Rheocarna treatment for non-dialysis patients
Abstract
Introduction
Rheocarna has attracted attention as an alternative therapy for severe chronic limb-threatening ischemia, and there have been reports of its usefulness. However, most of these reports have been on dialysis patients, and there are few reports on the treatment conditions and efficacy in non-dialysis patients.
Methods
Four non-dialysis patients who received Rheocarna were studied to determine the conditions under which it was administered and its effectiveness.
Results
The ulcer healed completely, but two patients required skin grafting. The treatment time and blood flow rate were 90–120 min and 50–80 mL/min, respectively. The processed volume was about 1.0 times the circulating blood volume, and the removal rate of low-density lipoprotein and fibrinogen was about 10%–15%.
Conclusion
The ulcers healed despite the removal rate of low-density lipoprotein and fibrinogen being small. To our knowledge, this is the first report on the treatment conditions and effectiveness of Rheocarna in non-dialysis patients.
1 INTRODUCTION
Comprehensive chronic limb-threatening ischemia (CLTI) is a clinical syndrome characterized by the presence of peripheral arterial disease combined with rest pain, gangrene, or leg ulceration for >2 weeks [1, 2]. The survival rates after major lower extremity amputation are 69.7% at 1 year, 34.7% at 5 years, and 75.4% and 42.2% in patients without renal failure, respectively [3], and major amputation still results in significant mortality. Due to recent technological advances, endovascular therapy (EVT) has become the first-line alternative treatment for CLTI [4], with wound healing rates ranging from 55.4% to 83.0% after 1 year of EVT [5] and improvement rates of 25.8% after a month of conservative treatment [6].
In recent years, the usefulness of the adsorptive blood purifier Rheocarna for improving refractory ulcers by improving hemorheology through low-density lipoprotein (LDL) and fibrinogen (Fib) adsorption in patients who are inadequate or non-responsive to revascularization procedures in the treatment of peripheral artery disease has been reported [7, 8]. However, most of these studies have been conducted in patients on dialysis, and there are not many reports on the treatment conditions and efficacy in non-dialysis patients.
2 MATERIALS AND METHODS
Four non-dialysis patients (mean age: 64.5 ± 14.5 years, three males), who underwent Rheocarna treatment between August 2022 and October 2024 at the Institute of Science Tokyo Hospital Hemopurification Center were included in the study. LDL and Fib levels before treatment initiation were 88–117 and 400–725 mg/dL, respectively (Table 1).
| Case no. | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Sex | Male | Male | Male | Female |
| Age | 72 | 62 | 41 | 83 |
| Body height (cm) | 168 | 165 | 175 | 149 |
| Body weight (kg) | 53.2 | 104.0 | 86.6 | 39.4 |
| Number of sessions | 18 | 13 | 12 | 10 |
| Frequency of sessions (/week) | 3 | 3 | 3 | 3 |
| Pre/post-WBC (/μL) | 5900/8000 | 5100/3500 | 8900/5900 | 8200/2300 |
| Pre/post-Plt (×104/μL) | 16.8/12.5 | 36.5/17.4 | 34.6/25.5 | 43.7/39.6 |
| Pre/post-LDL (mg/dL) | 98/51 | 88/54 | 98/57 | 84/66 |
| Pre/post-Fib (mg/dL) | 539/216 | 725/364 | 400/263 | 534/195 |
| Pre-CRP (mg/dL) | 0.32/0.03 | 0.51/0.05 | 0.18/0.06 | 0.06/0.03 |
| Treatment area | Left fourth and fifth toes | Left first and second toes | Right first toe | Left second toe |
| Outcome | Effective | Effective (STSG) | Effective (STSG) | Effective |
- Abbreviations: CRP, C-reactive protein; Fib, fibrinogen; LDL, low-density lipoprotein; Plt, platelet; Pre, before treatment; Post, after treatment; STSG, split-thickness skin grafting; WBC, white blood cell.
The conditions under which Rheocarna therapy was performed were as follows: treatment time: 120 min for Cases 1–3 and 90 min for Case 4. Vascular access was performed using peripheral veins in both hands in all cases, and the blood flow rate (Qb) started at 50 mL/min. In Cases 2 and 3, Qb was 80 mL/min after 30 min (Table 2).
| Case no. | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Sex | Male | Male | Male | Female |
| Average TV (L) | 5.9 | 8.4 | 5.7 | 4.5 |
| Average TV/CBV (BV) | 1.46 | 1.05 | 0.97 | 1.50 |
| Vascular access | Peripheral vein | Peripheral vein | Peripheral vein | Peripheral vein |
| Quantity of blood flow (mL/min) | 50 | 50–80 | 50–80 50 (from the fourth session) |
50 |
| Treatment time (min) | 120 | 120 | 120 | 90 |
| Anticoagulant | Heparin | Heparin | Heparin/NM (from the second session) | Heparin |
| Anticoagulant bolus dose (U/mg) | 1000 | 1000–2000 | 1000/0 | 1000 |
| Anticoagulant dose during procedure (U/h × mg/h) | 1000–2000 | 2000–4000 | 2000 × 20–40 | 1000 |
| Adverse event | Unpleasant feeling | Circuit coagulation | Circuit coagulation/lack of blood flow | ー |
| Adverse event time | First | First | First/third | ー |
| Response to the adverse events | End of the treatment (95 min) | Heparin dosage increase | Change to NM/change to TV | ー |
- Abbreviations: BV, blood volume; CBV, circulating blood volume; NM, nafamostat; TV, treatment volume.
3 RESULTS
3.1 Fib and LDL removal rate and outcome
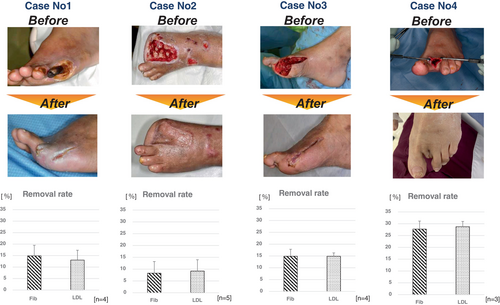
The treatment volume (TV) ranged from 4.5 to 8.4 L, and the TV corrected for TV by the patient's circulating blood volume (CBV) ranged from 0.97 to 1.5 blood volume (BV). The removal rate of Fib and LDL was approximately 10%–15% in Cases 1–3, but it exceeded 25% in Case 4. All patients also showed wound improvement, and in Cases 2 and 3, segmental skin grafting was performed as an adjunctive treatment (Figure 1).
3.2 Adverse events
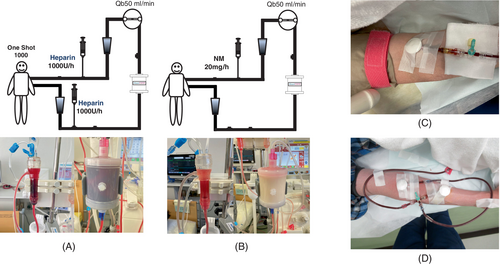
In Case 1, the patient experienced mood discomfort 95 min after the start of the first treatment session. However, the subsequent sessions did not result in any adverse events. In Cases 2 and 3, circuit coagulation was observed during the first session of treatment. In Case 2, the initial dose of heparin was increased to 2000 units, and the continuous dose was increased to 2000 U/h on the de-bleed and return side. Case 3 was treated with nafamostat mesylate (NM) after the second dose because heparin was administered continuously from the de-bleed and return side (Figure 2A,B). All patients lost blood by ejection from the upper arm (Figure 2C). However, in Case 2, during the third session, it became difficult to perform the treatment at a Qb of 80 mL/min, and another puncture was made into the vein on the side of the de-bleed, and the latter was performed from two points (Figure 2D). Therefore, Qb was maintained at 50 mL/min for 30 min after the fourth treatment.
4 DISCUSSION
Kobayashi et al. reported an ulcer healing rate of 50% in non-dialysis patients [7] but the ulcer healing rate in this study was 100%, although two patients had concurrent segmental layer transplantation. Excluding the two patients who also underwent segmental skin grafting, the healing rate was comparable at 50%. However, in terms of treatment conditions, Kobayashi et al. stated that the treatment was performed at a maximum flow rate of 200 mL/min for 1–2 h per session; however, the actual Qb and TV were not described. Furthermore, vascular access in non-dialysis patients was performed with a dialysis catheter in 9 of 10 patients, and in only one patient was it performed in a peripheral vein [7]. In the present study, all patients underwent the procedure in a peripheral vein, and although the TV is expected to be less than that reported by Kobayashi et al., there is no difference in therapeutic efficacy. Therefore, the results suggest that peripheral veins are the first choice for vascular access in patients not on dialysis. As for the device for peripheral vein access, on the de-bleed side, a Qb of 50–80 mL/min can be achieved by using the upper arm for hemodiafiltration and encouraging grip movements during the procedure. Conversely, if a patient has a high TV and Qb cannot be obtained, it is possible to perform two punctures into the vein and remove blood from both sides; however, since three punctures are required each time, optimal TV and Qb are issues for the future.
Rheocarna adsorbs lipoproteins such as LDL via electrostatic interactions with apoprotein B (ApoB), a positively charged substance, due to its negatively charged dextran sulfate, and Fib via hydrophobic interaction due to L-tryptophan, a hydrophobic amino acid. In particular, Fib is considered the molecule with the greatest effect on the plasma viscosity, with a 15%–20% decrease in Fib resulting in a plasma viscosity decrease of approximately 0.1 mPa s [9]. However, in our previous study, Fib was reduced by approximately 20% regardless of the TV, even when ≥6 L was administered [10]. Furthermore, the TV that results in a 20% Fib removal rate is about 1.5 times the CBV (1.5 CBV) [11], so from the perspective of hemorheological improvement, a TV of approximately 1.5 CBV suffices to ensure treatment efficacy. We thought that a TV of approximately 1.5 CBV would suffice to improve hemorheology. However, in this study, the minimum TV was about 1.0 CBV and the Fib removal rate was approximately 10%–15%; however, the ulcers healed regardless of the removal rate, suggesting that the Fib removal rate may not affect the treatment efficacy. In addition, since Fib increases as peripheral artery disease progresses but decreases with its improvement [12], future studies on Fib and treatment efficacy are required. However, in dextran sulfate LDL adsorption (DSAL), a correlation between erythrocyte deformability and LDL concentration has been demonstrated, and it has been reported that LDL apheresis reduces plasma viscosity and reflects rheological changes in erythrocyte deformability [13]. However, the removal rates of DSAL and Rheocarna differ significantly. Since Rheocarna's mechanism of action is currently unknown, it should be elucidated as soon as possible, which will hopefully also clarify the optimal treatment conditions.
Problems such as hypotension and circuit coagulation have been reported during Rheocarna treatment [7, 10, 14]. The decrease in blood pressure occurs almost simultaneously with the production of bradykinin (BK), which induces vasodilation when exposed to the negatively charged dextran sulfate and reaches the peak BK concentration [14], and the decrease in blood pressure is further contributed to by an increase in Qb [10]. In the present study, Qb was low and did not decrease excessively. NM was useful with respect to circuit coagulation. Circuit coagulation is particularly common in patients with high Fib [14] and has been improved by changing the anticoagulant from heparin to NM in patients who had not improved with increased heparin [15]. NM is a potent inhibitor of thrombin, XIIa, Xa, VIIa, kallikrein, plasmin, complement, trypsin, and other proteins. NM is useful in hypercoagulable cases because it inhibits thrombin, XIIa, Xa, VIIa, kallikrein, plasmin, complement, trypsin, other proteolytic enzymes, and phospholipase A2, thereby reducing hypercoagulability [15].
5 CONCLUSION
Our findings suggest that the first choice of vascular access in non-dialysis patients is peripheral veins, and a treatment volume of approximately 1.0 circulating blood volume may be sufficient to achieve therapeutic efficacy. However, since we included only four patients in our study, further studies with more participants are needed.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
This study was approved by the Ethics Committee of the Institute of Science Tokyo (M2019-313) and was conducted in accordance with the Declaration of Helsinki. As this is a retrospective study, the committee also approved the waiver of written informed consent and the use of the opt-out method.




