GACACGTTCTTCACGTTGAAACC | Pilgrim and Von Dohlen (2008) |
- a Internal primers used for cycle sequencing.
Mitochondrial COI gene was amplified with AccuPower PCR PreMix (Bioneer, Daejeon, Korea) under the following conditions: initial denaturation for 7 min at 94°C, followed by 35 cycles of 1 min at 94°C, 1 min at 48–53°C, and 1 min at 72°C, with a final 7-minute extension at 72°C. For other genes annealing temperatures were only variable (48–51°C for both 16S rRNA and 28S rRNA and 50–53°C for EF1-α). The PCR product was purified with a PCR purification Kit (Bioneer). The COI gene amplicons were directly sequenced, whereas those from other genes were cloned into a pGEM-T Easy vector (Promega). For the cloning process, XL1-Blue competent cells (Stratagene, Santa Clara, CA, USA) were transformed with the ligated DNA, and the resultant plasmid DNA was isolated using a Wizard Plus SV Minipreps DNA Purification System (Promega). DNA sequencing was conducted using the ABI PRISM BigDye Terminator ver. 3.1 Cycle Sequencing Kit with an ABI 377 Genetic Analyzer (PE Applied Biosystems, Foster City, CA, USA). For COI and 16S rRNA genes internal primers were used for cycle sequencing (Table 1). All products were sequenced from both strands.
Sequence analysis and alignment
Sequence delimitation and alignment were conducted using CLUSTAL W2 (Larkin et al. 2007). Each complete gene sequence was compared with available GenBank-registered sequences through Blast search (http://blast.ncbi.nlm.nih.gov/Blast.cgi) to verify the appropriacy of sequences. Additionally, COI sequences were searched for BOLD-IDS (Ratnasingham & Hebert 2007) to compare sequences to those of identical or similar species. In order to obtain codon-based alignment for each protein-coding gene (PCG), the nucleotide sequence of each PCG was subjected to RevTrans ver. 1.4 (Wernersson & Pedersen 2003). The well-aligned conserved blocks of each PCG were selected using GBlocks 0.91b (Castresana 2000), with the minimum length of a block set to 10 and allowed gap positions set to none for COI and EF1-α. On the other hand, the 16S and 28S rRNA genes were set for the parameters for rDNA alignments at minimum length of a block as 5, allowing gaps in half position, as recommended in Massana et al. (2004). Each gene and concatenated gene sequences were utilized to obtain sequence divergence at each taxonomic level using unrooted pairwise distance with PAUP* ver. 4.01b10 (Swofford 2002). Nucleotide composition was calculated by MEGA 4 (Tamura et al. 2011).
Phylogenetic analyses
Previous phylogenetic studies have shown uncertainty of the sister to Odonata, presenting Paleoptera hypothesis [Neoptera + (Ephemeroptera + Odonata)], Metapterygota hypothesis [Ephemeroptera + (Odonata + Neoptera)], and Chiastomyaria hypothesis [Odonata + (Ephemeroptera + Neoptera)] (Hennig 1981; Hovmöller et al. 2002; Kjer 2004; Kjer et al. 2006; Zhang et al. 2008). Selecting particular neopteran orders as outgroup is difficult to achieve practically with certainty, because they consist of about 30 orders that can be divided further into several groups (e.g., Hemipteroid assemblage, Orthopteroid assemblage, and Holometabola). Thus, we used two ephemeropteran species as outgroups (Ephemera orientalis and Cloeon dipterum) considering recent phylogenetic studies, which showed either a sister relationship between Ephemeroptera and Odonata (Ishiwata et al. 2011) or a rather basal position of Ephemeroptera compared to Odonata (Simon et al. 2012).
In order to select a substitution model via comparison of the Akalike Information Criterion (AIC) scores (Akaike 1974) Modeltest ver. 3.7 (Posada & Crandall 1998) was used. Individual models and data sets are, respectively, provided in Table 2. The maximum likelihood (ML) analyses were conducted using PHYML (Guindon et al. 2005), specifying the number of substitution rate categories as four and the starting tree as a BIONJ distance-based tree. The confidence values of the ML tree were evaluated via a bootstrap test with 1000 iterations. The Bayesian inference (BI) analyses were conducted using MrBayes ver. 3.1 (Huelsenbeck & Ronquist 2001). The model chosen by Modeltest was applied to each partition unlinked, when partition option was employed for BI analysis. When a best-fit model is absent either in BI, ML, or both methods, the next available model with the second highest AIC score was chosen for BI and ML analyses. Two independent runs of four incrementally heated Markov chain Monte Carlo (MCMC) chains (one cold chain and three hot chains) were simultaneously run for one-to-three million generations depending on the dataset, with sampling conducted every 100 generations. The convergence of MCMC, which was monitored by the average standard deviation of split frequencies, reached below 0.01 within one-to-three million generations depending on the dataset, and the initial 25% of the sampled trees were discarded as burn-in. The confidence values of the BI tree are presented as the Bayesian posterior probabilities (BPP) in percentages. Phylogenetic analyses were performed for each of the gene sequences and the concatenated sequences.
| Data sets | Partition | Selected model | Applied model |
|---|---|---|---|
| COI | – | GTR + I + G | GTR + I + G |
| 16S rRNA | – | HKY + I + G | HKY + I + G |
| 28S rRNA | – | GTR + I + G | GTR + I + G |
| EF1-α | – | SYM + I + G | GTR + I + G |
| COI + 16S rRNA | – | GTR + I + G | GTR + I + G |
| COI + 16S rRNA | 2 | Ind. | Ind. |
| 28S rRNA + EF1-α | – | GTR + I + G | GTR + I + G |
| 28S rRNA + EF1-α | 2 | Ind. | Ind. |
| All gene (COI + 16S rRNA + 28S rRNA + EF1-α) | – | GTR + I + G | GTR + I + G |
| All gene (COI + 16S rRNA + 28S rRNA + EF1-α) | 4 | Ind. | Ind. |
- –, not applied; Ind., individual model was applied for each gene.
Different tree topologies obtained through different dataset or tree construction methods was tested for statistical confidence using Treefinder (Jobb et al. 2004) applying respective models. The values of each topology were determined via six statistical tests each with 5000 replications: expected-likelihood weights (ELW) (Strimmer & Rambaut 2002), bootstrap probability (Felsenstein 1981), Kishino–Hasegawa (KH) (Kishino & Hasegawa 1989), Shimodaira–Hasegawa (SH) (Shimodaira & Hasegawa 1999), weighted SH (WSH) (Shimodaira & Hasegawa 1999), and approximately unbiased (AU) (Shimodaira 2002).
Results
Dataset characteristics
The sequence lengths of the 71 odonate species were 1147 bp in COI, 1545 bp–1598 bp in 16S rRNA, 822–842 bp in 28S rRNA, and 541 bp in EF-1α, respectively, revealing length variations due to insertion/deletion in the rRNAs, but not in the protein-coding COI and EF-1α (Table S2). The sequence length of the 16S rRNA and 28S rRNA increased to 2121 bp and 868 bp, respectively, when gaps were introduced to improved alignment. However, the conserved blocks selected with the inclusion of outgroups via GBlocks analysis (Castresana 2000) provided 1147 bp in COI (100% of original sequences), 1584 bp in 16S rRNA (74% of original sequences), 830 bp in 28S rRNA (96% of original sequences), and 541 bp in EF-1α (100% of original sequences), respectively (Table S2), providing a total of 4002 bp. These conserved blocks for each gene and concatenated gene were subsequently utilized for phylogenetic analysis.
Within-familial sequence divergence ranged from 12.44%–20.23% in COI, 4.84%–18.88% in 16S rRNA, 0.36%–4.60% in 28S rRNA, and 5.36%–9.93% in EF1-α, resulting in 5.92%–13.61% of divergence in concatenated sequences (Table 3). To the level of odonate families, thus, 28S rRNA showed the least divergence and EF1-α next, revealing the conserved nature of nuclear genes, whereas mitochondrial genes were more variable (Table 3). Considering the two types of data are unlinked and evolve under different evolutionary constraints, it would be a good practice to combine both mitochondrial and nuclear genes (Lin & Danforth 2004). In five odonate families, which were represented by multiple species the divergence of concatenated sequences ranked in the order of Coenagrionidae (13.61%), Gomphidae (13.37%), Libellulidae (12.11%), Lestidae (11.00%), Macromiidae (9.4%), and Aeshnidae (5.92%), although the number of taxonomic groups in each family differ from each other (Table 3).
| Taxon | No. of species | COI | 16S rRNA | 28S rRNA | EF-1α | Total genes |
|---|---|---|---|---|---|---|
| Anisoptera | ||||||
| Libelluloidea | 30 | 20.32 | 21.29 | 5.45 | 13.67 | 15.40 |
| Libellulidae | 26 | 20.23 | 16.26 | 3.03 | 9.93 | 12.11 |
| Corduliidae | 1 | |||||
| Macromiidae | 4 | 17.27 | 12.23 | 1.69 | 0.73 | 9.41 |
| Aeshnoidea | 7 | 12.44 | 4.84 | 0.36 | 5.51 | 5.92 |
| Aeshnidae | 7 | 12.44 | 4.84 | 0.36 | 5.51 | 5.92 |
| Gomphoidea | 11 | 18.97 | 17.56 | 4.60 | 6.65 | 13.37 |
| Gomphidae | 11 | 18.97 | 17.56 | 4.60 | 6.65 | 13.37 |
| Anisozygoptera | ||||||
| Epiophlebioidea | 1 | |||||
| Epiophlebiidae | 1 | |||||
| Zygoptera | ||||||
| Calopterygoidea | 2 | 17.18 | 13.85 | 0.50 | 2.95 | 10.31 |
| Calopterygidae | 2 | 17.18 | 13.85 | 0.50 | 2.95 | 10.31 |
| Coenagrionoidea | 15 | 19.78 | 22.04 | 4.30 | 11.64 | 15.77 |
| Coenagrionidae | 13 | 18.44 | 18.88 | 4.30 | 9.79 | 13.61 |
| Platycnemididae | 2 | 15.13 | 9.72 | 0.89 | 3.21 | 8.07 |
| Lestoidea | 4 | 18.89 | 14.76 | 1.34 | 5.36 | 11.00 |
| Lestidae | 4 | 18.89 | 14.76 | 1.34 | 5.36 | 11.00 |
The average A/T content of concatenated four genes was 62.2% (Table S2). In contrast, that of each mitochondrial gene was 65.7% in COI and 74.1% in 16S rRNA, respectively, showing higher A/T content than that of concatenated four genes. Between the two mitochondrial genes, the 16S rRNA gene evidenced obviously higher A/T content than was detected in the COI gene. Such a trend has also been reported in many mitochondrial genomes studies, including those of the Odonata (Hong et al. 2009; Lee et al. 2009; Kim et al. 2011). On the other hand, the nuclear gene demonstrates much lower A/T content compared to mitochondrial genes as 43.3 % in 28S rRNA and 50.2 % in EF1-α (Table S2). Relatively lower A/T content in the nuclear genes has also been reported in many previous studies including that of odonate phylogeny (e.g., Bybee et al. 2008).
The A/T content was almost similar among taxonomic groups. For example, the A/T content of the four concatenated genes between two suborders was 62.6% in Anisoptera, whereas it was 61.5% in Zygoptera (Table S2). Likewise, the range of the A/T content among all species was 62.6%–68.5% in COI, 71.2%–73.0% in 16S rRNA, 43.0%–43.8% in 28S rRNA, and 48.9%–51.7% in EF1-α, revealing a relatively small divergence range (Fig. 2).
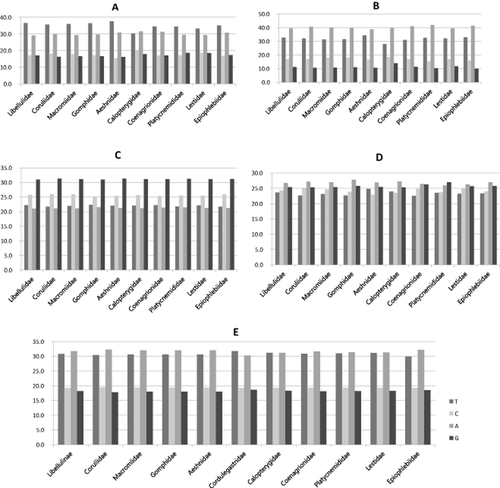
Comparison of nucleotide composition of each gene at family level. (A) COI, (B) 16S rRNA, (C) 28S rRNA, and (D) EF-1α, and (E) total genes. The values at y-axis indicate percentage of nucleotide composition.
Gene-based phylogenetic analyses
Phylogenetic analyses using individual genes (each COI, 16S rRNA, 28S rRNA, and EF-1α) and combination of each two genes based on origin (mitochondrial genes vs nuclear genes) and function (rRNA genes vs PCGs) were performed, but the resolving power of the single and each two-gene combination appeared to be insufficient, in that splitting among members of the same family, substantially low node support, and/or both were often observed. For example, mitochondrial DNA-based phylogeny (COI + 16S rRNA) placed Anisoptera non-monophyly in all analyses (Figs S1A–C) and Zygoptera non-monophyly in the BI method without partitioning and the ML method (Figs S1A,C). Nuclear DNA-based phylogeny (28S rRNA and EF-1α) was also unsatisfactory in that monophyletic Zygoptera was only obtained by the ML method and monophyletic Anisoptera was never recovered (Figs S1D–F). Further, non-monophyletic family was only observed when two concatenated nuclear genes were used. For example, among seven genera in Coenagrionidae that is divided into two subfamilies (Coenagrioninae and Psuedagrioninae), the genus Ceriagrion that belongs to the subfamily Psuedagrioninae was placed as the sister to the group composed of Platycnemididae and Calopterygidae, leaving other genera in the subfamily Coenagrioninae monophyletic in the nuclear DNA-based phylogeny, although this phenomenon is much lessened in the mitochondrial DNA-based phylogeny (Figs S1D–F). Previously, the family Coenagrionidae has been questioned for its monophyly, but the genera included in current study have never been questioned for their non-monophyletic status within the family (Bybee et al. 2008; Carle et al. 2008; Dumont et al. 2010). This may have occurred because nuclear genes have better resolution for deep phylogeny, whereas mitochondrial genes have some merit for recent phylogenetic divergence. When four genes were concatenated, such a non-monophyletic family was not observed (Fig. 3). Therefore, we limited our data presentation and discussion on the phylogenetic reconstruction to the trees generated on the basis of the four concatenated gene sequences.

The phylogenetic analyses using the concatenated mitochondrial COI, 16S rRNA, nuclear 28S rRNA, and EF-1α genes. (A) Bayesian Inference phylogram obtained with the dataset COI + 16S rRNA + 28S rRNA + EF-1α, which is not partitioned. The numbers at each node specify Bayesian posterior probabilities (BPP). (B) Bayesian Inference phylogram obtained with the dataset COI + 16S rRNA + 28S rRNA + EF-1α, which is divided into four partitions. The numbers at each node specify BPP. (C) Maximum Likelihood phylogram obtained with the dataset COI + 16S rRNA + 28S rRNA + EF-1α. The numbers at each node specify bootstrap percentage of 1000 pseudoreplicates. Two species of Ephemeroptera were used as outgroups. The subfamily names Lib, Bra, Sym, Tri, and Tri in Libellulidae were abbreviated for Libellulinae, Sympetrinae, Brachydiolacinae, Trithemistinae, and Trameinae, respectively. The scale bar indicates the number of substitutions per site.
Concatenated phylogenetic analyses and topological test
The analyses based on concatenation of the four genes, with different analytical methods (BI and ML methods) and BI-based partitioning strategy (four partitions based on genes) recovered monophylies of all families and revealed consistent familial and superfamilial relationships of Zygoptera as ((Coenagrionoidea + Calopterygoidea) + Lestoidea) in all analyses (Fig. 3). However, the relationships among Anisozygoptera and anisopteran superfamilies were fluctuating, presenting three topologies (termed tree A, B, and C; Figs 3A–C). Tree A was supported by the BI method without partitioning (Fig. 3A), tree B by the BI method with four partitions (Fig. 3B), and tree C by the ML method (Fig. 3C). Tree A clearly placed Anisoptera and Anisozygoptera as sisters to each other and supported the monophyletic Anisoptera by 0.82 BPP.
On the other hand, the anisopteran superfamilies Libelluloidea, Gomphoidea, and Aeshnoidea were unresolved, presenting the relationships (Anisozygoptera + (Aeshnidae + Gomphidae + (Macromiidae + (Libellulidae + Corduliidae)))) (Fig. 3A). Tree B obtained with the partition option was the least resolved in that the Anisozygoptera, Aeshnoidea, Gomphoidea, and Libelluloidea all were unresolved, presenting the relationships (Anisozygoptera + Aeshnidae + Gomphidae + (Macromiidae + (Libellulidae + Corduliidae))), although a close relationship of Anisozygoptera to Anisoptera was obvious (Fig. 3B). Tree C obtained by the ML method was most well resolved, supporting the sister relationship between Anisozygoptera and Anisoptera, the monophyletic Anisoptera, and the monophyletic Zygoptera, presenting (Anisozygoptera + (Gomphidae + (Aeshnidae + (Macromiidae + (Libellulidae + Corduliidae)))) (Fig. 3C). However, many nodes were very poorly supported. For example, the nodal support for the sister relationships between Libelluloidea and Aeshnoidea was only 31% and the sister group Anisoptera and Anisozygoptera was supported only by 72.4% (Fig. 3C).
In order to further clarify the inconsistency presented in the three trees, topological tests (Jobb et al. 2004) were performed, applying the GTR + I + G model. The six statistical tests performed (ELW, BP, KH, SH, WSH, and AU) confirmed that tree C (Fig. 3C) evidenced confidence values completely deviant from the range of 95%, providing 0% in six analyses (Table 4). Tree B (Fig. 3B) evidenced confidence values well within the range of the 0.95% only in the SH and WSH tests, and marginally in the KH, ELW, and BP tests, suggesting that topology B has the possibility to be accepted, but overall statistical confidence is comparatively very low (Table 4). On the other hand, tree A (Fig. 3A) was supported with the confidence values well within the range of 95% in all analyses, providing values of 0.95%–1.0%, suggesting that tree A, which was obtained by the BI method without partitioning, is comparatively well accepted in current data analysis.
| Topology | |||
|---|---|---|---|
| A | B | C | |
| ELW | 0.9539647 | 0.04763685 | 0 |
| BP | 0.95758 | 0.04448 | 0 |
| KH | 1 | 0.05748 | 0 |
| SH | 1 | 0.3555 | 0 |
| WSH | 1 | 0.10066 | 0 |
| AU | 0.9579511 | 0.04040324 | 0 |
- Topologies A, B and C represent Figure 3A–C.
Twenty-six species of Libellulidae included in this study belong to five subfamilies: Libellulinae, Sympetrinae, Trameinae, Brachydiplactinae, and Trithemistinae (Steinmann 1997). Three topologies obtained in this study consistently divided the Libellulidae into two groups: one that includes all Sympetrum and another that includes remaining subfamilies and two species of Sympetrinae (Crocothemis servilia and Deielia phaon), presenting the Sympetrinae non-monophyly (Figs 3A–C). In the latter group, each C. servilia and D. phaon grouped together with each Brachydiplactinae and Trithemistinae, both of which were represented by a single species.
Discussion
Subordinal relationships
The Anisozygoptera, which is represented by a relict family Epiophlebiidae and Anisoptera together are often named as Epiprocta on the basis of morphological characters (Lohmann 1996). Our analysis on the bases of four concatenated genes also supported Epiprocta (Fig. 3). This relationship has also been supported by several previous morphological studies (Fraser 1957; Carle 1982; Pfau 1991; Bechly 1996; Trueman 1996; Rehn 2003) and molecular studies (Hasegawa & Kasuya 2006; Fleck et al. 2008). Among them Rehn (2003) supported Epiprocta on the basis of cladistic parsimony method using the skeletal morphology and wing venation of adults, complemented with a few larval characters from 85 genera belonging to 45 families and subfamilies. Recent comprehensive molecular and morphological data by Bybee et al. (2008) has also shown the validity of Epiprocta under all analyses.
With regard to the monophyly of each odonate suborder, the monophyletic Anisoptera was supported with 0.82 BPP and the monophyletic Zygoptera was supported with 0.99 BPP (Fig. 3A). This result suggests each suborder monophyletic groups. Nevertheless, several previous studies suggested non-monophyletic Zygoptera (Fig. 1). In particular, Fleck et al. (2008) performed phylogenetic analysis based on 2026 bp of mitochondrial genes (16S rRNA, tRNAVal, and 12S rRNA) with an extensive taxon sampling also resulted in paraphyly of Zygoptera, placing the damselfly Lestidae as the basal lineage of the Epiprocta. On the other hand, Dumont et al. (2010) has shown two different topologies, depending on the sequences employed. Based on ∼1800 bp of 18S rRNA sequence they observed monophyletic Zygoptera, whereas ∼2400 bp from nuclear ribosomal 5.8S, 18S, ITS1, and ITS2, which definitely include rapidly evolving segments of sequences, has shown the zygopteran Lestidae the basal lineage of Anisoptera, supporting non-monophyletic Zygoptera. However, further extensive studies performed by Bybee et al. (2008) and Carle et al. (2008) have shown the Zygoptera monophyly and Bybee et al. (2008) suggested that a paraphyletic Zygoptera is an artifact caused by limited taxon sampling within the suborder, the use of a single molecular marker, and failing to perform concatenated molecular analyses. Furthermore, Dijkstra and Kalkman (2012) in the course of review of European Odonata supported monophyly of Zygoptera, placing Lestidae the sister to the remaining zygopteran families. In addition, Davis et al. (2011) using fossil data also supported each Anisoptera and Zygoptera monophyletic group. Our concatenated analysis always recovered the monophyletic Zygoptera (Fig. 3) and placed zygopteran Lestidae as the sister to the remaining damselflies, but paraphyletic Zygoptera was often observed in some analyses when either two mitochondrial or nuclear genes along were employed, emphasizing the importance of employing multiple genes (Fig. S1).
Familial and superfamilial relationships
Libelluloidea represented by three anisopteran families, Macromiidae, Libellulidae, and Corduliidae has consistently shown the relationships (Macromiidae + (Libellulidae + Corduliidae)) (Fig. 3). This relationship was also supported by morphological study by Rehn (2003), molecular studies by Fleck et al. (2008) and Dumont et al. (2010), and both molecular and morphological study by Bybee et al. (2008), indicating a robust phylogenetic relationship among families. With regard to the Zygoptera clear familial relationships are limitedly available in previously studies partially due to non-monophyly of Zygoptera (Fig. 1). However, the most extensive study by Bybee et al. (2008) consistently provided the relationships (((Coenagrionidae + Platycnemididae) + Calopterygidae) + Lestidae) both by MP and BI analyses. A recent molecular study (COI, 16S rRNA, and 28S rRNA) for comprehensive familial relationships of Zygoptera also evidenced similar relationships (Dijkstra et al. 2013b). This relationship is also supported by our concatenated analysis, providing relatively high nodal support (Figs 3A–B). Nevertheless, a recent morphological study using an anatomical dataset of the head morphology and endoskeletal features for Anisoptera has shown unresolved relationships among Libellulidae, Macromiidae, and Corduliidae as a clustered one clade (Blanke et al. 2013).
Superfamilial relationships of Anisoptera were not clearly resolved in our study (Fig. 3). The anisopteran superfamilies (Libelluloidea, Aeshnoidea, and Gomphoidea) were unresolved, presenting the relationships (Aeshnidae + Gomphidae + (Macromiidae + (Libellulidae + Corduliidae))) (Figs 3A–B). Previous phylogenetic studies using morphological data have also shown incongruent results. For example, Rehn (2003), Trueman (1996), and Carle (1982) have shown Gomphidae to be the most basal lineage of Anisoptera, whereas Fraser (1957) and Pfau (1991) placed Aeshinidae as the most basal lineage. Molecular data have also shown conflict relationships. For example, Saux et al. (2003) have shown Gomphidae to be the most basal lineage of Anisoptera in some analyses, whereas Hasegawa and Kasuya (2006) placed the Libellulidae as the most basal lineage, Dumont et al. (2010) placed Aeshnidae as the most basal lineage, and Fleck et al. (2008) placed the group Aeshnidae + Gomphidae as the most basal lineage of Anisoptera, showing conflict placement of the basal lineage of Anisoptera. The most extensive study, which incorporated molecular, morphological, and fossil data performed by Bybee et al. (2008) has shown the basal lineage of Anisoptera as the group Aeshnidae + Gomphidae by the MP method and Aeshnidae by the BI method. Taken these together, the exact relationships of Anisozygoptera to anisopteran families remained unclear, requiring further scrutinized study for this particular relationship.
Non-monophyly of Sympetrinae in Libellulidae
Due to the limited taxon sampling of below-familial level relationships (i.e., subfamily), the monophyly of each subfamily cannot evidently be discussed. However, two statistically acceptable topologies consistently divided the Libellulidae into two groups. The possibility of non-monophyly of Sympetrinae has also been suggested by molecular data (Ware et al. 2007) and both molecular and wing morphological data (Pilgrim & Von Dohlen 2008). For example, Ware et al. (2007) using 28S rRNA and 16S rRNA have shown the sister relationship between Crocothemis and the group composed of Brachydiplactinae + Palpopleurinae. This topology seems also to be held in our analysis in that the Crocothemis belonging to Sympetrinae and the Nannophya, belonging to Brachydiplactinae, was placed as the sister to each (Figs 3A–B). In the case of Deielia, our topologies supported the sister relationship between the Deielia and a member of Trithemistinae, but Ware et al. (2007) have shown the sister relationship between Deielia and a member of Brachydiplactinae, even though multiple species of Trithemistinae were included in the analysis. Pilgrim & Von Dohlen (2008) also tested the monophyly of the subfamily Sympetrinae using three molecular markers and 38 wing venation characters. They found non-monophyletic Sympetrinae, subdividing the subfamily into six lineages. They also found that other subfamilies such as Brachydiplactinae, Leucorrhininae, Trameinae, and Trithemistinae were not monophyletic, but found the Libellulinae monophyletic as seen in our analysis (Figs 3A–B). Consequently, they concluded that many of the wing venation characters that were employed to define the Sympetrinae were homoplasious characters, requiring synapomorphic characters for further progressed Sympetrinae phylogeny. The current study does not cover the diversity of species-rich Sympetrinae and also other subfamilies in Libellulidae. Thus, an expanded study that includes the diversity of Libellulidae is required for decisive and robust conclusion on the Sympetrinae non-monophyly.
Acknowledgments
This work was supported by research grants from the National Institute of Biological Resources- “Origin of Biological Diversity of Korea: Molecular Phylogenetic Analyses of Major Korean Taxa” awarded to Iksoo Kim. We, the authors, appreciate Professor Jong Wook Lee at Yeungnam University for providing Epiophlebia superstes.




