Atypical Hemolytic-Uremic Syndrome: An Update on Pathophysiology, Diagnosis, and Treatment
Abstract
Atypical hemolytic uremic syndrome (aHUS), a rare variant of thrombotic microangiopathy, is characterized by microangiopathic hemolytic anemia, thrombocytopenia, and renal impairment. The condition is associated with poor clinical outcomes with high morbidity and mortality. Atypical HUS predominantly affects the kidneys but has the potential to cause multi-organ system dysfunction. This uncommon disorder is caused by a genetic abnormality in the complement alternative pathway resulting in over-activation of the complement system and formation of microvascular thrombi. Abnormalities of the complement pathway may be in the form of mutations in key complement genes or autoantibodies against specific complement factors. We discuss the pathophysiology, clinical manifestations, diagnosis, complications, and management of aHUS. We also review the efficacy and safety of the novel therapeutic agent, eculizumab, in aHUS, pregnancy-associated aHUS, and aHUS in renal transplant patients.
Thrombotic thrombocytopenic purpura (TTP) is a thrombotic microangiopathy (TMA) and systemic disease characterized by formation of widespread von Willibrand Factor (vWF)-platelet thrombi in arterioles and capillaries of multiple organs resulting in thrombocytopenia, microangiopathic hemolytic anemia and neurologic symptoms 1-3. These vWF-platelet thrombi result from impaired function of the ADAMTS13 enzyme, which cleaves vWF into fragments. TTP may be congenital or acquired. In congenital TTP, deficiency of disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 causes impaired function of ADAMST13, while acquired TTP results from autoantibodies against ADAMTS13 3. Hemolytic uremic syndrome (HUS) is another type of TMA that chiefly presents as a triad of microangiopathic hemolytic anemia, thrombocytopenia and renal impairment 4-6. International Hemolytic Uremic Syndrome group classifies hemolytic uremic syndrome into: (i) infections-associated HUS due to Shiga toxin producing Escherichia coli (STEC), Streptococcus pneumonia, influenza A, H1N1, and HIV; (ii) HUS secondary to coexisting conditions, such as hematopoietic stem cell or solid organ transplantation, malignancy, autoimmune diseases, drugs (most commonly quinine, cyclosporine, and tacrolimus), malignant hypertension, and pre-existing nephropathy; (iii) cobalamin C defect-associated HUS; and (iv) atypical HUS due to dysregulation of alternative complement pathway and mutations of the gene, diacylglycerol kinase ε(DGKE) 7, 8.
Atypical hemolytic uremic syndrome (aHUS) is a rare variant of TMA that is caused by abnormalities of the alternative complement pathway resulting in endothelial cell dysfunction and formation of microvascular thrombi. Secondary HUS and aHUS were formerly considered the same disorder but are now considered to be separate conditions 7. While the clinical presentation is similar in aHUS and STEC-HUS, aHUS earned its name because it is not caused by either of the common etiological factors for typical HUS (Shiga toxin produced by E. coli O157:H7 or S. dysenteriae) 9-13. When patients are negative for Shiga toxin, other etiologies such as genetic and sporadic causes must be further investigated 4.
Genetic or acquired dysregulation of the complement alternative pathway is detected in 40–60% of patients with aHUS suggesting a genetic predisposition 14, 15. This dysregulation is caused by mutations in genes that encode complement regulatory proteins, Factor H (FH), Factor I (FI), membrane cofactor protein (MCP), complement 3 (C3), Factor B (FB) or thrombomodulin, or presence of anti-FH antibody resulting in activation of the complement system 7. The complement abnormality that is associated with aHUS is very rare with only roughly 1000 reported cases 4, 14, 16-22. DGKE and cobalamin C deficiency are rare genetic forms of HUS where the complement pathway is intact 23-25. The incidence of aHUS is estimated to be 0.23–0.42 cases per million; children (<18-year of age) constitute 0.10–0.11 cases per million 15, 26, 27. The disease has been triggered by pregnancy, viral illness, and sepsis among other causes; approximately 30% of aHUS results from unknown mechanisms 4. Regardless of the cause, aHUS is a rare disorder with poor clinical outcomes, and higher morbidity and mortality than infection-associated typical HUS. Atypical HUS has a mortality rate of 25% and about 50% of patients may show end stage renal disease (ESRD) 9-12. In this article the review of incidence, pathophysiology, clinical manifestations, diagnosis, complications, and management of aHUS is considered.
PATHOPHYSIOLOGY
In aHUS, a genetic or sporadic insult causes dysfunction in the complement cascade leading to complement deposition on endothelial cells, thickening of arterioles and capillaries and endothelial swelling and detachment 28. Consequently, proteins and cellular infiltrates such as neutrophils, macrophages, and platelets accumulate on the cell surface inducing a pro-thrombotic state. This causes formation of obstructive thrombi in the vessel lumina and shearing of red blood cells, creating schistiocytes, that results in the triad of Coombs negative hemolytic anemia, renal impairment, and thrombocytopenia 29. While aHUS affects multiple organ systems, renal involvement is studied most extensively 30. The renal endothelial cell, a common area for complement activation and immune complex deposition, has a major role in renal pathology since kidney biopsies of patients show complement fragments 29. Microthrombi in the vessels consume platelets causing thrombocytopenia, impede blood flow, and shear erythrocytes causing hemolytic anemia. Renal impairment in aHUS is also thought to be secondary to microthrombi formation in the kidney vasculature; endothelial damage is further exacerbated by anaphylotoxins (C3a and C5a) produced during complement activation 29, 31.
Complement abnormality
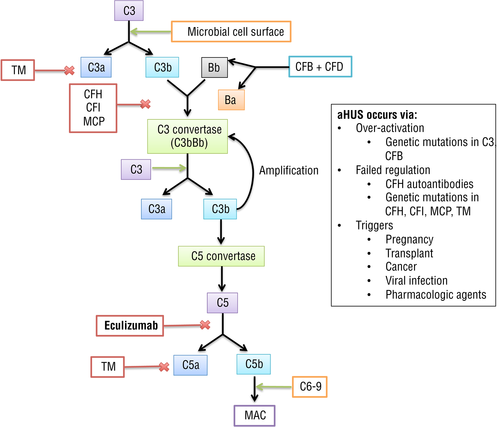
Complement is part of the innate immune response, helping host cells clear pathogens via three distinct pathways: classical, lectin, and alternative. These pathways ultimately converge to create C3 convertase, a complex that initiates membrane attack complex (MAC) C5-9 formation to destroy target cells via attachment and lysis 32. The complement alternative pathway activates the innate immune system in the absence of antibodies. In this pathway C3b, upon encountering a foreign surface, gets activated and combines with factor B to create C3 convertase (C3b) 32. C3 convertase then recruits more C3b to deposit on the cell membrane to create C5 convertase, which is responsible for MAC formation and subsequent cell death 32. The alternative pathway has built-in regulators that suppress unchecked C3b deposition and complement destruction of healthy cells 32. FH and FI regulate C3 convertase formation. FI acts by cleaving C3b into fragments, while FH binds to C3b and acts as a cofactor for FI to cleave C3b, preventing formation of C3 convertase 33-35. Thrombomodulin acts to degrade C3a and C5a. Any defect in these regulators leads to over-activation of the complement pathway.
Over-activation of the alternative complement pathway in aHUS occurs due to either the production of FH autoantibodies or due to genetic complement protein mutations such as FH, FI, FB, C3, and thrombomodulin (Fig. 1) 19. Granular C3 deposits in the glomeruli and arterioles during the intensive phase of the disease, leading to activation of complement and local C3 utilization 28, 36. Activation of the MAC (C5b-9) results in microvascular thrombosis, especially within the kidneys 37-39. The C3 convertase of the classical and lectin complement pathways is composed of C2 and C4 fragments; however, the C3 convertase of the alternative pathway splits C3, but has no effect on C4 37. Because low serum C3 levels mirror complement activation, reduced levels of C3 and normal C4 is characteristic of aHUS 28, 40-42. However, not all patients with aHUS show hypocomplementemia.
COMPLEMENT PROTEIN MUTATIONS
Complement dysregulation at the level of the cell surface is the main mechanism causing aHUS with familial causes accounting for approximately 20% cases 43. Although sporadic causes of aHUS are reportedly more common than the familial form, understanding the role of genetic mutations in aHUS offers prognostic value. To be considered familial aHUS, at least two members of a family must be diagnosed with aHUS within a 6-month period 10. Genetic predisposition to aHUS can be inherited as autosomal dominant, autosomal recessive, pathogenic variant(s) in a single gene, or rarely as polygenic inheritance 44. Although many mutations are implicated in the pathogenesis, the development of disease is multifactorial. While an individual may carry a mutation, a trigger (second-hit) is required for aHUS to occur. Thus, aHUS may be triggered by pregnancy, viral infection, cancer, organ transplantation, and use of certain medications 45. Mutations in CFH, CFHR3, MCP, CFI, CFB, and C3 genes predispose to occurrence of aHUS (Table 1) 43.
| Mutations | Mechanism of action 39 | % patients 40, 46 | % patients with ESRD 40 |
|---|---|---|---|
| CFH | Encodes factor H, which binds to C3b, inhibits production and accelerates decay of C3 convertase, and cofactor for FI | 21–22% | 60–80% |
| CFHR3 | Loss of complement regulation on the cell surface | 26% | 20–29% |
| MCP | MCP acts with FI to cleave C3b and C4b on surface of host cells | 15% | 6–38% |
| C3† | Constituent of C3 convertase and C5 convertase | 2–8% | >60% |
| CFB† | FB: component of C3 convertase | 1–4% | 70% |
| CFI | FI cleaves C3b and C4b with CFH and MCP | 4–8% | 58% |
- † Activating (gain of function) mutations, rest inactivating (loss of function).
CFH mutations
This gene encodes FH, which competitively binds to C3b to inhibit the production of C3 convertase, accelerates decay of C3 convertase, and acts as a cofactor of FI to cleave C3b 47. CFH mutations, chiefly affecting the C-terminal, account for 15–20% mutations in patients with aHUS. Antibodies against FH attach chiefly to the C-terminal, decrease FH binding to C3b, and increase alternative pathway-dependent cell lysis 46.
CFH-related gene 3 mutations (CFHR3)
Genes encoding CFH and CFH-related proteins, located on the long arm of chromosome 1, are highly homologous and prone to conversion and nonallelic homologous recombination 48, 49. The recombinations result in large deletions of genes such as CFHR1, CFHR3, and CFHR4 as well as presence of hybrid genes such as CFH-CFHR1; some of these changes are associated with formation of autoantibodies to FH 50-52.
MCP mutations
Membrane cofactor protein (MCP, CD46), together with CFI, cleaves C3b and C4b on the surface of host cells. Homo- or heterozygous mutations in the gene result in reduced expression of MCP on cell surfaces (type 1 mutation) or normal expression but decreased complement regulatory activity (type II mutation) 53.
C3 mutations
C3 is the cornerstone of complement cascade, since it is necessary for formation of both C3 convertase and C5 convertase 43. Several gain-of-function mutations have been identified within the gene that results in cleavage-resistant C3b, prevention of FI inhibition, and increased C3b affinity for FB 33, 35.
CFB mutations
Gain-of-function mutations result in increased formation of C3 convertase, and activation of the alternative pathway; blood levels of C3 are consistently low 43.
CFI mutations
This gene regulates the complement pathway through cleavage of C3b and C4b with help of FH and MCP (CD46) 43. Loss of function mutations in CFI result in unchecked C3b deposition with increased formation of C3 convertase 54. Mutations range from reduced levels of FI to complete absence.
Many of the above mutations are reported to be associated with familial aHUS. Patients with aHUS may show mutations in a single or multiple genes. Mutations in other genes have also been reported. Patients with mutations in CFH and CFI have worse outcome than those with MCP mutations; 80–100% of the former are at risk of disease recurrence in the renal allograft 4, 16.
CLINICAL FEATURES
Atypical HUS can present at any age and is of acute onset in 20% of cases. The clinical presentation depends upon the extent of microvascular injury and thrombosis, as well as ischemic injury to various organ systems 55. Patients with aHUS present with hemolytic anemia (hemoglobin <10 g/dL), thrombocytopenia (platelets<150 000/mm3), and impaired renal function. Renal impairment is frequent; most common manifestations are proteinuria, hematuria, hypertension, and azotemia 55. While proteinuria is typically mild, nephrotic range proteinuria may occur 55. A majority of patients require renal replacement therapy. Hypertension is often moderate to severe, due to vascular disease and volume expansion.
COMPLICATIONS
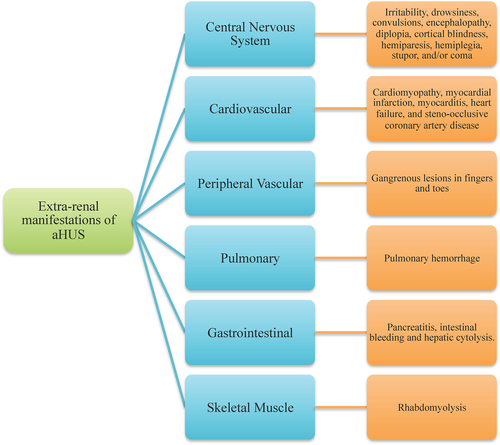
Atypical HUS presents as a systemic disease, and extra-renal features are seen in 20% and a catastrophic presentation with multi-organ involvement in 5% of patients (Fig. 2) 55.
Central nervous system
About 10–48% of patients with aHUS present with nervous system involvement, making it the most common extra-renal manifestation 55. Symptoms include irritability, drowsiness, convulsions, encephalopathy, diplopia, cortical blindness, hemiparesis, hemiplegia, stupor, and/or coma. The clinical features are similar to arterial hypertension and steno-occlusive diseases. Magnetic resonance imaging of the brain reveals symmetrical and bilateral lesions in basal ganglia, brain stem, and deep white matter 56.
Cardiovascular
Approximately 10% of patients show complications such as myocardial infarction, myocarditis, heart failure, cardiomyopathy, and occlusive coronary artery disease 57. Patients with mutations in CFH and C3 show high risk of involvement of the coronary vasculature 57.
Peripheral vascular
Rarely, children may present with gangrenous lesions of the fingers and toes. Ischemic changes are considered to be due to small vessel vasculopathy secondary to complement abnormalities 58-60.
Pulmonary
Pulmonary manifestations, chiefly pulmonary hemorrhage and hypoxemia, are reported in patients with multi-organ failure 61, 62.
Gastrointestinal
Typically, patients present post-solid organ transplantation (after treatment with a calcineurin inhibitor) with abdominal colic, constipation, abdominal distention, strictures, occlusion, and perforation 63.
DIAGNOSIS
The chief disorders of TMA include aHUS, thrombotic thrombocytopenic purpura (TTP), and Shiga toxin-associated HUS. The similarity in presentation can make difficult to distinguish aHUS from other conditions. Timely diagnosis is imperative due to different pathophysiology and therapy. Therapy must be initiated early for TTP and aHUS to prevent end-organ damage.
The diagnosis of TMA requires thrombocytopenia, microangiopathic hemolysis, and one or more of the following: neurological symptoms, renal impairment, or gastrointestinal symptoms. Neurological symptoms include altered sensorium, focal neurologic signs, or seizures. Renal impairment includes elevated creatinine, fall in eGFR, high blood pressure, and abnormal urinalysis. Gastrointestinal symptoms include diarrhea, nausea, vomiting, and abdominal pain. Once the diagnosis of TMA is established, distinction between Shiga toxin-associated HUS, TTP, and aHUS must be made.
The diagnosis of Shiga toxin HUS is based on clinical and lab findings following a history of prodromal diarrhea. The diagnosis is confirmed by serological testing and positive microbiological cultures for Shiga toxin producing E. coli. Other causes of TMA are considered if these tests are negative or in absence of history of diarrhea or bloody stools. The two important differential diagnoses of aHUS in adults include TTP and secondary HUS. TTP is characterized by persistent thrombocytopenia and defect in ADAMTS13 activity, determined through fluorometric or chromogenic assay. Distinguishing aHUS from TTP can prove challenging in adults. TTP is characterized by the triad of TMA with predominately neurologic features, as opposed to predominately renal impairment without neurologic symptoms in aHUS. ADAMTS13 assays are critical to differentiating between TTP and aHUS 3. Activity level < 50% indicates abnormal ADAMTS13 activity, 10–50% indicates low activity; severe deficiency (<5–10% activity) is highly indicative of TTP 64, 65. Secondary HUS may be considered in presence of autoimmune diseases, malignancies, hemopoietic stem cell or solid organ transplantation, malignant hypertension or use of drugs such as calcineurin inhibitors (cyclosporine, tacrolimus), gemcitabine, mitomycin, interferon, quinine, and cocaine 7, 66. The treatment of secondary HUS is withdrawal of offending drug or treating triggering condition 7, 66. Regardless of evidence, empirical plasma therapy and in resistant cases, complement blockade drugs have also been used successfully prompting immediate necessity of large-scale studies to investigate genetic mutations and dysregulation of alternative complement pathway 7, 66.
If the diagnosis of Shiga toxin or secondary HUS or TTP is not made clinically or confirmed by laboratory testing, then the diagnosis of aHUS must be considered. Since ADAMTS13 testing often takes several days to complete and severe end-organ damage may occur, more rapid methods of diagnosis have been proposed. Studies have shown that patients with a severe ADAMTS13 deficiency typically present with blood creatinine <2.26 mg/dL and a platelet count <30 000/mm3; approximately 50% also show antinuclear antibodies 67, 68. Diagnostic testing using blood creatinine, platelet count and presence of antinuclear antibodies had 98.1% specificity and 46.9% sensitivity. Figure 3 presents an algorithm to establish the diagnosis using this process 7.
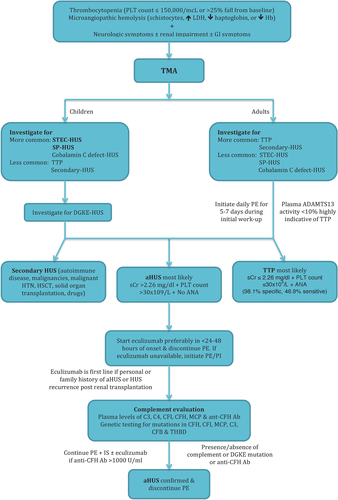
Patients with aHUS require relatively comprehensive evaluation of the complement pathway. These include estimation of blood levels of C3, factor H, I and B, and anti-factor H antibodies. Flow cytometry is done for MCP (CD46). Sequencing, using a next-generation approach, is done for the following genes: CFH, CFI, CFB, C3, MCP, DGKE, and THBD. A high proportion (approximately 85–90%) of patients with anti-FH antibodies show an 80 kB homozygous deletion of CFHR3-CFHR1; this deletion is best detected by multiplex ligation-dependent amplification of this region 69. Results of the above investigations might not be available immediately; therapy is however initiated promptly.
TREATMENT
Therapy of aHUS is supportive, with attention to management of acute kidney injury and systemic complications. The use of packed cells is necessary in patients with severe anemia. Platelet transfusions are rarely required, except in counts are <10 000/cu mm, or thrombocytopenia associated with active bleeding or in patients undergoing invasive procedures. Fluid and electrolyte management is important to maintain intravascular volume status and combat the consequences of aHUS, acute kidney injury, and multisystem organ failure. Electrolyte disturbances should be promptly corrected and nephrotoxic medications avoided. Hypertension should be managed with appropriate agents. Renal replacement therapy is required in patients with uremia, fluid overload, or electrolyte abnormalities. Plasma exchange and eculizumab, a complement inhibitor, offer specific forms of therapy 5, 55.
PLASMA EXCHANGE
Although plasma exchange and plasma infusions (PE/PI) have been the standard of care for aHUS, they do not address the underlying cause of complement dysfunction 70. PE is an effective form of acute management that enables removal of antibodies and other proteins; supportive data from clinical trials is, however, lacking 70. In patients with TTP, PE/PI eliminates vWF multimers, and autoantibodies in addition to providing normal ADAMTS13 to the patient. Empiric plasma therapy has thus been used in these patients, except for Shiga toxin-associated HUS where it is not usually required.
Therapy with PE/PI is widely available across the world, including in resource-limited countries. Therapy is generally begun within 24-h of diagnosis, and typically comprises of daily exchanges (1.5-times plasma volume, 60 mL/kg) for at least 5 days and until platelets >100 000/cu mm and schistocytes<2% for 2 days, followed by alternate days and then twice weekly 55. A multi-center audit of plasma therapy for aHUS showed significant complications of central venous catheterizationin in 31% of patients. The median time to hematological remission was 11.5 days; 11% of patients did not enter hematological remission and 17% were dialysis dependent by day 33 27.
Studies by Pishko et al. showed that empiric PE/PI therapy in patients with aHUS is unsatisfactory and poses risk for ESRD and premature death 71. Following treatment with PE/PI, 23% of patients with aHUS and none with TTP died. By day 21, 71% of patients with aHUS and 95% with TTP had recovery of platelet count >150 000/cu mm; 62% of patients with aHUS and 95% with TTP showed normal levels of LDH 71. In 75% of patients with aHUS and 29% with TTP, renal functions did not recover. These results suggest that empiric therapy with PE/PI in patients with aHUS might not be adequate to ensure hematological or renal remission, and be associated with a high risk of progressive renal impairment. Caprioli et al. also showed that approximately 70% of patients with aHUS with CFH mutation die, need dialysis, or have permanent renal damage at 1-year. Coincidentally, 94% of these patients had received PE/PI as the initial intervention 4.
Because of unsatisfactory recovery, experts in most developed countries do not recommend the empiric use of plasma (PE/PI) therapy for patients with aHUS. Exception is made for countries or centers where therapy with complement inhibitor, eculizumab, is not immediately available. In these cases, PE should be initiated as soon as a diagnosis of aHUS is made. Plasma therapy should be replaced by eculizumab, once the latter is available or if the patient shows lack of hematological remission despite 5–7 PE, life-threatening manifestations (seizures, myocardial dysfunction), or complications associated with PE or vascular access. The other exception is in patients with anti-FH antibodies, where most experts agree that the combination of PE and immunosuppression effectively reduces levels of circulating autoantibodies and inhibit their further production 72. PE has limited role in the management of patients with mutations in MCP. Finally, plasma exchange carries several adverse effects. It has been known to cause transfusion reactions including allergic, febrile, and anaphylactic reactions, transfusion-related acute lung injury, transfusion-associated circulatory overload, and infection 73.
ECULIZUMAB
Eculizumab is a humanized monoclonal IgG antibody that binds to complement protein C5, preventing cleavage into C5a and C5b. Blocking the formation of C5b prevents the continuation of the complement cascade and blocks formation of C5b-9 (MAC) (Fig. 2) 55. In patients with CFH, CFI, C3, or CFB mutations, eculizumab is the preferred intervention for therapy of current illness as well prevention of recurrences 74. It is also efficacious in patients with post-transplant disease recurrence, transplantation-associated aHUS 4, 75, 76, pregnancy-associated aHUS 7, 77-79, and HUS secondary to chemotherapy 66. By preventing MAC formation, eculizumab inhibits the mechanism by which aHUS causes pathology, making this novel drug the treatment of choice for aHUS patients. Table 2 summarizes studies on eculizumab.
| Study | Number | Clinical characteristics of patients | Dose and duration | Outcome |
|---|---|---|---|---|
Fakhouri et al. (2016) Open-label single-arm phase 2 trial |
41 | Twenty-four patients requiring baseline dialysis. Twenty-one (51%) patients had one or more identified complement genetic mutation, autoantibody, or deletion. Nine (22%) patients had a history of prior kidney transplantation. |
Eculizumab (900 mg/week for 4 weeks, 1200 mg at week 5 and then every 2 weeks) for 26 weeks. | Platelet counts and eGFR increased from baseline (P < 0.001). All 35 patients on baseline PE/PI discontinued by week 26. Of 24 patients requiring baseline dialysis, five recovered kidney function before eculizumab initiation and 15 of the remaining 19 (79%) discontinued dialysis during eculizumab treatment. No patients lost existing transplants. Quality-of-life measures were significantly improved. |
Cofiell et al. (2015) Open label, nonrandomized, single-group, multi-center trial |
41 | Twenty (49%) patients had 1 or more identified complement gene mutation and/or CFH autoantibody. Thirt-five (85%) patients received PE/PI before eculizumab. Twenty-four (59%) patients were receiving dialysis at baseline. Nine (22%) patients had a history of prior renal transplantation. Thirty-three (80%) patients had CKD stage 4 or 5. |
Eculizumab (900 mg/week for 4 weeks, 1200 mg at week 5 and then 1200 mg every 2 weeks) for 26 weeks with optional extension to 1 year. Thirty-eight (93%) completed the initial 26-week clinical study period and 21 (51%) continued treatment of 1 year during the optional extension period. |
Immediate and sustained reductions in C5a and sC5b-9 after first dose of eculizumab (P < 0.0001) to levels of healthy volunteers. Renal injury markers (clusterin, cystatin-C, b2- microglobulin, and liver fatty acid binding protein-1) were significantly reduced by week 6 (P ≤ 0.0005). Reduced inflammation (soluble TNF receptor-1), coagulation (prothrombin fragment F112 and D-dimer), and endothelial damage (thrombomodulin) markers to near-normal levels. Alternative pathway activation (Ba) and endothelial activation markers (soluble vascular cell adhesion molecule-1) decreased (P ≤ 0.0001, P < 0.0001, respectively) but remained elevated, reflecting ongoing complement activation in aHUS despite complete terminal complement blockade. |
Legendre et al. (2013) Two prospective phase 2 trials |
37 17 in trial 1 20 in trial 2 |
Patients with low platelet count and renal damage (trial 1) Patients with renal damage but no decrease in the platelet count >25% for at least 8 weeks during PE/PI (trial 2). A total of 24% of patients in trial 1 and 35% of patients in trial 2 had no identified complement gene mutation or CFH autoantibodies. In trial 1, six (35%) patients had one current kidney transplant and one (6%) patient had two transplants. In trial 2, three (15%) patients had one current transplant, three (15%) patients had two transplants, and two (10%) patients had four transplants each. |
Intravenous eculizumab (900 mg/week for 4 weeks, 1200 mg at week 5 then1200 mg every 2 weeks) for 26 weeks with an optional extension period. A total of 37 patients (17 in trial 1 and 20 in trial 2) received eculizumab for 26 weeks. Thirteen (76%) in trial 1 and 19 (95%) in trial 2 continued to receive during the extension period for a median of 64 and 62 weeks, respectively. | Platelet count increased; in trial 1 (P < 0.001). In trial 2, 80% of the patients had TMA event-free status. PE/PI was discontinued in all patients, and no new dialysis was required. In trial 1, dialysis was discontinued in four of five patients. In both trials, earlier initiation of eculizumab was associated with a significantly greater improvement in the eGFR throughout the treatment period (P = 0.007 in trial 1 and P < 0.001 in trial 2). Improvement in health-related quality of life noted (P < 0.001 in both trials). |
Licht et al. (2015) Two year outcome analysis of two phase 2 studies (above study) |
37 17 in trial 1 20 in trial 2 |
Patient characteristics same as above. | Eculizumab (900 mg/week for 4 weeks, 1200 mg at week 5 then1200 mg every 2 weeks) for 26 weeks with an optional extension period. Five patients in trial 1 and 16 in trial 2 continued the extension phase to the 2-year analysis. The median (range) duration of eculizumab exposure in trial 1 was 100 (2–145) weeks and in trial 2 was 114 (26–129) weeks. | In trial 1, significant increase in platelet count noted at week 26 (P < 0.001), 1 year (P < 0.001), and at the 2-year cutoff (P ≤ 0.001). Platelet count was normalized in 14 (82%) patients at 26 weeks and in 15 (88%) patients at the 1- and 2-year cutoffs. Hematologic normalization was met by 13 (76%) patients at week 26 and by 15 (88%) patients at the 1- and 2-year cutoffs. In trial 2, TMA event-free was observed in 16 (80%) patients at week 26, 17 (85%) patients at year 1, and 19 (95%) patients by the 2-year cutoff. Hematologic normalization was achieved by 18 (90%) patients’ at all three time points. |
Cavero et al. (2017) Retrospective analysis |
29 | Patients with secondary aHUS (15 drug-induced, 8 systemic diseases, 2 postpartum, 2 cancer-related, 1 associated with acute humoral rejection, and 1 with intestinal lymphangiectasia). Fourteen on baseline dialysis. Fifteen had solid-organ transplant. |
Eculizumab (900 mg/week for 4 weeks, 1200 mg at week 5 then1200 mg every 2 weeks) anywhere from two to 30 weeks. Eculizumab was discontinued after 8 weeks on average. The average number of doses was six. | A rapid improvement in hematological and renal abnormalities after initiation of eculizumab was observed in 20 patients (85%). The rate of response varied according to etiology of aHUS: 12/15 (80%) among drug-induced aHUS, 6/6 (100%) in postpartum, cancer-related, acute humoral rejection and intestinal lymphangiectasia, and 2/8 (25%) in aHUS associated with systemic diseases. The interval between eculizumab initiation and TMA resolution averaged 12 days. Resolution of hematological abnormalities with no improvement in renal function was observed in six patients. In three patients, both hematological abnormalities and renal function impairment persisted despite eculizumab treatment. The number of patients requiring dialysis decreased to four (13%). A ≥ 25% decrease in serum creatinine was seen in 20 (69%) patients and ≥ 50% decrease was seen in 15 (52%). There were a significantly higher number of patients with aHUS associated with systemic disease among non-responders. |
Greenbaum et al. (2016) Prospective analysis of open label phase 2 study |
22 | Twelve had no prior PE/PI. Eleven received baseline dialysis. Two had prior renal transplants. Eleven (50%) had ≥1 identified complement gene abnormality or CFH autoantibody. |
Eculizumab administered at doses prespecified by body weight (see pediatric dosing) for 26 weeks with an extension period up to 2 years. Patients were exposed to eculizumab for a mean of 5.5 months. | By week 26, 14 (64%) patients achieved a complete TMA response. TMA event-free status was achieved in 21 (95%) patients. By day 31, PE/PI was discontinued in all 10 patients who required it at baseline. Eighteen (82%) patients achieved hematologic normalization after a median of 55 days. Twenty-one (95%) patients achieved platelet count normalization after a median of 7 days. Eculizumab was associated with a rapid and sustained increase in platelet count from baseline to 27 weeks (P ≤ 0.0001). A decrease in serum creatinine level by ≥25% occurred in 16 (73%) patients after a median of 21 days. Dialysis was discontinued in 9 of 11 (82%) patients receiving it at baseline after a median of 7 days. None initiated new dialysis. Health-related quality of life was improved by week 27 (P < 0.0001) |
Walle et al. (2016) Post hoc analysis of four phase 2, open-label, single-arm, prospective clinical trials |
97 | Fifty-seven (59%) patients had a complement mutation or CFH autoantibody. Seventy-one (73%) receiving PE/PI at baseline. Forty-three (44%) receiving dialysis at baseline. Twenty-six (27%) had a history of kidney transplantation. |
Eculizumab (900 mg/week for 4 weeks, 1200 mg at week 5 then1200 mg every 2 weeks) for 26 weeks for all trials with an optional extension period. Twenty-one patients had eculizumab at >7 days of TMA presentation and 76 patients at ≥7 days of presentation. |
Patients receiving eculizumab ≤7 days after first signs of the aHUS manifestation showed a significantly (P < 0.05) greater mean improvement in eGFR from month 1 onwards. About 17/21 (81%) patients in the ≤7 group achieved a sustained increase in eGFR after 3 months that remained stable through 1 year. In contrast, 26/76 (34%) and 36/76 (47%) patients in the >7 days group achieved a sustained eGFR at 3 months and at 1 year, respectively. Mean eGFR change from baseline at 1 year was significantly higher in patients treated in ≤7 days than >7 days (57 vs. 23 mL/min/1.73 m2, P = 0.0098). The proportion achieving a sustained eGFR response (≥15 mL/min/1.73 m2) was significantly higher (P < 0.05) for the ≤7 day group compared to the >7 day group at all visits. Mean time to platelet count normalization was similar between groups. Younger age, higher baseline LDH and lower baseline hemoglobin were associated with greater eGFR improvements. |
Merill et al. (2017) Single center, retrospective review |
17 | Median ADAMTS13 activity was 60%. PE was initiated before eculizumab in 64% of patients. Eleven patients had a complement abnormality. |
Information on dosing not available. Median duration of therapy 90 days. |
Two patients died during eculizumab treatment, one from suspected infected vascular access and one from pulseless cardiac arrest during plasmapheresis. Of the 15 patients who stopped eculizumab due to non-adherence or physician directed cessation, three (20%) experienced relapse. No patient required dialysis after eculizumab cessation. About 94% of all patients achieved TMA event-free status and 82% remained dialysis-independent after last follow-up. Four of the patients that achieved clinical remission were found to have a persistently positive mHam assay of complement activation. |
Huerta et al. (2017) Retrospective study |
22 | Women with pregnancy-associated aHUS. In 16 (73%) patients aHUS occurred during the first pregnancy. In six (27%) patients, onset occurred during the antepartum period. In 16 (73%) patients the onset occurred during the postpartum period (≤1 week after delivery). Nine (41%) patients required acute hemodialysis. Associated glomerulonephritis was present in three cases. One patient had a solid organ transplant. A complement abnormality was detected in nine of 22 (41%) patients. |
Eculizumab 900 mg/week for 4 weeks, then 1200 mg every 2 weeks. Median treatment time of 10 months. |
Ten (45%) patients received eculizumab at the P-aHUS event and all of them (100%) had a positive response at both hematologic and renal levels. Three of the 10 patients treated with eculizumab required hemodialysis at P-aHUS onset, but renal functions recovered. Eculizumab was initiated with a median time of 17 days after the P-aHUS event and lasted 9.8 months on average. None of the eculizumab-treated patients reached ESRD at follow-up. Two patients treated with eculizumab had recurrences at 5 and 7 months after discontinuation, respectively. Within the group of patients that did not receive eculizumab (12 patients), 50% required hemodialysis at onset. Four of 12 patients (33%) reached ESRD during the first month and six patients (50%) required renal replacement therapy at the end of follow-up and subsequently received a renal transplant. Seven of 22 patients had aHUS recurrences after an average time of 240 months. |
Bruel et al. (2017) Retrospective study |
87 | Women with pregnancy-associated HUS. At diagnosis 56 (71%) patients required dialysis. Forty-nine patients had a complement gene variant. |
No information on dose or duration available. | Fifty-six (78%) patients underwent PE and 21 (41%) received PI. Four (5%) received eculizumab. Among the four patients treated with eculizumab, three (two with complement gene variants and one without) had a complete recovery of kidney function and one patient treated with eculizumab 2 months after diagnosis remained dialysis-dependent. |
Levi et al. (2017) Prospective study |
12 | Renal transplant patients with aHUS-related ESRD. A genetic mutation was found in nine patients. Anti-CFH antibodies were found in two patients. Five patients had previously received 15 renal transplants that were lost to aHUS recurrence. |
Eculizumab 1200 mg at day 0 and 900 mg at day 1 and then every week for 4 weeks. A maintenance dose of 1200 mg was administered every 2 weeks. All patients treated with the same dose and frequency for median follow-up time of 24.6 months. |
Ten patients were treated with eculizumab as prophylaxis of recurrence. Two patients were treated for aHUS recurrence within the first 30 days post-transplantation. Prompt initiation of eculizumab obtained complete and sustained remission of TMA features within a few days in these two patients. None of the 12 patients exhibited any hematological TMA features during eculizumab therapy. Three patients experienced antibody-mediated rejection despite appropriately dosed eculizumab treatment. One patient developed C3 glomerulonephritis, but under eculizumab therapy neither necrosis nor endo or extra capillary proliferation was observed. One patient experienced aHUS recurrence 1 month after discontinuation of eculizumab treatment for 55 months. |
Fakhouri et al. (2017) Retrospective study |
38 | Twenty-one of 38 (55%) patients carried novel or rare complement gene variants. Eleven patients had CFH variants. Eight patients had MCP variants. aHUS occurred postpartum in two patients and in the setting of pancreatitis in one patient. At presentation 47% of patients required dialysis. |
No information on eculizumab dose available. Median treatment duration of 17.5 months. | Renal outcome of ecuizumab-treated patients was excellent with median serum creatinine at time of discontinuation was 1 mg/dL in adults and 0.45 mg/dL in children. Only four patients retained chronic kidney disease. Twelve (32%) patients experienced aHUS relapse after discontinuation with a median time to relapse of 7.5 months. About 8/11 (72%) patients with CFH variants, 4/8 (50%) patients with MCP variants and none of the patients with no variants relapsed. Median time to relapse after eculizumab discontinuation was 6.5 months in patients with CFH compared to 10.5 months in patients with MCP (P = 0.66). The main differences between relapsers and non-relapsers were a history of previous aHUS episodes before eculizumab use and the frequency of complement genes variants. The 3-year risk of aHUS relapse after eculizumab discontinuation was significantly higher in patients with pathogenic variants in MCP (P = 0.001) or CFH (P < 0.001) genes compared with patients with no identified variants. In all relapsing patients, eculizumab was rapidly (<48 h) resumed. Platelet count normalized in a median time of 5.5 days. |
- CFH, complement factor H; eGFR, estimated glomerular filtration rate; ESRD, end stage renal disease; LDH, lactate dehydrogenase; P-aHUS, pregnancy-associated aHUS; PE, plasma exchange; PI, plasma infusion; TMA, thrombotic microangiopathy; TNF, tumor necrosis factor.
A review of controlled clinical trials, case series and case reports by Palma et al. found epidemiological evidence supporting the efficacy and safety of this agent in patients with aHUS. The results suggest that eculizumab controls aHUS in “the overwhelming majority of patients and likely at a higher frequency than with PE alone by historical database comparisons” 26. Moreover, eculizumab has better outcomes and a lower risk profile than PE/PI. This analysis substantiates eculizumab as the treatment of choice for all patients with aHUS. A recent systematic review of case reports in patients with aHUS compared outcomes between eculizumab, PE, non-eculizumab, and non-PE groups 80. The review found no significant difference in time to symptom resolution and normalization of serum creatinine and platelet counts between eculizumab and PE groups compared to non-eculizumab and non-PE groups, respectively. There was also no difference in these end points when comparing eculizumab to PE. However, there was significant reduction in mortality with use of eculizumab, and no difference in mortality between PE and non-PE groups 80.
In a study on 41 patients treated with eculizumab, 30 had complete response defined as hematologic normalization and preservation of kidney function 76. By week 26, all 35 patients on PE/PI were able to discontinue it. Additionally, no patients lost existing transplants and quality of life measures were improved. Cofiell et al. showed that 26-weeks therapy with eculizumab resulted in terminal complement blockade with reduced terminal complement activation and renal injury markers 81.
Legendre et al. conducted a two prospective phase 2 trials where 37 patients with aHUS received eculizumab for 26-weeks, with a long extension phase 82. Seventeen patients in trial 1 and 20 patients in trial 2 received eculizumab for a median of 64 and 62 weeks, respectively. They found that ecuizumab resulted in increased platelet counts in trial 1 and in trial 2, 80% of patients had TMA event-free status. Eculizumab was also associated with significantly improved glomerular filtration rate (GFR), discontinuation of dialysis and improved health-related quality of life 82. Licht et al. then performed a 2-year analysis of outcomes of the two phase 2 trials 83. They found that at all time points eculizumab inhibited terminal complement activity. In trial 1 the platelet count was significantly improved and hematologic normalization was achieved in 13 patients at 26 weeks and in 15 patients at 1 and 2 years 83. In trial 2, 16 patients achieved TMA event-free status at week 26, 17 patients at 1 year and 19 patients at 2 years. This study shows that the benefits of eculizumab treatment for aHUS are maintained at 2 years of follow-up 83. Cavero et al. identified 29 patients with secondary aHUS who had been treated with eculizumab due to worsening renal function and persistence of TMA despite plasmapheresis 84. Rapid resolution of TMA was observed in 20 patients and treatment was discontinued after a median of 8 weeks without recurrence of symptoms 84.
Eculizumab has also been shown to be safe and effective in pediatric patients. Greenbaum et al. prospectively evaluated the efficacy and safety of weight-based dosing of eculizumab in 22 children with aHUS over 26 weeks 85. By week 26, 14 achieved a complete TMA response, 18 achieved hematologic normalization, 16 had 25% or better improvement in serum creatinine, and PE/PI was discontinued in all patients 85. We recommend eculizumab as a first-line therapy for aHUS in all patients.
Several studies have demonstrated that immediate treatment with eculizmab in aHUS patients leads to better outcomes 82, 85, 86. A post hoc analysis by Walle et al. of 97 patients with aHUS showed that early eculizumab initiation lead to improved renal recovery and platelet count normalization 86. They also found that certain characteristics were associated with greater estimated GFR (eGFR) improvements including younger age, higher baseline LDH and lower baseline hemoglobin 86. These studies highlight the importance of early diagnosis and timely initiation of eculizumab in aHUS patients.
Current dosing guidelines for eculizimab are summarized in Table 3 87. While these dosing guidelines exist, there is currently no clinical data available to guide dose adjustments in aHUS patients to achieve or maintain optimal response. A case report suggests that these aHUS patients may require higher eculizumab doses 88. Six weeks after switching to maintenance dosing the patient developed signs and symptoms of aHUS. The patient was transitioned back to weekly 900 mg IV doses and remained clinically stable for 6 months 88. This case highlights a necessary increase in the dose of eculizumab if an optimal response is not achieved initially or if the initial response is lost following the transition to maintenance therapy.
| Pediatric patients |
|
| Adult patients |
|
The appropriate duration of eculizumab treatment in aHUS has not yet been established. Merill et al. administered eculizumab therapy to 17 patients with active aHUS and found the median duration of therapy was 90 days before physician directed cessation when a patient achieved TMA remission 89. Three patients experienced relapses in the setting of active inflammatory bowel disease, non-adherence to antihypertensives for malignant hypertension, and after liver transplant with medication non-adherence. For the patients that achieved clinical remission, it is worth noting that some converted from positive to negative in the assay of complement activation while others remained persistently positive 89. This indicates that ongoing complement activation does not necessarily predict relapse and that eculizumab may be safely discontinued in some patients despite persistent complement activation. The results of this study support an approach of early eculizumab initiation and a trial of cessation with regular monitoring when patients achieve clinical remission. When early initiation of eculizumab is not possible, there is evidence to suggest that renal recovery can still be improved by initiating long-term eculizmab therapy. In a case report, eculizumab therapy was started 29 days after aHUS onset and plasma exchange 90. The patient required long-term dialysis, however, long-term treatment with eculizmab allowed for discontinuation of dialysis after 18 months 90. This case demonstrates that renal function can be slowly recovered with long-term eculizumab even when initiation is delayed. Fakhouri et al. analyzed the risk of relapse associated with complement gene variants after eculizumab discontinuation in 38 patients 91. They found that the 3-year risk of aHUS relapse after eculizumab discontinuation was significantly higher in patients with pathogenic variants in MCP (P = 0.001) or CFH (P < 0.001) genes compared to patients with no identified variants 91. These results suggest that pathogenic variants in complement genes are associated with a higher risk of aHUS relapse after eculizumab discontinuation and clinicians should be cautious when discontinuing eculizumab in this patient population.
The cost of eculizumab must be considered, as it is very expensive at > $300 000/year of treatment 92 and many insurance companies have budget restrictions. Van den Brand et al. performed a cost-effectiveness analysis of treatment alternatives for aHUS patients with ESRD. They concluded that eculizumab treatment in conjunction with kidney transplantation results in a substantial gain of quality-adjusted life years (QALYs) 93. For recurrent aHUS there was no difference in QALYs between eculizumab upon recurrence (kidney transplantation plus 3 months of eculizumab therapy in cases of recurrent aHUS), eculizumab induction (kidney transplantation plus 12 months of eculizumab prophylaxis and retreatment if a recurrence occurred), and lifelong eculizumab prophylaxis (kidney transplantation plus lifelong eculizumab prophylaxis), however, induction and prophylaxis resulted in significantly higher costs 93. Thus, eculizumab upon recurrence is the most cost effective treatment.
As with all pharmacologic agents, there are risks and side effects associated with eculizumab. Eculizumab blocks the activation of the terminal complement cascade rendering patients more susceptible to infections from encapsulated bacteria 87. It has been known to cause serious meningococcal infections, therefore, meningococcal vaccination is strictly recommended before initiating eculizumab therapy. Ideally, patients are vaccinated at least 2 weeks prior to starting eculizumab therapy. Any patients with current or unresolved Neisseria meningitides infection should not receive this therapy 87. Furthermore, immunizations against Streptococcus pneumoniae and Haemophilus influenza type B should be considered before initiating therapy.
The most commonly reported adverse events with eculizumab therapy include infections (24%), hypertension (5%), chronic renal impairment (5%), and renal impairment (5%). Because eculizumab contains protein products there is a potential risk for infusion reactions 87. Thus far no patients in the clinical trials have reported any infusion reactions; however, some reported adverse reactions including headache, diarrhea, hypertension, upper respiratory infection, abdominal pain, vomiting, nasopharyngitis, anemia, cough, peripheral edema, nausea, urinary tract infections, and pyrexia 87.
ECULIZUMAB IN PREGNANCY-ASSOCIATED AHUS
Pregnancy-associated TMA, including TTP and HUS, is rare with an incidence of roughly one in 25 000 pregnancies 79. Bruel et al. retrospectively analyzed the presentation, outcome and frequency of complement alternative pathway gene variants in a large international cohort of patients with pregnancy-associated HUS and concluded that it is a form of aHUS triggered by pregnancy 77. Additionally, Fakhouri et al. found that 85% of patients with pregnancy-associated HUS had complement pathway abnormalities 94. Huerta et al. retrospectively analyzed a cohort of patients with pregnancy-associated aHUS and found that all of the 10 patients treated with eculizumab had normalization of all hematologic parameters and preservation of renal function 78. Moreover, none of the patients required dialysis or transplantation at the end of the follow-up. Eculizumab was discontinued in 7 of the 10 cases after a median treatment time of 10 months 78.
ECULIZUMAB FOR AHUS AFTER RENAL TRANSPLANTATION
Caprioli et al. found that patients with CFH, CFI, or C3 mutations who do not respond to the aforementioned treatment modalities are more likely to progress to ESRD 4. In these patients, aHUS reoccurs in up to 50% of kidney transplants while graft failure occurs in nine out of 10 patients 70. The high failure rates in this specific population necessitates genotyping all patients with aHUS prior to renal transplantation. A study by Levi et al. administered eculizumab to 12 renal transplant patients with histories of aHUS 75. Ten patients received eculizumab as prophylaxis for recurrence and two patients received eculizumab for aHUS recurrence. All but one patient had inherited or acquired dysregulation of the complement pathway (CFH, CFI, and C3). None of the patients experienced aHUS recurrence during treatment, confirming that eculizumab is highly effective in preventing post-transplantation aHUS recurrence 75. The patients received eculizumab once a week for 4 weeks and then maintenance dose every 2 weeks. Therapy was discontinued after a median follow-up of 24.6 months 75.
CONCLUSION
Atypical hemolytic uremic syndrome is a rare and devastating disease caused by failed regulation and over-activation of the alternative complement pathway. While the kidney is predominately affected, the disease can involve multiple organ systems and cause severe morbidity and mortality when left untreated. The clinical presentation and management of aHUS is the same whether the disease is genetic, acquired or caused by an unknown mechanism. Eculizumab, a C5 inhibitor, offers a novel therapeutic approach to aHUS and is the preferred treatment. Understanding the approach to diagnosis and management of aHUS is critical as early diagnosis and intervention decreases disease morbidity and mortality.
Conflict of Interest
None.
Financial Support
None.




