Peritoneal Lavage With Low GDP Neutral pH PD Solution Reduces Mortality and Severity in a Lethal Peritonitis Model
Funding: This work was supported by Terumo Corporation.
ABSTRACT
Introduction
The efficacy of peritoneal lavage monotherapy using PD solution for severe peritonitis remains unproven.
Methods
We evaluated the effects of 3-times and 6-times lavage using low GDP neutral PD solution on a Methicillin-sensitive Staphylococcus aureus (MSSA) lethal peritonitis model rats without antibiotic use. Changes in effluent viable bacteria numbers dependent on lavage frequency were determined in a further 10 rats.
Results
Compared to the lethal peritonitis model without lavage, both 3-times and 6-times lavage significantly reduced 24-h mortality, effluent FDP and creatinine concentrations. Furthermore, 6-times lavage significantly prevented the rough fur development, intraabdominal adhesion, and pus formation which developed in both the 0-times and 3-times lavage groups. The effluent viable bacterial counts showed significant step-ladder decreases with an increasing number of the lavages.
Conclusion
We demonstrated for the first time that lavage monotherapy using PD solution reduces mortality and severity in a lethal peritonitis model by rapidly eliminating bacteria without antibiotic use.
Abbreviations
-
- CFU
-
- colony forming unit
-
- EPS
-
- encapsulating peritoneal sclerosis
-
- FDP
-
- fibrin degradation products
-
- ISPD
-
- international society for peritoneal dialysis
-
- LD100
-
- 100% lethal dose
-
- MSSA
-
- methicillin-susceptible Staphylococcus aureus
-
- PD
-
- peritoneal dialysis
-
- PD peritonitis
-
- peritoneal dialysis-associated peritonitis
-
- PLF
-
- peritoneal lavage fluid
-
- S. aureus
-
- Staphylococcus aureus
1 Introduction
Peritonitis is a state of critical urgency that requires immediate treatment in both surgical and medical fields in patients undergoing peritoneal dialysis (PD). Mortality rates are still high, 17%–20% in surgical patients [1] and 9% in PD patients [2]. In PD patients, the main cause of death is peritonitis with sepsis [3, 4], indicating antibiotic monotherapy cannot always cure these patients, though the International Society for Peritoneal Dialysis (ISPD) guidelines recommend antibiotic monotherapy [5].
Indeed, mild peritonitis can generally be cured with antibiotics alone. However, severe PD peritonitis has a high mortality rate [2-4], even when appropriate antibiotics are used. Additional treatment other than antibiotics is expected to be developed for severe PD peritonitis. In Japan, many Japanese nephrologists implement a combination treatment of lavage and antibiotics for severe PD peritonitis with severe turbid effluent, but the ISPD guideline does not recommend lavage treatment, creating a discrepancy between the guideline and actual clinical practice in Japan.
To prevent death from a fatal infection, early elimination of the causative organism is essential for saving the patient's life [4]. As a combination treatment with antibiotics, lavage treatment via a PD catheter has long been expected to directly remove both bacteria and inflammatory mediators from the intra-abdominal cavity to the outside of the body.
However, the effectiveness of intraperitoneal lavage has been denied in both the surgical [6] and PD fields [5, 7, 8]. In the field of PD, the ISPD guideline recommends that augmented peritoneal lavage via a PD catheter should not be performed for the purpose of improving peritonitis cure [5]. However, we identified several issues with the references [7, 8] cited in this ISPD guideline.
Firstly, they administered antibiotics first and delayed lavage for two or three days [7, 8], this delay may have made it difficult to detect the effects of the lavage treatment.
Secondly, they uniformly excluded patients with fatal peritonitis [7, 8], which may be the reason for the failure to demonstrate that lavage has a mortality-lowering effect. In fact, Wong et al. [7] concluded that adjunctive lavage did not improve the overall outcome, although it may be beneficial for the more severe peritonitis patients who have high CRP.
Third, Ejlersen E. et al. [8] did not assign a non-lavage group, so it is likely that the effectiveness of the lavage could not be detected. They assigned participants to either the 2-times or 15-times lavage group only and could not detect a significant difference in treatment effect between the two lavage groups. In addition, the species of the causative organisms differed between the two groups.
Based on these reports, we concluded that the efficacy of lavage without antibiotics use in the treatment of severe peritonitis must first be verified using an experimental model of lethal peritonitis (most severe peritonitis), because it is difficult to conduct clinical studies only on patients with lethal peritonitis. If antibiotics were administered in combination with the lavage, the interpretation of the results would be unclear whether peritoneal lavage or antibiotics were more effective. Thus, we designed a study without antibiotics use to detect the efficacy of lavage alone.
To date, no clinical studies on lethal peritonitis have been reported, but one study has been conducted using an animal model. This animal study showed that intraperitoneal saline lavage was effective in reducing mortality and intraperitoneal adhesion in a one-shot E. coli lethal peritonitis model [9]. However, the lavage with saline is not usually used for PD patients because of its potential to cause congestive heart failure in PD patients. The only lavage solution that can be used for intraperitoneal lavage in actual clinical practice is PD dialysate. However, no studies have examined the effect of PD dialysate on lethal peritonitis model to date.
Surprisingly, there are no reports that have validated the efficacy of intraperitoneal lavage in models of lethal peritonitis caused by MSSA (the most common and lethal causative organism of PD peritonitis), which is often treated with antibiotics alone, without catheter removal [4]. Therefore, MSSA is likely to be a good treatment target organism for lavage treatment.
Furthermore, it should also be noted that the references [7, 8] used in the ISPD guideline only included users of old conventional acidic bioincompatible PD solutions that are not currently used in Japan. In 2001, low GDP neutral PD solution, Midpeliq (Terumo Corporation, Tokyo, Japan), was developed and started to be used in Japan [10]. This biocompatible PD solution has been reported to reduce the risk of encapsulating peritoneal sclerosis (EPS), a life-threatening complication of PD, more than conventional acidic solutions [11], so this PD solution may be the best lavage solution for severe peritonitis at present. However, the effectiveness of peritoneal lavage with this new PD solution on lethal peritonitis in both human and animal models has not been reported and needs to be verified.
Among the 4 representative lethal peritonitis models [12-15], we selected the one-shot lethal model [15] which is relatively similar to severe PD peritonitis. We developed a lethal peritonitis model with high dose intraperitoneal inoculation of MSSA and investigated the effectiveness of lavage monotherapy using Midpeliq 135 without antibiotic use in the MSSA lethal peritonitis model.
Based on this information, we decided to conduct a comparative study of a lavage versus a non-lavage group using MSSA lethal peritonitis model. The study was designed to detect the efficacy of lavage treatment alone without antibiotics use.
2 Methods
The viable bacterial count experiment was performed at Terumo Corporation and the other experiments were performed at HAMRI CO. LTD.
2.1 Animals
Specific pathogen-free 6-week-old male Crl: CD (SD) rats (The Jackson Laboratory Japan) were used in the experiments. The care and handling of the animals were in accordance with the National Institutes of Health guidelines and the Animal Care and Use Guidelines of either HAMRI CO. LTD. or Terumo Corporation. All experimental protocols were approved by the animal ethics committee of each institution. Animals were maintained under constant temperature (24°C ± 3°C) and humidity (50% ± 20%) with a 12-h light/dark cycle.
2.2 Bacterial Preparation
Methicillin Susceptible Staphylococcus Aureus (MSSA) JCM 2413 was grown overnight at 37°C and stocked in glycerol. Then 100 μL of the stock solution was added to 20 mL of Mueller-Hinton liquid medium (Becton, Dickinson and Company) and pre-cultured at 37.0°C for 1 day. Then 1 mL of the pre-cultured bacterial solution was added to 100 mL of Mueller-Hinton liquid medium, and the mixture was cultured at 37°C for 12 h. At the end of the culture, the bacterial solution was centrifuged at 1700 × g for 15 min at 4°C and resuspended in Midpeliq 135 and adjusted to the target concentration. CFU per milliliter values were determined by colony counting of serial dilutions on Mueller-Hinton agar plates incubated overnight at 37°C.
2.3 Development of the MSSA Lethal Peritonitis Model
A mouse model of Escherichia coli lethal peritonitis by intraperitoneal inoculation of 5 × 108 CFU has been previously reported [16]. Therefore, we preliminarily investigated a 100% lethal dose (LD100) using different concentrations of S. aureus by the Probit method [17]. 3 groups of rats (n = 3 each) were injected intraperitoneally with 15 ml of a sterile PD solution containing 1 × 108, 1 × 109, and 1 × 1010 CFU S. aureus. All rats receiving either 1 × 108 or 1 × 109 CFU showed rough hair, but none died within 24 h of inoculation. On the other hand, all rats inoculated with 1 × 1010 CFU S. aureus died within 24 h of inoculation. The LD100 at 24 h post inoculation was estimated to be 1 × 1010 CFU [18].
2.4 Experiment Protocol
24 SD rats were randomly assigned to the following 4 groups (n = 6 each) and their general state and survival were monitored for 24 h.
Group 1: only 1 × 1010 CFU MSSA/15 mL Midpeliq 135 inoculation without any lavage.
Group 2: 3-times lavage with 25 mL of Midpeliq 135 after MSSA inoculation at the same dose.
Group 3: 6-times lavage with 25 mL of Midpeliq 135 after identical dose MSSA inoculation.
Group 4: only 6-times lavage with 25 mL of Midpeliq 135 without MSSA inoculation.
All procedures were performed after the skin sterilization under 2%–3% isoflurane (isoflurane inhalation anesthesia liquid, Mylan Inc.) inhalation anesthesia.
In groups 1, 2, and 3, rats were intraperitoneally inoculated with 15 mL of 1 × 1010 CFU MSSA. In group 1, no further lavage was performed after MSSA inoculation. For groups 2 and 3, the inoculated MSSA solution was immediately drained, and the peritoneal cavity was lavaged 3- or 6-times with a 25 mL syringe of Midpeliq 135 prewarmed to 37° using the custody needle (Surflo custody needle 21G). Group 4 rats were lavaged 6-times with 25 mL of Midpeliq 135 alone without MSSA inoculation. Following these procedures, the animals were monitored for signs of mortality and severity at 0, 3, 6, and 24 h, and their general state was recorded as follows:
N: no abnormality, A: rough fur, B: swelling of orbit, C: increased respiration rate, D: listless. And each degree of abnormality was recorded as follows: 1: Mild, 2: Moderate, 3: Severe.
After a 24-h observation period, a further 15 mL Midpeliq 135 was infused intraperitoneally into all rats, including the dead rats, to collect an effluent sample. Two hours later, effluent samples were collected from the peritoneal cavity of all rats and used to measure effluent creatinine and effluent FDP concentrations.
2.5 Assessment of Intra-Abdominal Conditions
Finally, all animals were examined for intra-abdominal conditions by necropsy immediately after euthanasia; the organs were photographed, and the images were archived. Peritoneal adhesion and pus formation were scored as present or absent.
2.6 Measurement of Effluent Creatinine and FDP Concentration
Effluent creatinine concentrations were measured using an animal dry-chemistry multi-analyzer, Fuji-DRI-CHEM 7000VZ. Effluent FDP concentrations were determined using an enzyme-linked immunosorbent assay kit (R&D Systems Inc. or LifeSpan Biosciences Inc.). Where data were below the limit of quantification, the lower limit was used for further statistical analysis.
2.7 Viable Bacterial Counts Study
A further viable bacterial counts study was performed. We inoculated 10 rats with 15 mL of 1 × 1010 CFU MSSA containing Midpeliq 135 immediately after euthanasia. After draining the inoculated MSSA solution, the peritoneal cavity was lavaged 6 times with 25 mL of Midpeliq 135, and each peritoneal lavage fluid (PLF) was collected by immediate cold processing of the PLF. One mL of each PLF was serially diluted with 9 mL of cold sterile saline and repeatedly diluted to a final concentration of 107. 103 ~ 107 diluted solution was plated on Mannitol Salt Agar Plates [Becton, Dickinson and Company, BBL (TM) Mannitol Salt Agar 211 407]. Two days after inoculation, bacterial colonies were counted and CFU of bacteria per milliliter of fluid were calculated [16].
2.8 Statistics
Data were analyzed using JMP software (JMP Pro 16 for Windows, SAS Institute, Cary, North Carolina). Analysis of variance (ANOVA) was performed between different scoring categories, and post hoc Dunnet's test or Tukey HSD test was performed as appropriate. Graphs were generated using Graph Pad Prism 9 software (GraphPad, San Diego, USA) and data were expressed as mean ± standard deviation or as numbers (percentages).
3 Results
3.1 General State of Animals
Table 1 shows the time courses of the changes in general state at 0, 3, 6, and 24 h after the intervention. Group 1 (inoculation only without lavage) showed rough fur at 6 h after inoculation and a higher mortality rate at 24 h after inoculation (5/6: 83.3%), and the resultant survived 1 rat (animal number 13) showed a morbid state (positive for rough fur, swelling of orbit, increased respiration rate, listless) at 24 h after MSSA inoculation. Group 2 (3-times lavage) showed transient rough fur at 6 h after inoculation (6/6: 100%), but their rough fur disappeared at 24 h after inoculation, and all rats survived at 24 h after MSSA inoculation (mortality rates 0/6: 0%). On the other hand, both group 3 (6-times lavage after MSSA inoculation) and group 4 (no inoculation) showed no abnormality in the general state, including rough fur, and all survived within the 24-h observational period (mortality rates 0/6: 0%).
| Group | MSSA inoculation | Times of lavage | Animal number | 0 h | 3 h | 6 h | 24 h |
|---|---|---|---|---|---|---|---|
| 1 | YES | 0 | 11 | N | N | A1 | Death |
| 12 | N | N | A1 | Death | |||
| 13 | N | N | A1 | A1, B1, C1, D2 | |||
| 14 | N | N | A1 | Death | |||
| 15 | N | N | A1 | Death | |||
| 16 | N | N | A1 | Death | |||
| 2 | YES | 3 | 21 | N | N | A1 | N |
| 22 | N | N | A1 | N | |||
| 23 | N | N | A1 | N | |||
| 24 | N | N | A1 | N | |||
| 25 | N | N | A1 | N | |||
| 26 | N | N | A1 | N | |||
| 3 | YES | 6 | 31 | N | N | N | N |
| 32 | N | N | N | N | |||
| 33 | N | N | N | N | |||
| 34 | N | N | N | N | |||
| 35 | N | N | N | N | |||
| 36 | N | N | N | N | |||
| 4 | NO | 6 | 41 | N | N | N | N |
| 42 | N | N | N | N | |||
| 43 | N | N | N | N | |||
| 44 | N | N | N | N | |||
| 45 | N | N | N | N | |||
| 46 | N | N | N | N |
- Note: Animals were observed for signs of mortality and severity at 0, 3, 6, 24 h after each procedure and their general state was recorded as follows: N: no abnormality, A: rough fur, B: orbital swelling, C: increased respiratory rate, D: listless. And each degree of abnormality was recorded as follows: 1: mild, 2: moderate, 3: severe.
Table 2 shows the statistical analyses of general state. Compared with group 1, groups 2, 3, and 4 (no inoculation) showed significant reductions of 24-h mortality (6/6: 83.3% vs. 0/6: 0%*, 0/6: 0%*, 0/6: 0%*, *p < 0.01), 24-h rough fur development (6/6: 100%, 0/6: 0%*, 0/6: 0%*, 0/6: 0%*. *p < 0.01). Compared to both groups 1 and 2, groups 3 and 4 showed no rough fur development after 6 h (6/6: 100%, 6/6: 100% vs. 0/6: 0%*, 0/6: 0%*, *p < 0.01).
| Group 1 | Group 2 | Group 3 | Group 4 | p value | |
|---|---|---|---|---|---|
| MSSA inoculation | YES | YES | YES | NO | |
| Times of lavage | 0 | 3 | 6 | 6 | |
| Rough fur | |||||
| 3-h n, (%) | 0/6 (0) | 0/6 (0) | 0/6 (0) | 0/6 (0) | NS |
| 6-h n, (%) | 6/6 (100) | 6/6 (100) | 0/6 (0) * | 0/6 (0) * | * < 0.01 |
| 24-h n, (%) | 6/6 (100) | 0/6 (0) * | 0/6 (0) * | 0/6 (0) * | * < 0.01 |
| Mortality | |||||
| 6-h n, (%) | 0/6 (0) | 0/6 (0) | 0/6 (0) | 0/6 (0) | NS |
| 24-h n, (%) | 5/6 (83.3) | 0/6 (0) * | 0/6 (0) * | 0/6 (0) * | * < 0.01 |
- Note: Compared with group 1, groups 2, 3 and 4 (negative control) showed significant reductions of 24-h mortality (6/6: 83.3% vs. 0/6: 0%*, 0/6: 0%*, 0/6: 0%*, *p < 0.01), 24-h rough fur development (6/6: 100%, 0/6: 0%*, 0/6: 0%*, 0/6: 0%*. *p < 0.01). Compared to both groups 1 and 2, groups 3 and 4 showed no rough fur development after 6 h (6/6: 100%, 6/6: 100% vs. 0/6: 0%*, 0/6: 0%*, *p < 0.01). And compared with group 1, groups 2, 3 and 4 showed no rough fur development after 24-h (6/6: 100% vs. 0/6: 0%*, 0/6: 0%*, 0/6: 0%*, *p < 0.01).
3.2 Necropsy Findings
All animals underwent necropsy to examine their intra-abdominal condition immediately after all procedures were completed. Figure 1 shows representative photographs of intra-abdominal findings at necropsy 26 h after the procedure.

Figure 1a,b show the intra-abdominal conditions in group 1 (MSSA inoculation only without lavage) and group 2(3 times lavage after MSSA inoculation). As indicated by the white arrow, spider web-like adhesion was observed around the intestine. And as indicated by the black arrow, intraperitoneal white pus formation was observed.
Figure 1c,d show the intra-abdominal conditions in group 3(6 times lavage after MSSA inoculation)and group 4(only 6 times lavage without MSSA inoculation). No adhesion and no pus formation observed in the intra-abdominal space.
Table 3 summarizes the intra-abdominal findings at necropsy. Although all rats in groups 1 and 2 developed intra-abdominal adhesion and pus formation, groups 3 and 4 showed a significant reduction in intra-abdominal adhesion (6/6: 100%, 6/6: 100% vs. 0/6: 0%*, 0/6: 0%*, *p < 0.01) and pus formation (6/6: 100%, 6/6: 100% vs. 0/6: 0%*, 0/6: 0%*, *p < 0.01).
| Group 1 | Group 2 | Group 3 | Group 4 | p value | |
|---|---|---|---|---|---|
| Adhesion n, (%) | 6/6 (100) | 6/6 (100) | 0/6 (0) * | 0/6 (0) * | * < 0.01 |
| Pus n, (%) | 6/6 (100) | 6/6 (100) | 0/6 (0) * | 0/6 (0) * | * < 0.01 |
- Note: Although all rats in groups 1 and 2 developed intra-abdominal adhesion and pus formation, groups 3 and 4 did not show any intra-abdominal adhesion (6/6: 100%, 6/6: 100% vs. 0/6: 0%*, 0/6: 0%*, *p < 0.01) and pus formation (6/6: 100%, 6/6: 100% vs. 0/6: 0%*, 0/6: 0%*, *p < 0.01).
3.3 Effluent Creatinine Levels
Figure 2a shows that the effluent creatinine levels were significantly higher only in group 1 (MSSA inoculation without lavage) compared with groups 2, 3, and 4 (6.60 ± 8.06 vs. 0.17 ± 0.05, 0.13 ± 0.05, 0.20 ± 0.24 mg/dL, p < 0.05). There were no significant differences in the effluent creatinine levels between groups 2, 3, and 4. The original effluent data are available in the Table S1.
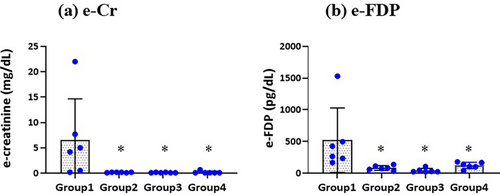
3.4 Effluent FDP Levels
As shown in Figure 2b, the effluent FDP levels were significantly higher only in group 1 (no lavage) compared with groups 2, 3, and 4 (520.2 ± 510.2 vs. 83.4 ± 37.4, 42.4 ± 32.7, 122.6 ± 50.9 pg/mL, p < 0.05). There were no significant differences in the effluent FDP levels between groups 2, 3, and 4.
3.5 Changes in Viable Bacterial Numbers by a Series of Lavage
Figure 3 shows the changes in viable bacterial numbers following a series of lavage. A Tukey Kramer analysis for log10 bacterial numbers revealed a significant stepwise decrease in viable bacterial numbers with increasing lavage time (8.68 ± 0.10, 7.7 ± 0.17*, 7.17 ± 0.26*, 6.91 ± 0.44 and 6.34 ± 0.47* log 10 CFU/mL for 0-, 1-, 2-, 3-, 6-times lavage, *p < 0.01). However, there was no significant difference between groups 2 and 3 (p=0.39). Viable bacterial numbers were reduced to approximately 1/10, 1/30, 1/50, and 1/150 concentrations after 1-, 2-, 3-, and 6-times lavage. The original bacteria count data are available in the Table S1.
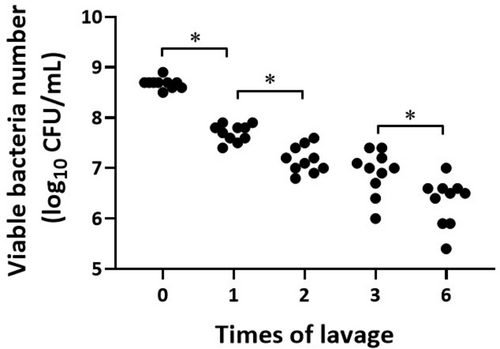
4 Discussion
In the present study, the lethal peritonitis model (Group 1) developed higher mortality, rough fur development, intra-abdominal adhesion, and pus formation, elevated effluent FDP and creatinine, indicating severe peritonitis and AKI induction.
Surprisingly, 6-times lavage (Group 3) completely reduced all abnormalities above without the use of antibiotics. The viable bacterial numbers were significantly reduced to approximately 1/150 concentrations after 6-times lavage.
In contrast, the 3-times lavage is partially effective in reducing the severity of lethal peritonitis because the 3-times lavage group developed rough fur transiently and their intra-abdominal adhesion and pus formation still remained in spite of a significant reduction in mortality, as well as in effluent creatinine and FDP concentration. The viable bacterial numbers were significantly reduced to approximately 1/50 concentrations after 3-times lavage.
Thus, although 6-times lavage was more effective in adequately curing the lethal peritonitis, even 3-times lavage showed a lifesaving effect in this lethal peritonitis model.
An important effluent marker, FDP level has been reported to increase rapidly in peritonitis and is associated with the development of EPS [19]. We observed a significant increase in effluent FDP in the lethal peritonitis group 1, compared with group 4 (no MSSA inoculation control group), suggesting a severe peritonitis induction by this one-shot lethal peritonitis model. Also, 3- and 6-times lavage significantly reduced effluent FDP levels to the same level as group 4, indicating an effective reduction in the severity of peritonitis even in 3-times lavage.
Previously, we reported that a long duration of peritonitis may cause EPS and it would be important to shorten the duration of peritonitis to prevent EPS development [20]. In the present study, we showed that short-term frequent lavage monotherapy with low GDP neutral PD solution would shorten the duration of peritonitis by significant reduction of severity of peritonitis. In this study, peritoneal lavage with low GDP neutral PD solution, in addition to the previously reported saline [9, 21], glycyrrhizic acid [22], Seprafilm, and heparin [23], was effective in reducing intraperitoneal adhesions and pus formation, and may reduce EPS development in cases with severe peritonitis.
The viable bacteria count study revealed that viable bacterial numbers were significantly reduced lavage-time dependent manner to approximately 1/10, 1/30 concentrations after 1- and 2-times lavage. These results suggest that even low-frequency lavage may contribute to achieving an earlier cure of PD peritonitis.
In real clinical practice for PD patients, a single lavage with 2 L of PD solution could be completed within 20 min. Antibiotics could not achieve a similarly rapid reduction in bacterial counts as quickly as lavage, suggesting an early start of both lavage with antibiotics would accelerate early recovery from the severe phase of peritonitis and shorten its duration.
Once foreign pathogens are introduced into the normally sterile peritoneal cavity, invading bacteria gain access to the circulating blood via transdiaphragmatic absorption into the lymphatic circulation [24]. Early elimination of intra-abdominal bacteria by lavage would decrease transdiaphragmatic bacterial absorption and reduce the severity of sepsis.
The possible mechanisms by which our lavage treatment successfully reduced mortality in this study might include the inhibition of septic shock via the reduction of MSSA α-toxin activity [25] by the hyperosmolarity of the PD solution, in addition to the elimination of bacteria.
Interestingly, optimizing the composition of irrigation fluid to reduce the potency of Staphylococcus aureus α-toxin has been performed by Liu et al. [26]. They observed that increasing the osmolarity of the saline from 300 mOsm (0.9% NaCl) to 400, 600, or 900 mOsm (sucrose addition) significantly reduced MSSA α-toxin activity in an osmolarity-dependent manner. Inoue T, et al. also reported a higher survival rate by peritoneal lavage with saline than with twice-distilled water (lower osmolarity) using a lethal dose of Escherichia coli in a one-shot model [15].
These suggest that lavage with hyperosmolar Midpeliq 135 (353 mOSm: glucose addition) used in this study might contribute to our successful results. In this regard, Midpeliq 250 (417mOSm) may be more effective due to its higher osmolarity.
- Whether these effects of lavage are specific to low GDP neutral pH solution is unknown. Midpeliq 250, icodextrin, or the other PD solutions might show better results.
- As this study was conducted on animals, clinical trials are required to confirm the effectiveness of intra-abdominal lavage using a low GDP PD neutral solution for treating severe peritonitis in clinical practice.
In conclusion, this study demonstrated for the first time that the lavage treatment with low GDP neutral PD solution significantly reduces the severity and mortality in a lethal peritonitis model through its rapid bacterial elimination effect without antibiotic use. Further research is needed to determine the details of the most effective implementation of lavage treatment.
Ethics Statement
The care and handling of the animals were in accordance with the guidelines of the National Institutes of Health and the Guide for Animal Experiments of either HAMRI CO. LTD. or Terumo Corporation. All experimental protocols were approved by the animal ethics committee of each institution.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.




