Impact of therapeutic and low volume plasma exchange on clinical laboratory parameters in patients treated for Alzheimer's disease from the AMBAR study
Abstract
Introduction
Little is known about the impact of plasma exchange (PE) on clinical laboratory parameters in Alzheimer's disease (AD) patients.
Methods
AD patients in the AMBAR trial (N = 322) received weekly therapeutic PE (TPE) for 6 weeks followed by monthly low-volume PE (LVPE) for12 months. Treatment were placebo (sham PE), low-albumin, low-albumin + IVIG (i.e., albumin alternated with intravenous immunoglobulin) and high-albumin + IVIG.
Results
Coagulation parameters transiently increased post-TPE. Blood calcium, platelets, and albumin levels decreased but remained within the reference range. Leukocyte counts increased. Fibrinogen, hemoglobin, total protein, gamma globulin, and IgG, transiently dipped below the reference range. Hypogammaglobulinemia (7.2 g/L) persisted in pre-TPE measurements. No changes were observed during the LVPE period. Cerebrospinal fluid parameters and vital signs were unchanged throughout.
Conclusion
Laboratory parameters of AD patients were affected by TPE similarly to effects of PE-treatment for other pathologies. These effects were less pronounced or non-existent for LVPE.
1 INTRODUCTION
Alzheimer disease (AD) is a degenerative brain disorder that is the most common cause of dementia [1]. The main hallmarks of AD are the accumulation of amyloid plaques formed from extracellular aggregates of amyloid β peptides (Aβ) and the deposits of intracellular neurofibrillary tangles (NFT) of phosphorylated tau protein [2, 3]. Both amyloid deposits and NFT are suspected to be responsible for cell death in the AD brain, although which one triggers the pathological sequalae remains unclear. Reduction of Aβ accumulation as a logical therapeutic target to improve cognitive function in AD patients had been marred by failures until recently [4-6]. A series of studies using anti-Aβ antibodies have shown a potential clinical benefit and could improve our understanding of the Aβ pathophysiology [7-10]. Currently available treatments, which are purely symptomatic, include cholinesterase inhibitors and N-methyl-d-aspartate (NMDA) receptor antagonists [11].
The AMBAR (Alzheimer Management By Albumin Replacement) study is a novel therapeutic approach to AD treatment that has been recently developed on the basis of performing plasma exchange (PE) with albumin replacement. The main rationale behind this strategy hinges on the existence of a dynamic equilibrium between brain, cerebrospinal fluid (CSF), and plasma Aβ [12, 13], which circulates mostly bound to albumin [14, 15]. Therefore, decreasing brain Aβ burden might be achieved through PE-mediated removal of albumin-bound Aβ in plasma which, to restore equilibrium, would induce transport of free Aβ from CSF to plasma. Replacement with therapeutic Aβ-free albumin [16] would provide new potential binding sites for plasma Aβ and promote further sequestration of Aβ. PE is routinely and safely used in many neurological diseases, such as severe acute inflammatory demyelinating polyneuropathy, Guillain–Barré syndrome, multiple sclerosis, and others [17-21].
Clinical efficacy and safety results of the AMBAR study have been published elsewhere [22, 23]. These analyses demonstrated that PE with albumin slowed the decline or stabilized AD symptoms as assessed by cognitive, functional, neuropsychological, neuropsychiatric, and global assessment scores, while improving the patient's quality of life. Importantly, the AMBAR study combined the use of therapeutic plasma exchange (TPE) with a new technique, low-volume plasma exchange (LVPE). LVPE removes a lower volume of plasma compared to conventional TPE, an amount similar to regular plasma donation. Although TPE is a routine procedure with a safety profile that has been extensively studied [24-26], there is little information concerning its effects on AD patients, typically an aged population with associated health issues. Moreover, the impact of LVPE on laboratory analytics routinely performed during PE is unknown.
In this AMBAR sub-study, we report the changes in plasma and CSF laboratory parameters and vital signs observed across the PE program and the differences between TPE and LVPE and how they can contribute to our better understanding of the clinical outcomes of this treatment.
2 METHODS
2.1 The AMBAR study
The AMBAR study was a multicenter, randomized, blinded, and placebo-controlled parallel-group trial that enrolled patients with mild-to-moderate AD between 2012 and 2016. Institutional Review Boards or Ethics Committees from the sites and health authorities from both countries approved the protocol, the patient information sheets, and the informed consent form, in agreement with the Declaration of Helsinki as well as the standards of Good Clinical Practice (GCP) [27].
2.2 Patients
Participants in the AMBAR trial (N = 322) were both men (46%) and women (54%) with an average age of 69.0 ± 7.7 (mean ± SD) years. Patients were diagnosed with AD according to the National Institute of Neurological and Communication Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) criteria [28]; were free of cerebrovascular disease; had a Mini-Mental State Examination (MMSE) [29] score from 18 to 26; and were under current treatment with acetyl-cholinesterase-inhibitors (AchEIs) and/or memantine within the previous 3 months. Full details of demographic and clinical characteristics of patients and the itemized inclusion and exclusion criteria have been published elsewhere [23, 30].
2.3 PE treatment
Patients were randomized to one of the three PE treatment groups or a control group (sham PE) in a (1:1:1:1) distribution. The intervention regime included an initial 6-week period with one session of TPE per week (six TPE sessions in total), followed by a second 12-month period with one session of LVPE per month (12 LVPE sessions in total). In the initial TPE treatment phase, all the patients assigned to any of the treatment groups follow the same treatment regime.
TPE was performed using the commercial continuous-flow cell separator used in each center. Either centrifugation- or filtration-based technology was permitted to achieve separation of plasma from concentrated cells. Peripheral or central access was used based on the individual characteristics of the patient. LVPE was carried out through a peripheral line by means of a prototype apheresis device based on regular plasma donation that uses a rotating membrane and tangential filtration.
The replacement volume of PE was calculated based on patient's weight, sex, and hematocrit. In TPE, 2500–3000 mL of patient's plasma was replaced with the same volume of albumin (Albutein 5%, Grifols). In LVPE, 690–880 mL of patient's plasma was replaced with 100–200 mL of albumin (Albutein 20%, Grifols). While one treatment arm received 20 g of albumin 20% (“Low-albumin” group; N = 78), the other two arms received 20 g or 40 g of albumin 20% alternated with 10 g or 20 g of intravenous immunoglobulin (IVIG) (Flebogamma 5% DIF, Grifols) (“Low-albumin + IVIG”, N = 86; and “High-albumin + IVIG” groups, N = 79; respectively) every 4 months to correct a possible immunological deficit. For the placebo group (sham PE; N = 79), simulated non-invasive PE treatments that did not involve any fluid transfer were performed. Table 1 summarizes the treatment modalities in the various active groups including plasma and albumin exchanged volumes of TPE and LVPE.
| TPE | LVPE | |||
|---|---|---|---|---|
| Treatment group | All active groups | Low-albumin | Low-albumin + IVIG | High-albumin + IVIG |
| Duration of treatment | 6 weeks | 12 months | 12 months | 12 months |
| Plasma exchange frequency | Weekly | Monthly | Monthly | Monthly |
| Plasma exchanged per session | 2500–3000 mL | 690–880 mL | 690–880 mL | 690–880 mL |
| Plasma exchanged at the end of treatment | 18 L | 8–11 L | 8–11 L | 8–11 L |
| Type of albumin infused | 5% | 20% | 20% | 20% |
| Albumin infused per session | 2500–3000 mL | 100 mL | 100 mL | 200 mL |
| IVIG infusion every 4 months | No | No | 20 g | 40 g |
- Abbreviation: IVIG, intravenous immunoglobulin.
Further details of the AMBAR trial apheresis devices and interventions have been published elsewhere [23, 30, 31].
2.4 Variables of interest and sampling
Coagulation parameters analyzed included prothrombin time (Quick), activated partial thromboplastin time (aPTT), fibrinogen concentration, platelet count, and total calcium. The PE procedure was postponed 24 h if Quick time was <60% of the control value (INR > 1.5), if fibrinogen level was <1 g/L, or if platelet count was below 100 000/μL. No further sessions were carried out until the parameters returned to adequate levels. In the case of hypocalcemia, for calcium levels below 8.7 mg/dL, protocol-specified oral or intravenous supplementation was administered based on the total calcium level.
In addition to the hemostasis-related measurements, standard laboratory parameters analyzed in blood samples included hemogram (hematocrit, hemoglobin, and leukocyte count), and biochemistry (albumin, total protein, gamma globulin, immunoglobulin G [IgG], creatinine, and troponin I).
For all laboratory parameters, EDTA-blood samples (42 mL) were collected at screening (baseline visit; Month 0); before each PE session (TPE and LVPE), after each TPE session, and after the LVPE period (final visit; Month 14). Samples after the TPE period (intermediate visit; Month 2) were also collected for IgG. Samples after each LVPE session were also collected for Quick time, aPTT, calcium, and IgG.
Standard laboratory parameters analyzed in CSF samples included albumin, total protein, and glucose. CSF samples were collected at baseline, intermediate, and final visits. Each participating center used their standard testing methods.
Vital signs monitored (per protocol) included heart rate, respiratory rate, body temperature, and blood pressure. In this study, systolic and diastolic blood pressure were analyzed. Blood pressure was monitored 15–30 min before each PE session, during, and again 15–30 min after each PE session, and more often if deemed necessary by the site investigator. Vital signs were collected at baseline, intermediate, and final visit as well as before, during, and after each PE session.
2.5 Data analysis
For each variable of interest, values at every time point (mean ± standard error of the mean [SEM]) were plotted longitudinally for all four treatment groups. Comparisons between treatment groups and the profile of changes during TPE and LVPE periods were descriptively analyzed. Values outside normal ranges were also considered. For this analysis, the normal ranges from the central laboratory were utilized.
3 RESULTS
3.1 Coagulation parameters
Baseline Quick time and aPTT for all groups ranged from 12.6 ± 0.2 to 13.4 ± 0.8 s, and from 29.7 ± 0.4 to 31.7 ± 0.5 s, respectively. During the TPE period, as expected, both post-PE PT and aPTT increased, but remained within the normal range (10–20 s) for PT (Figure 1A) and above the normal range (25–42 s) for aPTT (Figure 1B). Values returned to baseline levels at next pre-PE measurement. No changes were observed during the LVPE period.
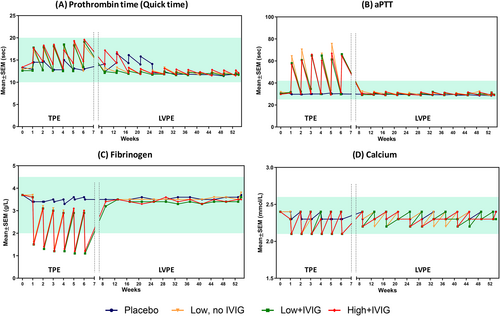
Baseline fibrinogen level was 3.7 g/L, identical in all groups. During TPE period, fibrinogen levels dropped below normal range (2.0–4.5 g/L) in post-PE measurements in all treatment groups: range 1.1–1.6 g/L. Levels remained unchanged in the placebo group. Values returned to near baseline levels at subsequent pre-PE measurements. No changes were observed during the LVPE period. Figure 1C summarizes the changes in fibrinogen levels.
All PE-treated groups showed a post-PE decrease of calcium levels during the TPE period (baseline: 2.4 mmol/L in all groups), close to the lower limit of reference range (2.1 mmol/L). Calcium levels recovered during the LVPE period, during which a slight saw-tooth pattern was observed in both the PE-treated and placebo groups (Figure 1D).
No changes in platelet count were observed across the study and no differences between the treatment groups (ranges: 213.2 ± 6.4 to 244.6 ± 7.9 × 109/L in placebo group; 195.8 ± 5.8 to 265.1 ± 8.7 × 109/L across the PE-treated groups).
3.2 Hemogram parameters
Mean hematocrit remained constant at 40% in all treatment groups across the study. In contrast, mean hemoglobin levels decreased progressively during the TPE period from baseline levels (from 138.8 ± 1.5 to 141.8 ± 1.3 g/L across all groups) in a saw-tooth pattern (Figure 2A). At the end of the TPE period, mean hemoglobin reached values close to lower threshold of normal (115 g/L) in the PE-treated groups. Mean hemoglobin levels partially recovered and stabilized across the LVPE period, although the PE-treated groups consistently remained slightly below placebo. Placebo group showed a similar but less marked pattern as PE-treated groups.
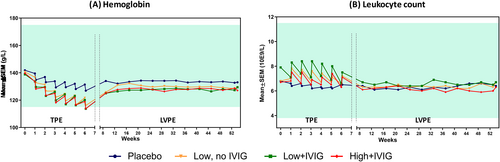
Mean leukocyte count at baseline in all groups ranged from 6.7 ± 0.2 × 109/L to 7.9 ± 0.7 × 109/L. During the TPE period, a rise in post-PE count was observed in the three PE-treated groups, returning closer to baseline at the subsequent pre-PE measurement, and during the LVPE period. No changes were observed in the placebo group across the study (Figure 2B).
3.3 Biochemical measurements in plasma
Mean albumin levels remained stable and in the normal range in all PE-treated groups (between 41.7 ± 0.3 and 46.1 ± 0.3 g/L) across the entire study. However, the placebo group showed a slight post- vs. pre-PE drop (saw-tooth pattern) in mean albumin levels during the TPE period, with a slight progressive decrease compared to the PE-treated groups. Levels normalized during the LVPE period (Figure 3A).
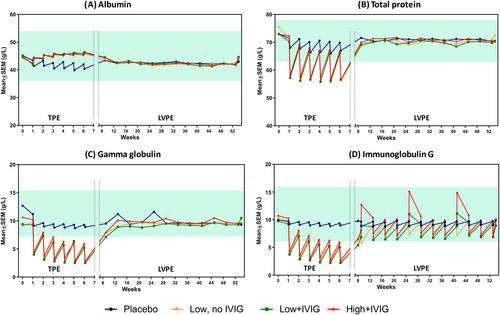
Conversely, mean total protein level dropped markedly below normal levels after PE in all treated groups during the TPE period (baseline: 73.7 ± 0.4 g/L; post-PE: 56.0 ± 0.5 to 57.8 ± 0.4 g/L). As with mean albumin levels, a slight drop in post-PE protein levels was also observed in the placebo group, but still within the normal range. No changes were observed during the LVPE period (Figure 3B).
Both mean gamma globulin and IgG levels markedly dropped below the threshold of hypogammaglobulinemia (7.2 g/L [720 mg/dL]) in all the PE-treated groups during the TPE period. During the LVPE period, levels recovered to normal baseline levels (around 10 g/L [1000 mg/dL]). Mean IgG levels showed a post-PE drop but remained within the normal range including the peak in mean IgG levels after the PE sessions in the groups receiving IVIG infusions (LVPE 1, Week 9; LVPE 5, Week 25, and LVPE 9, Week 41). Details are provided in Figure 3C,D. The placebo group did not show changes in mean gamma globulin and IgG levels across the study.
Mean creatinine levels remained well within the normal range (35–105 μM/L) all across the study in all treatment groups (average: 76.3 ± 2.3 μM/L in the placebo; 67.4 ± 2.0 to 71.3 ± 2.4 μM/L across the PE-treated groups). Most troponin I values were below the detection limit. No difference between control and PE-treated groups in frequency of detectable troponin values was observed.
3.4 CSF biochemistry
None of the parameters measured in CSF showed changes in this study, neither in the placebo nor in the PE-treated groups (Table 2). Mean glucose measurements ranged between 3.5 ± 0.1 and 3.8 ± 0.1 mmol/L (normal range: 2.2–4.2 mmol/L). Mean albumin measurements remained in the range of 0.22 ± 0.01 to 0.29 ± 0.02 g/L (normal range: 0.1–0.3 g/L). Mean total protein measurements ranged between 0.42 ± 0.03 and 0.46 ± 0.04 g/L. However, these values were slightly above normal (normal range below 0.4 g/L).
| Parameter and visit | PE-treatment | |||
|---|---|---|---|---|
| Placebo | Low albumin | Low albumin + IVIG | High albumin + IVIG | |
| Glucose (n; mmol/L) | ||||
| Baseline | 72; 3.62 ± 0.08 | 74; 3.67 ± 0.1 | 82; 3.8 ± 0.11 | 76; 3.68 ± 0.09 |
| Intermediate | 72; 3.52 ± 0.11 | 69; 3.57 ± 0.1 | 74; 3.74 ± 0.12 | 67; 3.66 ± 0.08 |
| Final | 62; 3.63 ± 0.08 | 56; 3.7 ± 0.12 | 51; 3.73 ± 0.13 | 51; 3.53 ± 0.08 |
| Albumin (n; g/L) | ||||
| Baseline | 70; 0.25 ± 0.01 | 74; 0.22 ± 0.01 | 81; 0.24 ± 0.01 | 75; 0.24 ± 0.01 |
| Intermediate | 71; 0.26 ± 0.03 | 68; 0.25 ± 0.01 | 73; 0.29 ± 0.02 | 65; 0.26 ± 0.01 |
| Final | 61; 0.26 ± 0.02 | 56; 0.23 ± 0.02 | 51; 0.25 ± 0.02 | 49; 0.25 ± 0.02 |
| Total protein (n; g/L) | ||||
| Baseline | 53; 0.44 ± 0.02 | 62; 0.43 ± 0.02 | 72; 0.42 ± 0.02 | 56; 0.43 ± 0.02 |
| Intermediate | 63; 0.46 ± 0.04 | 62; 0.42 ± 0.02 | 64; 0.45 ± 0.02 | 55; 0.43 ± 0.02 |
| Final | 60; 0.43 ± 0.02 | 54; 0.42 ± 0.03 | 49; 0.42 ± 0.03 | 47; 0.43 ± 0.03 |
3.5 Vital signs
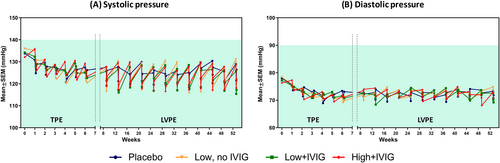
Neither mean systolic nor diastolic pressure values were outside the normal range during the study. However, the PE-treated groups tended to show higher pre- and lower post-PE values of systolic pressure than the placebo group, particularly during the LVPE period. All groups, including placebo, showed a tendency for a decrease in both systolic and diastolic pressure during the TPE period. These data are summarized in Figure 4A,B.
4 DISCUSSION
The AMBAR study demonstrated that PE with albumin replacement could slow the decline in cognitive, functional, global assessments, verbal learning memory, language fluency, and processing speed in patients with mild-to-moderate AD [22, 23]. The safety results of the AMBAR study also demonstrated that PE with albumin replacement was a feasible and safe technique for AD patients, since it induced expected and, therefore, preventable and controllable adverse events [24-26, 32]. However, considering the particular characteristics of AD patients, vital signs and laboratory test parameters were monitored more frequently than in typical clinical setting. In the present analysis, we report the changes observed in the treatment groups and the differences between TPE and LVPE. Overall, the studied parameters did not show alterations different or unexpected than those observed in younger populations undergoing PE.
We observed longer coagulation times (particularly in aPTT) and lower fibrinogen levels in the post-PE measurements during the TPE period. This was not unexpected, given that one plasma volume is removed with this PE modality. Low fibrinogen levels after TPE have previously been described [33, 34], although the impact on bleeding risk has not been established (i.e. transient coagulopathy post-PE with albumin replacement is expected and does not translate to increased bleeding). In the induction period with weekly TPE, the coagulation times and fibrinogen levels were normal in the pre-PE measurements, which has been previously reported. Fibrinogen has approximately 72-h half-life and is not stored and, thus, it is expected that return to baseline may take several days. Fibrinogen synthesis is increased in similar situations to PE, such as hemodialysis [35]. In addition, the fact that coagulation times and fibrinogen levels were unaffected by the LVPE procedure suggests that a decrease in removed plasma volume in LVPE was compensated by the patients' fibrinogen synthetic capacity. This observation may also translate into a lower risk of post-procedure bleeding after LVPE.
PE procedures are known to produce hypocalcemia due to chelation of ionized calcium by sodium citrate, an anticoagulant used during both TPE and LVPE procedures [36]. This phenomenon is transient and it is usually well tolerated by the patient [37]. In our study, post-PE calcium levels dropped but never decreased below the normal range. The variation of calcium levels in the LVPE procedures was milder, likely due to shorter duration and lower citrate dose delivered.
Platelets count did not change either in TPE or LVPE procedures. In PE, the majority of blood morphotic elements (i.e., cells and platelets) are returned to the patient after plasma separation. For similar reasons, hematocrit remained constant across the study.
Interestingly, hemoglobin levels were not only decreased post-PE during the TPE period, but the recovery at the subsequent PE measurement was insufficient to return to baseline hemoglobin level. Considering that hematocrit was stable, this effect could be ascribed to a differential volume between the exchanged plasma and the amount of replacement fluid. Remarkably, a similar but milder pattern was observed in the placebo group, which did not undergo fluid exchange at all, and was probably due to the blood samples collected. In addition, since brain iron homeostasis is perturbed in AD [38, 39], a deleterious effect on hemoglobin levels could be induced by the stress [40] during weekly TPE period in both placebo and PE-treated groups, an effect that can be exacerbated in aged AD patients.
Leukocytes are also part of the corpuscular component of blood which are reinfused during PE. However, instead of the expected stable levels, we observed increased post-PE leukocyte counts during the TPE period. Past studies have reported an increased number of white blood cells in the peripheral blood of patients during PE [41] or, recently, no change [42]. However, these studies were reported three decades apart. Our leukocyte count included migrating cells such as neutrophils, therefore increased post-TPE counts may reflect the mobilization of neutrophils residing in the blood vessels (i.e., a marginal pool) that were mobilized by the PE procedure [43]. The increased leukocyte count was greater during the TPE period than during the LVPE period (no changes observed). Since the residing neutrophils are ready to migrate through the blood vessels to the tissues as a part of the inflammatory response [44], their eventual mobilization back to the bloodstream during TPE might have anti-inflammatory effects.
Albumin makes up 35%–50% of the total normal plasma protein level (~60–80 g/dL). In our analysis, albumin levels were stable across the study in the PE-treated groups. By contrast, total protein levels dropped after PE during the TPE period. This effect was expected, since the removed plasma containing all proteins, but was replaced only by albumin at a volume based on patient's hematocrit, weight, and sex. Effective resynthesis and re-equilibration of proteins has occurred since levels were normal at every pre-PE measurement. However, the pre–post saw-tooth pattern observed in the placebo group during the TPE period is difficult to explain. As a discussion of hemoglobin levels, psychological stress due to the PE procedure could have an influence [45]. In any case, these variations, though intriguing, lack clinical relevance.
Gamma globulin and IgG levels also dropped below normal values in post-PE measurements during the TPE period. However, levels did not recover prior to the subsequent pre-PE measurements. This suggests that the synthesis rate of immunoglobulins in this patient population was slower than other proteins. This might be explained by the site of synthesis: plasma cells vs. hepatocytes. Nevertheless, the kinetics of protein synthesis in vivo is incompletely understood [46]. In the AMBAR trial, IVIG was infused during LVPE period to correct an anticipated immunological deficit caused by plasma depletion. However, during the LVPE period, variations of gamma globulin and IgG levels did not exceed the limits of the normal range. This suggests that IVIG supplementation may not be required if a LVPE approach is followed and only administered if patient's IgG levels are below the normal range and meet the criteria for prophylactic infusion.
Creatinine is a breakdown product of creatine phosphate from muscle and protein metabolism. It is released at a constant rate by the body and is a small molecule [47], which may explain why no changes in creatinine levels based on PE procedures were observed.
In CSF, no changes in glucose, albumin, or total protein levels were observed across the TPE and LVPE periods. Total protein values were slightly above the normal range, which could reflect an intrinsic pathology of AD and/or dehydration and CSF volume changes in aged patients [48].
Blood pressure, particularly systolic, showed a complex pattern associated with PE in both TPE and LVPE periods. Although PE procedure is known to increase systolic blood pressure [49], in our study, the variations were too small to have clinical relevance.
Analysis of the clinical outcomes and CSF AD biomarker levels of the AMBAR study had limitations as reported elsewhere [22, 23]. Although Aβ plasma levels were not reported, results of the Phase 2 trial demonstrated no changes in the placebo group, whereas, in TPE-treated patients, Aβ levels showed a saw-tooth pattern linked to sampling the before and after TPE. This pattern was more apparent in Aβ40 than in Aβ42, which suggested a faster clearance for Aβ40 [50]. Additional limitations of this sub-study of the laboratory parameters include the possible imperfection of the blinding procedure. However, we observed marked differences between the placebo and active treatments, which suggests that blinding was effective. In addition, post-LVPE and intermediate visit values were not available for all parameters, and data analysis was descriptive. Although statistical significance of differences between the groups was not assessed, the qualitative analysis performed on many parameters allowed getting a comprehensive picture of the effect of TPE and LVPE on AD patients.
5 CONCLUSION
In summary, most blood laboratory parameters and vital signs tested in AD patients were affected by TPE as expected from observations in other patients treated with PE for other indications. Specifically, post-TPE levels of calcium, hemoglobin, total protein, gamma globulin, and IgG transiently dropped below, or close to the lower limits of, the normal range. By contrast, gamma globulin and IgG remained low between TPE sessions, whereas there was an intriguing rise of post-TPE leukocyte count. The effects were much milder or even negligible in the LVPE treatment phase.
ACKNOWLEDGMENTS
The authors thank patients for their indispensable contribution. Jordi Bozzo PhD CMPP and Michael K. James PhD (Grifols) are acknowledged for medical writing and editorial assistance in the preparation of this manuscript.
FUNDING INFORMATION
The AMBAR study is funded by Grifols, a manufacturer of therapeutic human serum albumin and intravenous immune globulin.
CONFLICT OF INTEREST STATEMENT
Mercè Boada has been a consultant for Araclon, Avid, Bayer, Elan, Grifols, Janssen/Pfizer, Lilly, Neuroptix, Nutricia, Roche, Sanofi, Biogen, and Servier; and received fees for lectures and funds for research from Araclon, Lilly, Grifols, Janssen, Novartis, Nutricia, Piramal, Pfizer-Wyett, Roche, and Servier. Oscar L. López has been a consultant Grifols and Lundbeck. Zbigniew M. Szczepiorkowski has been on the board of directors or an advisory committee for, ICCBBA, Fenwal/Fresenius Kabi, Grifols, and Novartis; and has received grants and/or contract research for Fresenius Kabi, Erydel, Cellphire, and Cytosorbents. Laura Núñez, Miquel Barceló, Carlota Grifols, and Antonio Páez are employees of Grifols.
PATIENT CONSENT
The patient and a close relative or legal representative read the patient information sheet, agreed to participation in the trial, and then signed the informed consent form.
CLINICAL TRIAL REGISTRATION
EudraCT#: 2011-001598-25; ClinicalTrials.gov ID: NCT01561053.
Open Research
DATA AVAILABILITY
Data reported in this manuscript are available within the article. Additional data from the AMBAR study are available from the corresponding author upon reasonable request.




