A lesson from Bass Strait on connectivity conservation
Abstract
Isolation effects on the distributions of plant species in fragmented forests appear to be weak over tens to hundreds of years and strong over geological eras. The 250 km wide, 6.5 millennia old Bass Strait, and other millennium-scale disjunctions in the range of Eucalyptus regnans forests, were used to determine the effects of intermediate periods of isolation on plant species occurrence and composition. Three of six floristic communities were found on both sides of Bass Strait. The residuals from multiple regression models using climatic variables on the latitude vector were not explained by latitude, indicating negligible isolation effects from Bass Strait. However, there was a lesser compositional effect of disjunctions within land masses than between land masses, suggesting some effect of the larger barrier. No species that commonly occurred with E. regnans and were largely confined to wet forest exhibited absences from any region where the climate and soils were within their range. If areas isolated from each other for millennia can maintain their vascular plant biota, the expenditure of conservation funds on creating corridors to connect large areas separated by anthropogenic landscape modification might require more justification than it is currently afforded.
Key insights
As isolation of 250 km for many millennia has not substantially affected vascular plant species composition, creating corridors to connect large areas separated by anthropogenic landscape modification might require more justification than it is currently afforded.
1 INTRODUCTION
The maintenance or improvement of both the size and connectivity of natural landscapes is suggested to be essential for successful nature conservation, especially in the context of accelerating climate change (Hilty et al., 2019; International Union for the Conservation of Nature, 2020; Mackey et al., 2008, 2013; McQuillan et al., 2009; Soule et al., 2004), although it has been noted that connectivity can be a twin-edged sword (Lindenmayer et al., 2010). I have expressed some doubts on the scientific foundation of conservation programs that promote improvements in connectivity (Kirkpatrick, 2011). These doubts were based on studies of tracheophytes, bryophytes, and avifauna in fragmented landscapes in Tasmania, Australia (Gilfedder & Kirkpatrick, 1998; Kirkpatrick & Gilfedder, 1995; MacDonald & Kirkpatrick, 2003; Pharo et al., 2005; Woolley & Kirkpatrick, 1999), all of which exhibited no, or very weak, landscape effects related to geometric variables, such as distance to the nearest remnant, distance to the nearest larger remnant and remnant size, compared with effects related to variation in fire and grazing management. More recent work on the distributions of species in several taxonomic groups in relation to variation in patterns of landscape-scale disturbance by logging concludes that many species are more likely to recolonise logged areas where unlogged areas form a larger part of the matrix but that species in unlogged forest were resilient to the scale of landscape disturbance (Wardlaw et al., 2018), a conclusion consistent with a strong management effect and a weak effect of distance.
Nevertheless, the existence of biotic realms provides evidence that a lack of connectivity can result in highly divergent biotas as a result of vicariant evolution over hundreds of thousands to millions of years, although, at a geological time scale, plants may be less influenced by connectivity changes than vertebrate animals (Cody et al., 2010; Sanmartin & Ronquist, 2004).
Landscapes isolated over geological time periods are very different in the likelihood of low probability long distance dispersal events from landscapes that have been fragmented or isolated for decades or centuries. If there are few apparent effects of loss of connectivity on plant species composition at the decade to century time scale and many at the geological time scale, at what time scale do strong effects occur? Nature conservation planning at the millennium scale is likely to be important, as in the case of protecting glacial refugia (Kirkpatrick & Fowler, 1998). If strong connectivity change effects can be identified at this time scale, it may be worth expending resources on restoring or maintaining connections. Conversely, if they are weak or undetectable, biodiversity might be better maintained by concentrating resources on satisfying the needs of threatened species and ecosystems.
The sea level and climatic changes that occurred during the Quaternary provide an opportunity to identify isolation effects at the millennium timescale. As glaciers waxed and waned, land masses were joined, then parted by the formation of straits. Nakamura et al. (2009) investigate the island floras on either side of two such barriers in the Ryukyu Archipelago of Japan and conclude that one of the two barriers affected plant species composition independent of distance and environmental effects. The present paper uses quadrat floristic data from Eucalyptus regnans F. Muell. forest over its full range on both sides of Bass Strait, Australia, to undertake a more refined test of the hypothesis that isolation, over millennia, results in compositional changes independent of environment.
Tasmania was separated from Australia approximately 12,000 years ago, with present day sea level being approximately attained 6,500 years ago (Blom, 1988). Bass Strait, a shallow east to west body of water between the mainland of Tasmania and the mainland of Australia, is currently a 250 km wide barrier to dispersal of terrestrial organisms, although stepping stone islands occur at its eastern and western ends. During the colder periods of the Quaternary, a land bridge connected Australia to Tasmania, isolating marine life to the east and west (Waters, 2008) and providing an occasional migration route for eucalypt species and clades (McKinnon et al., 2004; Nevill et al., 2010). There is transgression of some clades of E. regnans across Bass Strait (Nevill et al., 2010). In Eucalyptus, most seeds fall within twice tree height, with a possibility of carriage for kilometres encased in capsules carried on small branches in the fierce winds of bushfires (McArthur, 1967). There are many gaps greater than 30 km in the range of E. regnans. The stands in the Otway Ranges of Victoria are currently among the most isolated within the two landmasses (Figure 1). Disjunctions also pertain to the many understorey species associated with wet eucalypt forest.
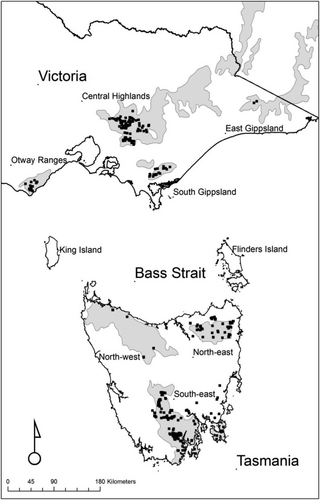
If the north–south disjunction caused by Bass Strait affects the distribution of the plant species of E. regnans forest, it could be expected that species composition would be predicted by latitude after the effects of climatic predictors were removed. If the east–west disjunctions between the Otways and the rest of Victoria, and the northeast and the rest of Tasmania, had an effect on the distribution of species in E. regnans forest, species that occur both to the north and to the south in either or both of the northeast of Tasmania and the Otways could be expected to be absent. Overall, the geographic distances between disjunct regions of occurrence of the forest type would be expected to predict the species composition distances between them.
This article uses quadrat data over the full range of E. regnans (Figure 1) to determine the degree to which floristic variation across Bass Strait, and between other disjunct occurrences of the taxon, is attributable to environmental conditions. The null hypothesis was that floristic variation, and the distributions of species confined to wet eucalypt forest, was affected by isolation, rather than being a response to environmental variation. Floristic variation is described, the level of explanation of floristic variation from environmental data is tested, and whether the residual variation is related to the presence of barriers to migration is determined.
2 METHODS
The database developed by Kirkpatrick et al. (1988) was curated to ensure constancy of taxonomic treatment. The floristic data were typically from 10 × 10 m quadrats and constituted as complete a list of vascular plant species as was possible to gain at the time of sampling. Terrestrial orchids and other wet forest geophytes were unreliably observed, as they are not usually evident throughout the year. They were therefore excluded from analyses.
The grid location (converted from Australian Geodetic Datum [AGD] 66 to decimal degrees), elevation (m), aspect (degrees), and slope (degrees) were recorded in association with the original floristic field survey data using the best available topographic map sheets at that time and field measurements. These data were used as inputs into the program BIOCLIM (Nix & Busby, 1986), a subprogram of ANUCLIM (Hutchinson et al., 2000; Hutchinson & Kesteven, 1998), and used to generate interpolated estimates for six climatic parameters, selected to represent a larger set of strongly inter-correlated variables: mean daily minimum temperature of coldest period (°C), mean daily maximum temperature of the warmest period (°C), mean annual rainfall (mm), mean rainfall of the driest month (mm), highest period radiation (Mj/m2/day), lowest period radiation (Mj/m2/day).
DECODA (Minchin, 1990) was used for distance calculations, ordination, and vector fitting. Minitab16 was used for all other analyses. The floristic data were ordinated using global non-metric multidimensional scaling with default options. Linear vectors were fitted for all environmental variables in four dimensional ordination space. The significance of R for each variable was determined using 1,000 randomisations. Scores were derived on the latitude vector. Multiple regression was used to develop the best multivariate equation to predict the latitude vector scores. The Best Subsets procedure was used to develop a candidate set. Adjusted R2 was used to select the best model for which the slopes of all variables were significant. The residuals from this model were then regressed against latitude.
The scores on each of the four axes of the ordination were used as the input to an agglomerative classification based on Ward’s method and Euclidean distance. The pattern of increase in error with agglomeration was used to choose the six group level. One-way analysis of variance (ANOVA) with Tukey’s test was used to determine differences between groups on spatial and environmental variables.
The percentage frequencies of all species in the Otways, the rest of Victoria, the northwest of Tasmania, the northeast of Tasmania, and the south of Tasmania were calculated. Species that were common outside wet forest ecosystems were excluded from the table, as the disjunction distances used in the analyses did not apply to them. These disjunction distances pertained to wet eucalypt forest (Figure 1) and were measured in kilometres. Climatic distances were calculated from the scores on the first two axes of a principal component ordination based on a correlation matrix of the climate variables described above. Axis 1, which was heavily loaded on maximum temperature and the two radiation variables, accounted for 51.7% of the variance. Axis 2, which was heavily loaded on the precipitation variables, accounted for 28.8% of the variance. These scores were used to calculate the Euclidean climatic distance between each pair of the regions. The mean scores for regions on Axes 1 and 2 on the species composition ordination were used to calculate the Euclidean compositional distance between regions. A variable (InternalExternal) was created differentiating within Tasmania or Victoria geographic distances from between Tasmania and Victoria distances. Linear regression was used to describe and test the relationships between compositional distance and each of geographic distance and climatic distance. The residuals from within State distances and those from between State differences were compared using one way ANOVA, to test for an effect of Bass Strait on species composition. One-way ANOVA with Tukey’s test was used to determine differences between regions on climatic variables.
The regional table was searched for possible instances of species absence because of isolation. Range-wide distributions for all such species were examined using the full wet eucalypt forest data base (Kirkpatrick et al., 1988), reliable species lists, and herbarium records to determine whether an apparent gap was the product of a shift out of vegetation dominated by E. regnans or inadequate sampling, rather than a real absence.
3 RESULTS
The six communities were floristically and environmentally distinct from each other (Tables 1 and 2). Communities 1–3 were recorded from both sides of Bass Strait. Communities 1 and 3 had 1% and 3%, respectively, of their quadrats in Victoria, whereas Community 2 had 96%. The remaining communities were confined to Victoria. Latitude formed the strongest vector in ordination space, followed by June radiation, December radiation, mean daily maximum temperature, and mean daily minimum temperature (Table 2). The scores on the latitude vector were highly predicted by a combination of environmental variables (R2 adjusted = 81.3%), most strongly by those associated with summer temperatures (Table 3). The residuals were unrelated to latitude.
| 1 | 2 | 3 | 4 | 5 | 6 | Tas | Vic | |
|---|---|---|---|---|---|---|---|---|
| Pomaderris apetala Labill. w | 92 | 0 | 18 | 0 | 0 | 0 | 66 | 0 |
| Pimelea drupacea w | 49 | 4 | 32 | 0 | 0 | 3 | 44 | 1 |
| Zieria arborescens w | 44 | 22 | 14 | 16 | 42 | 19 | 33 | 24 |
| Gahnia grandis (Labill.) S.T.Blake m | 39 | 4 | 14 | 0 | 0 | 0 | 30 | 0 |
| aBedfordia salicina (Labill.) DC. w | 26 | 0 | 6 | 0 | 0 | 0 | 19 | 0 |
| aMonotoca glauca (Labill.) Druce w | 21 | 0 | 17 | 0 | 0 | 0 | 20 | 0 |
| Blechnum wattsii Tindale f | 46 | 96 | 45 | 45 | 55 | 61 | 46 | 60 |
| Notogrammitis billardierei | ||||||||
| (Willd.) Parris f | 11 | 81 | 68 | 44 | 0 | 29 | 31 | 39 |
| Nothofagus cunninghamii w | 25 | 78 | 68 | 10 | 3 | 68 | 40 | 33 |
| Hymenophyllum australe Willd. f | 4 | 52 | 17 | 11 | 0 | 0 | 9 | 14 |
| Pittosporum bicolor Hook. w | 34 | 48 | 23 | 34 | 23 | 32 | 30 | 34 |
| Parsonsia brownii (Britten) Pichon w | 7 | 48 | 0 | 19 | 16 | 6 | 4 | 21 |
| Asplenium bulbiferum G.Forst. f | 4 | 48 | 11 | 29 | 0 | 13 | 5 | 24 |
| Carex appressa m | 3 | 48 | 3 | 21 | 6 | 23 | 3 | 23 |
| Blechnum nudum f | 41 | 44 | 11 | 23 | 16 | 29 | 31 | 25 |
| Rumohra adiantiformis | ||||||||
| (G.Forst.) Ching f | 25 | 44 | 32 | 19 | 0 | 3 | 29 | 16 |
| Blechnum fluviatile | ||||||||
| (R.Br.) E.J.Lowe ex Salomon | 1 | 41 | 3 | 5 | 0 | 23 | 2 | 14 |
| Blechnum chambersii Tindale f | 3 | 37 | 3 | 15 | 3 | 10 | 3 | 15 |
| Todea Barbara (L.) T. Moore f | 1 | 37 | 0 | 6 | 13 | 3 | 1 | 12 |
| Hymenophyllum cupressiforme Labill. f | 8 | 33 | 27 | 15 | 0 | 0 | 15 | 12 |
| Hymenophyllum flabellatum Labill. f | 7 | 33 | 33 | 16 | 0 | 3 | 17 | 13 |
| Lastreopsis acuminata | ||||||||
| (Houlston) C.V.Morton f | 1 | 30 | 0 | 23 | 0 | 0 | 1 | 14 |
| Isolepis inundata R.Br. m | 0 | 22 | 0 | 8 | 3 | 13 | 0 | 10 |
| Dicksonia antarctica Labill. f | 85 | 96 | 97 | 84 | 55 | 94 | 89 | 82 |
| Atherosperma moschatum w | 38 | 78 | 94 | 11 | 6 | 32 | 58 | 27 |
| Histiopteris incisa (Thunb.) J.Sm. f | 47 | 52 | 76 | 40 | 35 | 74 | 58 | 48 |
| Hypolepis rugosula (Labill.) J.Sm. f | 25 | 26 | 55 | 8 | 3 | 16 | 37 | 11 |
| aPhyllocladus aspleniifolius | ||||||||
| (Labill.) A.Rich. ex Hook.f. w | 4 | 0 | 29 | 0 | 0 | 0 | 13 | 0 |
| Hymenophyllum rarum R.Br. f | 6 | 11 | 26 | 3 | 0 | 0 | 14 | 3 |
| Hymenophyllum peltatum | ||||||||
| (Poir.) Desv. f | 2 | 0 | 24 | 0 | 0 | 3 | 10 | 1 |
| Nematolepis squamea w | 17 | 4 | 20 | 2 | 3 | 3 | 18 | 3 |
| Coprosma quadrifida | ||||||||
| (Labill.) B.L.Rob w | 73 | 41 | 24 | 92 | 45 | 19 | 56 | 58 |
| Olearia argophylla | ||||||||
| (Labill.) F.Muell. ex Benth. w | 75 | 15 | 53 | 90 | 45 | 45 | 66 | 59 |
| Polystichum proliferum | ||||||||
| (R.Br.) C. Presl f | 80 | 41 | 77 | 90 | 55 | 90 | 79 | 75 |
| Cyathea australis (R.Br.) Domin f | 13 | 48 | 2 | 85 | 84 | 19 | 9 | 64 |
| Clematis aristata R.Br. ex Ker Gawl. w | 33 | 56 | 18 | 84 | 81 | 81 | 28 | 77 |
| Hedycarya angustifolia A. Cunn. w | 0 | 56 | 0 | 82 | 48 | 35 | 0 | 60 |
| Pomaderris aspera Sieber ex DC. w | 0 | 41 | 0 | 73 | 68 | 26 | 0 | 56 |
| Acacia melanoxylon R.Br. w | 58 | 41 | 12 | 69 | 29 | 16 | 42 | 44 |
| Sambucus gaudichaudiana DC. w | 0 | 19 | 2 | 60 | 19 | 45 | 0 | 41 |
| Bedfordia arborescens Hochr. w | 0 | 0 | 0 | 56 | 39 | 23 | 0 | 35 |
| Microsorum pustulatum | ||||||||
| (G.Forst.) Copel. f | 22 | 52 | 38 | 55 | 0 | 16 | 28 | 35 |
| Lepidosperma laterale R.Br. m | 24 | 15 | 2 | 34 | 42 | 23 | 16 | 29 |
| Geranium potentilloides | ||||||||
| L’Hér. ex DC. h | 2 | 4 | 0 | 31 | 29 | 19 | 2 | 22 |
| Rubus fruticosus L, w | 4 | 11 | 0 | 24 | 3 | 6 | 3 | 13 |
| Tetrarrhena juncea R.Br. m | 0 | 56 | 0 | 74 | 90 | 55 | 0 | 69 |
| Pteridium esculentum | ||||||||
| (G.Forst.) Cockayne f | 55 | 7 | 9 | 40 | 84 | 39 | 39 | 42 |
| Viola hederacea Labill. h | 14 | 48 | 2 | 42 | 77 | 55 | 10 | 52 |
| Pimelea axiflora F. Muell. ex Meisn. w | 0 | 19 | 2 | 31 | 68 | 10 | 0 | 32 |
| Prostanthera lasianthos Labill. w | 14 | 26 | 3 | 27 | 65 | 23 | 10 | 34 |
| Eucalyptus obliqua L’Hér. w | 39 | 11 | 14 | 13 | 55 | 3 | 30 | 19 |
| bAcacia obliquinervia Tindale w | 0 | 7 | 0 | 0 | 52 | 6 | 0 | 13 |
| bEucalyptus cypellocarpa | ||||||||
| L.A.S. Johnson w | 0 | 0 | 0 | 19 | 52 | 19 | 0 | 22 |
| Olearia lirata (Sims) Hutch. w | 36 | 7 | 0 | 40 | 48 | 13 | 23 | 30 |
| Correa lawrenceana w | 2 | 11 | 0 | 27 | 45 | 23 | 1 | 27 |
| Goodenia ovata Sm. w | 7 | 0 | 0 | 8 | 42 | 3 | 4 | 12 |
| Gonocarpus teucrioides DC. h | 3 | 11 | 0 | 6 | 42 | 6 | 2 | 14 |
| bLomatia fraseri R.Br. w | 0 | 22 | 0 | 19 | 39 | 39 | 0 | 27 |
| Notelaea ligustrina Vent. w | 11 | 7 | 3 | 16 | 35 | 23 | 7 | 20 |
| Billardiera longiflora Labill. w | 11 | 0 | 5 | 10 | 32 | 13 | 8 | 13 |
| Acacia verticillata (L’Hér.) Willd. w | 19 | 7 | 0 | 3 | 29 | 0 | 12 | 9 |
| Gahnia sieberiana Kunth m | 1 | 7 | 0 | 6 | 29 | 3 | 0 | 11 |
| Galium leiocarpum I. Thoms. h | 0 | 0 | 0 | 3 | 26 | 3 | 0 | 7 |
| Acacia dealbata Link w | 72 | 44 | 30 | 42 | 71 | 84 | 57 | 57 |
| Olearia phlogopappa (Labill.) DC. w | 4 | 19 | 0 | 15 | 52 | 81 | 2 | 36 |
| Australina pusilla (Poir) Gaudich. h | 0 | 52 | 0 | 66 | 6 | 81 | 0 | 54 |
| Polyscias sambucifolia w | 0 | 11 | 0 | 6 | 48 | 74 | 0 | 29 |
| Hydrocotyle hirta R.Br. ex A.Rich. h | 3 | 30 | 2 | 37 | 35 | 71 | 3 | 41 |
| Stellaria flaccida h | 2 | 19 | 0 | 40 | 10 | 58 | 1 | 33 |
| Cassinia aculeata (Labill.) R.Br. w | 27 | 7 | 0 | 18 | 48 | 55 | 17 | 29 |
| Dianella tasmanica m | 16 | 4 | 8 | 6 | 23 | 45 | 12 | 18 |
| Dryopoa dives (F.Muell.) Vickery f | 0 | 7 | 0 | 5 | 6 | 45 | 0 | 14 |
| Tasmannia lanceolata | ||||||||
| (Poir.) A.C.Sm. w | 12 | 41 | 12 | 5 | 16 | 42 | 12 | 22 |
| Senecio linearifolius A.Rich. h | 4 | 4 | 0 | 11 | 19 | 42 | 3 | 18 |
| Poa poiformis (Labill.) Druce m | 0 | 4 | 0 | 6 | 26 | 42 | 0 | 17 |
| Leptinella filicula (Hook.f.) Hook.f. h | 0 | 4 | 0 | 2 | 10 | 42 | 0 | 12 |
| Urtica incisa Poir. h | 10 | 11 | 9 | 15 | 0 | 39 | 10 | 16 |
| Acaena novae-zelandiae Kirk h | 29 | 11 | 2 | 24 | 19 | 39 | 20 | 23 |
| Oxalis perennans Haw. h | 2 | 0 | 0 | 21 | 16 | 32 | 1 | 18 |
| bAcacia frigescens J.H.Willis w | 0 | 7 | 0 | 2 | 10 | 26 | 0 | 9 |
| Mentha laxiflora Benth. h | 0 | 0 | 0 | 6 | 10 | 26 | 0 | 10 |
| Coprosma hirtella Labill. w | 2 | 4 | 0 | 2 | 10 | 23 | 1 | 8 |
- Note: F = fern, h = herb, m = monocot, w = woody plant; species are ordered by abundance in the community in which they are most abundant. Species that did not occur in at least 20% of the quadrats in a community are not listed.
- a Tasmanian endemic.
- b Mainland Australian endemic.
| Community | R | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Min temp (°C) | 2.1b | 2.3b | 0.5d | 3.8a | 2.0b | 1.2c | 0.7244 |
| Max temp (°C) | 20.2c | 22.7a | 19.6d | 22.4ab | 22.9a | 22.0b | 0.7426 |
| Annual rainfall (mm) | 1170d | 1443b | 1443b | 1294c | 1382bc | 1613a | 0.5846 |
| Driest month (mm) | 62b | 72a | 70a | 67a | 69a | 71a | 0.4963 |
| Radiation maxmth | 22.1b | 23.1a | 20.9c | 23.3a | 23.2a | 23.0a | 0.7657 |
| Radiation minmth | 4.5b | 5.4a | 4.2c | 5.3a | 5.4a | 5.3a | 0.8586 |
| Latitude | 42.1a | 37.9bc | 42.3a | 38.3b | 37.8c | 37.7c | 0.9301 |
| Longitude | 147.2a | 145.9c | 146.6b | 145.3d | 146.0c | 145.8c | 0.6124 |
| Altitude | 301d | 529b | 495b | 373c | 516b | 789a | 0.7441 |
- Note: Communities with the same letters for variables are statistically identical at p > 0.05 using Tukey’s test on ANOVA results (all p < 0.001).
| Response/predictor | Coef | SE coef | T | p |
|---|---|---|---|---|
| Latitude vector score | ||||
| Constant | 1.9558 | 0.6533 | 2.99 | 0.003 |
| MinTemp | 0.1204 | 0.0197 | 6.12 | <0.001 |
| MaxTemp | −0.0948 | 0.0188 | −5.03 | <0.001 |
| AnnRainfall | −0.0003 | 0.0001 | −2.17 | 0.031 |
| MinRainMnth | 0.0067 | 0.0033 | 2.03 | 0.043 |
| MaxRadmth | 0.1082 | 0.0356 | 3.04 | 0.003 |
| MinRadMth | 0.3852 | 0.0927 | 4.16 | <0.001 |
| Altitude | −0.0009 | 0.0002 | −4.96 | <0.001 |
- Note: R2 (adj.) = 81.3%.
Northwest and northeast Tasmania had very similar climates (Table 4). The other regions were more climatically different from each other (Table 4). There was a stronger correlation between climatic distance and floristic distance (r = 0.843, d.f. = 8, p = 0.002) than between geographic distance and floristic distance (r = 0.743, d.f. = 8, p = 0.014). The residuals from the regression of climatic distance against compositional distance were strongly explained by Internal/External (F = 13.441, 8, p = 0.006, r2 [adj] = 58.02%), indicating that geographic distances internal to the two states had a lesser effect on compositional distance than distances between them.
| Region | |||||
|---|---|---|---|---|---|
| EVict | Otways | NWT | NET | SET | |
| Min temp (°C) | 2.3b | 4.5a | 3,0b | 2.4b | 1.0c |
| Max temp (°C) | 22.7a | 20.6b | 20.1bc | 20.7b | 19.6c |
| Annual rainfall (mm) | 1415a | 1442abc | 1277abc | 1244bc | 1267c |
| Driest month (mm) | 70a | 69abc | 58bc | 62c | 67ab |
| Radiation maxmth | 23.2a | 22.9ab | 22.6ab | 23.3a | 23.2a |
| Radiation minmth | 5.4a | 5.0b | 4.4d | 4.7c | 4.2d |
- Note: Communities with the same letters for variables are statistically identical at p > 0.05 using Tukey’s test on ANOVA results (all <0.001).
- Abbreviations: Evict, eastern Victoria; NET, northeastern Tasmania; NWT, northwest Tasmania; SET, southeastern Tasmania.
There were several instances of a species missing in the data from E. regnans forest in a disjunct region with a suitable climatic and edaphic environment. Atherosperma moschatum Labill., Blechnum nudum (Labill.) Mett. ex Luerss., Dianella tasmanica Hook.f., Nothofagus cunninghamii (Hook.) Oerst., Parsonsia brownii (Britten) Pichon, Nematolepis squamea (Labill.) Paul G. Wilson, Pimelea drupacea Labill., Polyscias sambucifolia (Sieber ex DC.) Harms, and Zieria arborescens Sims were absent from the E. regnans quadrat data set for the Otways (Table 5), while being otherwise recorded for the area (Lunt, 1992; Parsons et al., 1977). Carex appressa R. Br., Correa lawrenceana Hook., and Stellaria flaccida Hook. were absent from the northeastern Tasmania quadrat data set, but all occur within the wet forests in the region. The northwest Tasmanian data set lacked 11 species that were found to the north and south (Table 5), but all were recorded from the region.
| Species | e Victoria | Otways | ne Tas | nw Tas | se Tas | p |
|---|---|---|---|---|---|---|
| n | 139 | 14 | 55 | 10 | 113 | |
| Acacia melanoxylon | 39.57 | 92.86 | 69.09 | 60.00 | 26.55 | <0.001 |
| Billardiera longiflora | 12.95 | 14.29 | 3.64 | 20.00 | 9.73 | 0.302 |
| Blechnum wattsii | 60.43 | 57.14 | 34.55 | 80.00 | 47.79 | 0.005 |
| Clematis aristata | 76.98 | 78.57 | 27.27 | 50.00 | 25.66 | <0.001 |
| Coprosma quadrifida | 53.24 | 100.00 | 72.73 | 80.00 | 45.13 | <0.001 |
| Cyathea australis | 64.03 | 64.29 | 18.18 | 30.00 | 2.65 | <0.001 |
| Dicksonia antarctica | 81.29 | 92.86 | 90.91 | 100.00 | 87.61 | 0.191 |
| Geranium potentilloides | 23.02 | 14.29 | 1.82 | 10.00 | 0.88 | <0.001 |
| Notogrammitis billardierei | 34.53 | 78.57 | 9.09 | 50.00 | 40.71 | <0.001 |
| Histiopteris incisa | 49.64 | 35.71 | 45.45 | 60.00 | 63.72 | 0.062 |
| Hymenophyllum flabellatum | 12.23 | 21.43 | 10.91 | 10.00 | 20.35 | 0.317 |
| Hypolepis rugosula | 10.07 | 21.43 | 29.09 | 30.00 | 40.71 | <0.001 |
| Microsorum pustulatum | 30.94 | 78.57 | 36.36 | 30.00 | 23.01 | 0.001 |
| Olearia argophylla | 56.12 | 92.86 | 72.73 | 50.00 | 64.60 | 0.024 |
| Olearia lirata | 31.65 | 14.29 | 63.64 | 20.00 | 3.54 | <0.001 |
| Pittosporum bicolor | 33.09 | 42.86 | 32.73 | 40.00 | 27.43 | 0.695 |
| Polystichum proliferum | 71.94 | 100.00 | 90.91 | 50.00 | 75.22 | 0.003 |
| Rumohra adiantiformis | 14.39 | 28.57 | 30.91 | 30.00 | 27.43 | 0.041 |
| Polyscias sambucifolia | 32.40 | 0.00 | 0.00 | 0.00 | 0.00 | <0.001 |
| Australina pusilla | 56.10 | 28.60 | 0.00 | 0.00 | 0.00 | <0.001 |
| Bedfordia arborescens | 34.50 | 42.90 | 0.00 | 0.00 | 0.00 | <0.001 |
| Hedycarya angustifolia | 59.00 | 71.40 | 0.00 | 0.00 | 0.00 | <0.001 |
| Lomatia fraseri | 28.80 | 14.30 | 0.00 | 0.00 | 0.00 | <0.001 |
| Pimelea axiflora | 28.80 | 64.30 | 0.00 | 0.00 | 0.00 | <0.001 |
| Pomaderris aspera | 54.00 | 71.40 | 0.00 | 0.00 | 0.00 | <0.001 |
| Sambucus gaudichaudiana | 41.70 | 35.70 | 0.00 | 0.00 | 0.00 | <0.001 |
| Tetrarrhena juncea | 67.60 | 85.70 | 0.00 | 0.00 | 0.00 | <0.001 |
| Atherosperma moschatum | 29.50 | 0.00 | 34.55 | 20.00 | 73.45 | <0.001 |
| Blechnum nudum | 28.06 | 0.00 | 47.27 | 60.00 | 20.35 | <0.001 |
| Dianella tasmanica | 19.42 | 0.00 | 3.64 | 40.00 | 14.16 | 0.004 |
| Nothofagus cunninghamii | 35.97 | 0.00 | 23.64 | 40.00 | 48.67 | 0.001 |
| Nematolepis squamea | 2.88 | 0.00 | 1.82 | 10.00 | 26.55 | <0.001 |
| Pimelea drupacea | 0.72 | 0.00 | 52.73 | 70.00 | 37.17 | <0.001 |
| Zieria arborescens | 25.90 | 0.00 | 47.27 | 30.00 | 25.66 | 0.003 |
| Parsonsia brownii | 23.02 | 0.00 | 12.73 | 0.00 | 0.88 | <0.001 |
| Asplenium bulbiferum | 23.74 | 28.57 | 1.82 | 0.00 | 7.08 | <0.001 |
| Hymenophyllum australe | 13.67 | 14.29 | 5.45 | 0.00 | 11.50 | 0.394 |
| Hymenophyllum cupressiforme | 8.63 | 50.00 | 10.91 | 0.00 | 17.70 | <0.001 |
| Notelaea ligustrina | 17.27 | 50.00 | 14.55 | 0.00 | 4.42 | <0.001 |
| Olearia phlogopappa | 38.85 | 7.14 | 5.45 | 0.00 | 0.88 | <0.001 |
| Prostanthera lasianthos | 35.25 | 21.43 | 9.09 | 0.00 | 10.62 | <0.001 |
| Tasmannia lanceolata | 23.02 | 7.14 | 20.00 | 0.00 | 8.85 | 0.014 |
| Urtica incisa | 15.83 | 14.29 | 9.09 | 0.00 | 10.62 | 0.426 |
| Correa lawrenceana | 26.62 | 28.57 | 0.00 | 0.00 | 1.77 | <0.001 |
| Stellaria flaccida | 33.09 | 35.71 | 0.00 | 0.00 | 1.77 | <0.001 |
| Carex appressa | 23.74 | 14.29 | 0.00 | 10.00 | 3.54 | <0.001 |
| Bedfordia salicina | 0.00 | 0.00 | 12.73 | 20.00 | 22.12 | <0.001 |
| Gahnia grandis | 0.00 | 0.00 | 10.91 | 60.00 | 37.17 | <0.001 |
| Monotoca glauca | 0.00 | 0.00 | 12.73 | 50.00 | 20.35 | <0.001 |
| Pomaderris apetala | 0.00 | 0.00 | 87.27 | 80.00 | 53.98 | <0.001 |
- Note: Species are ordered by their patterns of absence. Absences are shown in bold. p = probability of differences between regions deviating from expected evenness (Chi2). Only species that occurred in at least 10% of the quadrats in at least one region were included in this analysis.
4 DISCUSSION
The fact that variation in the latitude vector can be logically explained by climatic variables, with latitude having no predictive power with the residuals from the multiple regression analysis, suggests that the null hypothesis should be rejected, thereby accepting that there is a negligible millennium-scale isolation effect of Bass Strait on compositional variation in E. regnans forest. However, there was a definite lesser effect on species composition differences of disjunctions within states than between them, indicating that water barriers might be more effective than land barriers. On land, there may be possibilities for stepping stones for dispersal of wet forest species between locally moist situations, options less available in Bass Strait with its very scattered, small, dry islands.
A partial blindness of variation in the species composition of E. regnans forest to the existence of Bass Strait and other substantial disjunctions seems to be mirrored in genetic variation in the species itself, with several clades having toeholds on either side of the strait (Nevill et al., 2010), as with the communities described in this article. The species that co-occur with E. regnans also occur widely under other dominants of wet forests (Kirkpatrick et al., 1988), so a disjunction in E. regnans does not necessarily represent a disjunction in their ranges. However, none are marine, and few can survive in the dry basalt plains that enclose the Otway Ranges.
The rainforest tree, A. moschatum, has been thought to have been absent from the Otway Ranges (Howard & Ashton, 1973; Parsons et al., 1977), and its absence attributed to exposure to frequent fires (Howard & Ashton, 1973). However, the less fire-adapted, and less vagile, N. cunninghamii has survived in the Otways, and A. moschatum was collected in the past from the region (Lunt, 1992). It appears that land clearance is responsible for its contemporary absence (Lunt, 1992).
The present results indicate that the current emphasis on maintaining spatial continuity in Australian nature conservation may be largely misplaced for vascular plants. Nevertheless, there are some convincing cases in southeastern Australia in which isolation at the millennium scale seems to have affected plant species distributions. These include the absence of Huon pine (Lagarostrobus franklinii [Hook.f.] Quinn) from the Arthur River catchment (Gibson et al., 1991) and the pre-European invasion absence of Pittosporum undulatum Vent. from Tasmania (Gleadow & Ashton, 1981). Northeastern Tasmania has absences of Tasmanian endemic rainforest species, such as Eucryphia lucida Labill. and Anodopetalum biglandulosum A. Cunn. ex Endl. that are not readily explicable by current climate and soils. However, in these cases, the barrier to migration from western Tasmania to northeastern Tasmania seems likely to have persisted through the Pleistocene, as the barrier at the height of the last glacial period was wider than that at present (Kirkpatrick & Fowler, 1998).
Given the exceptions above, landscape effects should not be totally ignored in conservation planning, but attempts should be made to determine the conservation productivity of corridor development in contingent circumstances and to find alternatives to expensive attempts to restore connectivity. To some extent, translocation and introduction can be used as a test of the validity of the isolation hypothesis. For example, the success of P. undulatum in colonising eastern Tasmanian forests could suggest that Bass Strait has formed a strong barrier to its spread, although introduced birds may have increased seed dispersal and changes in land use may also have facilitated its spread. The flip side of this argument is that connectivity is unnecessary if translocation is cheaper and as effective.
Landscape effects are not just those related to isolation. The reduction in area of local habitat that results from fragmentation may cause the gradual loss of species, a phenomenon poetically labelled by biogeographers as relaxation, although island-like habitats do not necessarily result in this effect, even for highly sessile organisms (Driscoll et al., 2010). Relaxation may be reduced by maintenance of connectivity with other habitat islands, but the main cause of relaxation is likely to be inadequate resource availability within the normal spatial range of activity of an organism, as with the 30% remnant threshold for survival of woodland birds in the eastern Australian wheat belt (Ford, 2011).
The data from E. regnans forests strongly suggest that, where the individual areas that have been isolated are large, extensive barriers present over many thousands of years have a negligible effect on species distributions. Thus, it is only where a lack of connectivity is combined with an isolated small habitat area for species known to be in trouble that one might wisely contemplate a response, which may not be the creation of corridors.
ACKNOWLEDGEMENTS
Dr Jayne Balmer kindly provided Figure 1 and critically appraised the manuscript. Dr Graeme Newell kindly extracted the BIOCLIM data for Victoria. Miranda Kellett helped with the production of the tables. There were no specific funds dedicated to this project. Open access publishing was facilitated by University of Tasmania, as part of the Wiley - University of Tasmania agreement via the Council of Australian University Librarians. Open access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
ETHICS STATEMENT
No approvals were required or morally appropriate.
Open Research
DATA AVAILABILITY STATEMENT
Data are available by request to the author. They are also lodged in Tasmanian and Victorian government databases.




