2011 update Japanese Society for Dialysis Therapy Guidelines of Vascular Access Construction and Repair for Chronic Hemodialysis
The first edition of the Guidelines for the Construction and Repair of Vascular Access in Chronic Hemodialysis was published by the Japan Society for Dialysis Therapy (JSDT) in 2005, under Seiji Ohira, Chairman. Recently, many presentations making reference to the guidelines have appeared in conferences and journals. The 2011 version uses this first edition as a foundation and was formed with a collection of data following the publication of the first edition. During this time, in 2008, the JSDT conducted a survey on the conditions of vascular access (VA), where it found that native arteriovenous fistula (AVF) made up 89.7%, synthetic arteriovenous grafts (AVGs) were 7.1%, superficialization 1.8%, other direct arterial puncture 0.1%, long-term indwelled catheters 0.5%, temporary venous catheters 0.5%, single-needle dialysis 0.2%, and other methods 0.1% 1. In a survey conducted 10 years earlier, in 1998, AVF usage was at 91.4%, AVGs were 4.8%, superficialization was 2.5%, external shunts 0.2%, and other methods 1.1% 2 (Table 1). In other words, with an increase in long-term dialysis cases and damage to native vessels due to advanced age, internal shunts with native vessels are on the decrease; this implies that the use of AVG will increase. In the 2008 survey, other methods made up 0.1%: catheters were not categorized within this survey but may have been included.
| AVF | AVG | Superficialization | Direct venipuncture | Long-term indwelled catheters | Temporary catheters | Single-needle dialysis | External shunt | Others | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1998 | 91.4% | 4.8% | 25% | – | – | – | – | 0.2% | 1.1% | 100.0% |
| 2008 | 89.7% | 7.1% | 1.8% | 0.1% | 0.5% | 0.5% | 0.2% | – | 0.1% | 100.0% |
In these guidelines, in addition to the level of evidence, the level of recommendation has been included, and the categories are evaluated with both. As the Kidney Disease: Improving Global Outcomes (KDIGO) 3 uses the Grading of Recommendations Assessment, Development and Evaluation (GRADE) as a method of evaluating evidence, the Evidence Level Evaluation Committee of the JSDT made considerations 4 and based their method on that. With regard to study design, random controlled trials (RCT) were rated as high, observational studies as low, and all others as very low. Considerations for contents, quality, bias, etc. were given a two-level plus or minus point; in the end, they were divided into four levels, “A–D” (Table 2). “A” may represent high, “C” low, and “D” very low, and plus and minus points assigned accordingly. Where evidence is not shown, the committee has assigned an expert opinion (O). Because the number of RCTs in kidney-related fields is extremely limited, the evidence level was expected to be low, and there was a possibility that most proposals would be weak. Levels of recommendation have been divided into Level 1: Strong and Level 2: Weak, but if an “expert judgment” has shown them to be clinically important, even though the evidence level may be weak, they were strongly recommended. The opposite is also true. In this manner, the level of recommendation is denoted together with the evidence level. As an example, where the evidence level is “low” but the level of recommendation is “strong,” would be written as “1C.” In the case where the evidence level is “medium” and the level of recommendation is “weak,” would be written as “2B” (Table 2). “Expert judgment” was decided in various meetings as listed.
| Grade for level of recommendation | ||
|---|---|---|
| Strength | Notation | |
| Level 1 | Strong | … is recommended |
| Level 2 | Weak | … is preferred |
| The committee denotes “no grade” as an opinion “O” | ||
|---|---|---|
| Grade | Quality of evidence | Definition |
| A | High | We are confident that the true effect lies close to that of the estimate of the effect. |
| B | Moderate | The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. |
| C | Low | The true effect may be substantially different from the estimate of the effect. |
| D | Very low | The estimate of effect is very uncertain, and often will be far from the truth. |
Chapter 1 Vascular Access-Related Informed Consent
Statements
- The dialysis physician must provide an adequate explanation to the patient and family regarding the necessity of vascular access (VA), which is indispensable for the initiation and continuation of hemodialysis, as well as construction and maintenance methods. It is recommended that the degree of understanding of the patient be confirmed throughout the explanation process (O).
- It must be understood that this explanation be included in the process of informed consent when the patient has agreed to the initiation of dialysis therapy (O).
- When the dialysis physician entrusts the construction of VA to an access surgeon, the access surgeon has the duty to again explain VA in detail to the patient. The topics that should be included in the explanation are shown in Table 1 (O).
- Topics (1)–(7) are essential in the explanation to the patient, but the others may be omitted in the early stage of the disease in consideration of the patient's understanding and be explained after stabilization of the patient's physical and mental condition. It is recommended that all topics be discussed with a representative of the family (O).
- An additional explanation of the chosen surgical procedure must be performed (O).
- Used frequently (cannulation) over a long period, the occurrence of complications over time is unavoidable because of the nature of VA; it is recommended that the topics listed in Table 2 be explained in advance (O).
|
|
Commentary
1. 2. The attending physician has the duty to explain to end-stage renal disease (ESRD) patients about the treatment methods deemed necessary for them 5, 6.
3. Topics that the explanation should cover are listed in Table 1; however, it is best to discuss these based upon the stage of disease, condition, and degree of understanding of the patient. Information should not be limited to what the medical side thinks is necessary; information the patient desires or what we imagine they would want should be included as part of the explanation. It is best not to explain everything at one time but to repeat the explanation in a series of meetings 7, 8.
4. The dialysis physician should provide a broad general explanation, but it falls upon the access surgeon to provide a detailed explanation of the actual VA construction.
5. When the method of VA has been decided upon, a detailed explanation of the necessity and characteristics should be provided, especially in the case of grafts 9.
6. The explanation of VA should include discussion of the various complications that could develop.
Chapter 2 The Basics and Timing of VA Construction
Statements
- If the patient has been diagnosed with chronic renal failure based upon clinical course, or 1) eGFR < 50 mL/min/1.73 m2, 2) proteinuria > 0.5 g/gCr, 3) testing positive for proteinuria as well as hematuria, etc., then the patient should be promptly referred to a nephrologist to improve the prognosis (1-C).
- If hemodialysis has been selected as the treatment for end-stage renal failure, the nephrologist should explain the role and importance of VA in this treatment and refer the patient to an access surgeon as soon as possible (O).
- The access surgeon is required to have sufficient experience and expertise (O).
- The access surgeon should closely examine the veins and arteries in the forearm of the patient through visual inspection, palpation, ultrasound, etc., record the course of the vessels, and make a plan for VA construction. It is important that peripheral circulation and cardiac function be sufficiently evaluated at this time also (1-C).
- VA construction should be considered when eGFR is less than 15 mL/min/1.73 m2 (CKD stages 4 and 5) as well as taking into account clinical conditions (O). In patients with diabetic nephropathy, who have a tendency to show overhydration, VA construction should be considered at a higher eGFR (O).
- When considering VA for hemodialysis on the basis of patency, resistance to infection, and associated complications, AVF should be selected as the first choice whenever possible (1-B).
- Anticipating the start of hemodialysis from the results of various laboratory tests and clinical symptoms, ideally the AVF should be constructed at least 2 to 4 weeks before the initial puncture (O). In the case of an AVG, the time from construction to initial puncture should be 3 to 4 weeks (O).
- AVF and AVG construction may cause adverse effects on cardiac function (1-B). Therefore, in cases where cardiac function is clearly reduced, superficialization of the artery or intravascular indwelling catheter should be selected (1-C).
- VA by vascular catheterization should be well thought out, and indwelling should be performed immediately prior to use (1-C).
Commentary
VA construction basics and timing
1. Taking into consideration the availability of the simple eGFR conversion equation, giving importance to convenience, it was decided to make use of eGFR 8, 10, 11.
Patients who have been introduced to a specialist at the early stages of the onset of renal disease and have received education and guidance as well as overall management have more time until the initialization of dialysis and, when introduced to dialysis, they will have a better survival rate 10. An explanation to each medical department regarding this, as well as obtaining their cooperation, is also vitally important.
2. In the case that hemodialysis (HD) has been chosen as the form of renal replacement therapy (RRT) by the ESRD patient, the nephrologist should explain the necessity and importance of VA to the patient and then refer them immediately to a VA surgeon (Fig. 1) 8, 11.
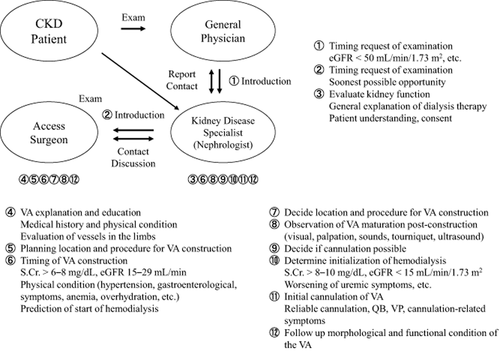
A part and classification of access related pain
3. The patency of the VA is affected by the patient's age, gender vascular condition, underlying disease, as well as other factors and is clearly affected by the surgeon's ability 12.
4. Angiography clarifies the path, diameter, and secondary route of the vessels. It is extremely useful when choosing the vessels for VA construction 13.
5. 6. The VA surgeon is in constant contact with the nephrologist and, when eGFR is 15 mL/min/1.73 m2, considers VA construction. This eGFR value takes into account the time needed for the AVF to mature for cannulation. In cases of diabetic nephropathy, VA construction should be considered at a lower value 13-15.
7. Currently, AVF is the best choice for VA if cardiac function and vascular conditions allow it 16, 17.
8. AVF and AVG construction can have adverse effects on cardiac function such as an increase in cardiac output, etc. Therefore, thorough examination of pre- and post-surgery cardiac function is required 18, 19.
9. Although intravascular indwelled catheters are simple and very useful, because of the various complications reported (injury to the vascular wall, thrombosis, luminal narrowing), their use should be avoided as much as possible 20-25.
Chapter 3 vascular access construction and pre-/postsurgical management
Chapter 3-1 Overall and Local Blood Vessel Evaluation before Construction (Newly Constructed VA)
Statements
- The type of surgery and timing should be determined after performing a preoperative evaluation of overall condition and peripheral circulation (1-D).
- Preoperative evaluation through physical examination (visually and through palpation) is a requirement (1-C).
- In the case the type of VA and location cannot be determined by visual examination or palpation of the vessels, examination by ultrasound is preferred (2-B).
- Angiography is useful in the diagnosis of central vein stenosis or occlusion but it should be performed only after considering residual renal function and possible complications (2-D).
Commentary
1. Preoperative evaluation of overall condition is a requirement. In case of systemic infection or clear cases of malnutrition, dehydration, overhydration, or edema, treatment should be undertaken, and once symptoms have improved, only then should surgery be performed.
In patients where diabetes, collagen disease, or arteriosclerosis has led to peripheral circulation disorders, it is necessary to consider the possible development of steal syndrome following AVF/AVG construction. After construction of AVF or AVG, especially in the elbow, because access blood flow volume increases, the risk of steal syndrome becomes higher 26.
2. Physical examination of the vessels prior to the construction of AVF is the most important aspect in determining AVF's success or failure, and it is vital to spend time performing it. Examination of not only the lower arm but also the upper arm is required. Before performing an evaluation of the vessels, examination of the entire arm is necessary (Table 1).
|
If AVF/AVG were to be constructed on the side where a hemodialysis catheter or pacemaker was placed in the subclavian vein or there has been a past history of breast cancer surgery, venous hypertension 27-29 can occur following construction.
3. For vascular evaluation, as stated previously, visual inspection and palpation are most important. In recent years, ultrasound has gained attention as a method for objectively evaluating vasculature 30-33. The goals of examination by ultrasound are shown in Table 2.
|
Research on the diameter of the radial artery needed for a successful radiocephalic AVF (RCAVF) has shown that the smallest size is between 1.5 mm and 2.0 mm 30, 34-36. The diameter of the radial artery should be at least 1.5 mm: anything smaller will have a lower success rate. Construction in the central region should be considered. But the diameter of the artery is only one indicator for a successful AVF; wall thickness and function should also be included for consideration and then evaluated. The required blood flow for a radial artery anastomosed for use in an AVF is between 20 and 40 mL/min with no set rate 37, 38; at this time there is no satisfactory indicator.
The differences in the size of the veins are recorded before and after application of a tourniquet. Veins of diameter between 1.6 and 2.5 mm after tourniquet application are recommended 30, 34, 35, 39. Ultrasound examination is effective but whether or not it should be applied to all cases is a subject of debate. Nursal et al. 40 evaluated the effectiveness of ultrasound evaluation in an RCT of patients in whom AVF construction was shown possible by physical examination. There was no significant difference in the results between a group that underwent ultrasound examination and a group that did not. They concluded that if the patients had arteries and veins that were in good condition, ultrasound examination was not a requirement.
4. Conditions where central venous stenosis can be suspected are shown in Table 3.
|
Chapter 3-2 AVF Construction and Perioperative Management
Statements
- AVF of the wrist or the tabatière (anatomical snuff box) would be the first choice, but the site of VA construction should eventually be determined by a comprehensive evaluation of the patient's background, general conditions, and local conditions (1-B).
- If AVF construction in the forearm is judged to be difficult or impossible, then consider AVF in the elbow or upper arm (1-C).
- VA should be constructed considering those patient characteristics that will have an effect on patency (1-A).
- The recommended method of anastomosis is an efficient arterial side to venous end type (2-C).
- Arterial spasm can easily occur immediately following surgery; appropriate measures should be taken (O).
- Appropriate monitoring and evaluation of access function should be performed following surgery. In patients where the VA has poorly matured, appropriate treatment is recommended (1-B).
- Cannulation of a primary AVF should take place 2 weeks or more following surgery (2-C).
- Antiplatelet agents should be administered based on the individual requirement of the patient (2-C).
Commentary
1. The advantages of an RCAVF in the distal forearm are listed in Table 1.
|
If the arteries and veins of the anastomosed snuff box are of adequate size, construction of a tabatière AVF is also possible, but in this case an RCAVF can also be chosen. Hatakeyama et al. 41. reported that of 1560 AVF examples, the 1-, 3-, and 5-year secondary patency rates listed by modalities were 61%, 53%, and 44% for tabatière AVFs and very much similar at 70%, 59%, and 54% for Brescia-Cimino AVFs. However, in contrast, there are also reports stating that the secondary patency rates of the tabatière AVF are significantly poorer 42, 43. Because there has been no RCT, it is difficult to choose between the two, and at this point, whichever can be chosen as a first choice.
If no appropriate vessels can be located on the radial side, an AVF constructed on the ulnar side should be considered. An AVF on the ulnar side generally consists of the ulnar artery anastomosed to the basilic vein (ulnobasilic AVF: UBAVF). But if the ulnar arteries are too narrow, an extended length of the basilic vein can be mobilized and anastomosed to the radial artery. The patency rate of the UBAVF is lower than that of RCAVFs 44, but by performing PTA, the secondary patency rates are reported to show no significant difference when compared with RCAVFs 45.
The site of construction should be decided based on the background of the patient, their overall condition, and local findings. Factors that should be considered when deciding on the site for construction are shown in Table 2.
|
2. If the cephalic vein in the upper arm is unusable for AVF construction, the basilic vein can be superficialized and anastomosed to the brachial artery at the elbow to form a transposed brachial-basilic arteriovenous fistula (TBBAVF). If a TBBAVF is to be constructed, ultrasound or angiography should be used to check the path, size continuity, and anastomosis point with the brachial artery of the basilic vein. It is also important to determine if an adequate length can be mobilized. Patency rates of the TBBAVF have been reported to be better than an AVG 46, 47, but stenosis easily develops at cannulation sites. If occlusion occurs, the vein can no longer be used for graft placement; therefore, application of a graft (AVG) should be carefully examined.
3. Numerous reports have found the patency of primary accesses to be low in females, the elderly, and diabetic patients. The patency rates of AVFs in the elderly are significantly low 48, 49. Woods et al. 48 studied 245 examples of AVF failures. In a comparison of the patency rates in those over 65 years of age and those below 65 years of age, they report that those over 65 years of age had a significantly lower patency rate. In a meta-analysis of the AVF outcomes in elderly patients, elderly and younger patients were compared, and at 12 months and 24 months, elderly patients were shown to have a significantly greater number of RCAVF failures 50. Also, there are numerous studies reporting that the patency rates for females 51, 52 and diabetes patients 52, 53 are significantly low.
4. The general method of anastomosis for an AVF is an artery side to vein end anastomosis. The blood flow rates of end-to-end, side-to-end, and side-to-side anastomoses were compared by Kukita et al. 54, and although they found no significant difference, the blood flow in the end-to-end anastomosis was shown to be low. Taking into consideration the ease of anastomosis, low initial failure rate, and lack of complications, we recommend a side-to-end anastomosis.
5. Because of this, the access should be checked for thrill immediately following surgery. If the thrill is weak, an intravenous injection of 2000–3000 units of heparin or low-molecular-weight heparin should be administered.
6. Ohira et al. 55 conducted a questionnaire survey of 23 facilities, calculating the primary failure rates in 5007 RCAVFs. The primary failure rate was reported to be 0.8–23.6% (average: 7.6%), varying widely between different facilities. Approximately 70% of these were reported to be salvageable. In a meta-analysis by Rooijens et al. 56, the primary failure rate was 15.3% (6–34%). Allon and Robbin 57 reported a primary failure rate of 2–53%, showing a wide variation even among authors.
In a recent systematic review regarding the effects of non-maturity following surgery, 21% were reported to be attributed to pre-operation clinical risk factors, 24% to pre-operation hemodynamic factors, while 50% can be attributed to postoperative hemodynamic factors 58. Murakami et al. 59 measured the resistive index (RI) within 1 week of AVF surgery and compared it to the 6-month patency rate. For the 39 examples whose RI was less than 0.6, the patency rate was 92.3%, significantly higher than the 46 examples whose RI was greater than 0.6 with a patency rate of 69.6%. It was reported that in examples where the RI measured soon after surgery was greater than 0.6, the patency rate was low, and examination should be performed for the presence of stenosis. It has been reported that if the cross-sectional area of the radial artery is greater than 8.5 mm2 or venal blood flow rate is greater than 425 mL/min, the rate of maturity for a functional access is 95–97%, respectively 60.
7. From the Dialysis Outcomes and Practice Patterns Study (DOPPS) data presented by Rayner et al. 61, a 2.27 times increased risk of access failure in AVFs that were cannulated within 14 days of construction was reported as opposed to those that were cannulated 43–84 days after construction.
8. A number of RCTs regarding antiplatelet agents following AVF surgery have taken place with ticlopidine and aspirin being reported to be effective 62-65.
Chapter 3-3 AVG Construction and Perioperative Management
Statements
- If an AVF cannot be constructed even though the cardiac function can withstand the burden of access and there are no peripheral circulation disorders, an AVG should be constructed (1-B).
- The arm should be the first choice for the location of the implant but it can also be located in the leg (1-B).
- The shape of the implant can be straight or looped (1-C).
- Artificial vessels can be made of ePTFE, PU, or PEP. These should be used knowing the characteristics of the material (1-C).
- The graft can be placed under local anesthesia (involving brachial nerve block) (2-C).
- Systemic heparinization during surgery may be necessary in some cases (2-C).
- Prophylactic administration of antibiotics prior to and during surgery is recommended (O).
- The goal for primary patency rates 1 year after surgery is 60%. The goal for secondary patency rates (assisted patency) is 80%, 60%, and 40% for 1 year, 3 years, and 5 years, respectively (1-C).
Commentary
1. As an access, the AVF has a better patency rate and lower frequency of complications. For these reasons, AVF is the first choice for an access, but if the appropriate veins cannot be located, an AVG would be the next choice 66-68. Because the access blood flow rate of the AVG is approximately 1000 mL/min 3 weeks after construction, it places a burden on cardiac function soon after surgery 69, 70. However, the limit of the burden placed on the heart with respect to cardiac function that can be tolerated is still unclear.
2. If the AVG is implanted where the vessels are of larger diameter, in a more proximal location, blood flow will be increased and the patency rates improved 71. In order to have as many possible sites for future implants, normally start in the forearm. If the AVG is placed in the thigh, problems caused by infection or circulation disorders are more serious than if the AVG were placed in the arm. Because of this, it is applicable to those cases in which the AVG can no longer be placed in the arm 68.
3. The technically, relatively simple straight type has few applications, while the loop type offers more choices for the vessels that can be anastomosed as well as a larger area for cannulation, giving it a higher frequency of use 72.
4. In Japan, there are currently three types of artificial vessels that can be used 73. Experience with expanded polytetrafluoroethylene (ePTFE) grafts up to now has shown them to be better than other material with regard to resistance to infection, long-term patency, and ease of use 66. However, following implant, a 2- to 3-week waiting period is required before cannulation can take place, and in 5% of the cases, seroma formation is a problem. Polyurethane (PU) grafts, however, can be cannulated early and have early and midterm patency rates equivalent to those of ePTFE 74, 75. However, the graft has a problem as it easily kinks 76. There are ePTFE grafts that are tapered or stepped on the arterial end and others that are cuffed with a hooded style on the venous end. It has been reported that the cuffed grafts improve graft patency rates 77-79. Heparin coating is also reported to improve the patency rate 80. In 2006, polyolefin-elastomer-polyester (PEP) grafts, which can be used early, are easily cannulated, and have good hemostasis characteristics, became available in Japan. The patency rates are nearly equivalent or better than ePTFE or PU grafts 81, 82.
5. Approaches with the axillary vein or internal jugular vein will, however, require general anesthesia 83.
6. Intravenous infusion of approximately 2000 U of heparin following the surgical procedure was found to be effective in those cases in which spasms occurred 84. In a report by Dixon et al. 85, the 1-year primary patency rate improved to 28% (23% for the control group, P < 0.05) with the administration of dypyridamole plus aspirin. Compared with the 1-year patency rates achieved in Japan 82 of 46.3%, it is clearly inferior.
7. Prophylactic administration of antibiotics prior to and during surgery is recommended.
8. One-year primary patency rates for AVG are reported to be 35.3–64.5% 82, 86 with secondary patency rates reported to be 52–85.5% 86-91. The major cause of occlusion is the development of stenosis in the output vein. Secondary patency rates can be improved with monitoring for stenosis and aggressive treatment before occlusion occurs. With this, the goal of secondary patency rates at 1 year of 80%, 3 years of 60%, and 5 years of 40% is attainable.
Chapter 3-4 Arterial Superficialization Construction and Perioperative Management
Statements
- The application of arterial superficialization is recommended for the conditions shown in Table 1 (1-C).
- The first choice for the location of construction with arterial superficialization is the upper arm (1-C).
- The incision for arterial superficialization should be made maintaining adequate distance from the artery so it does not coincide with the area for cannulation (1-C).
- It is important that arterial superficialization not be too deep or not too shallow causing gangrene. The length required should provide an adequate length for cannulation (1-C).
|
Commentary
The superficialized artery is used as the outflow route, and cannulation of a superficial subcutaneous vein is necessary at each hemodialysis session 92.
1. Cases for which arterial superficialization (Table 1) are applicable can be divided into four groups. Group 1 includes those patients in whom cardiac function is insufficient, and construction of an AVF (AVG) will lead to cardiac failure 93. Group 2 includes cases in which vascular damage has occurred, making AVF (AVG) construction difficult 94, 95. Group 3 consists of those whose AVF (AVG) has caused steal syndrome to occur, and Group 4 includes those where it is used as a backup because of frequent VA trouble.
It is known that AVF (AVG) has an effect on cardiac function 93, 96. However, there is little evidence to show what blood flow rate causes a detrimental effect and is a direct reason for cardiac failure.
2. The arteries used in superficialization are the brachial artery located between the elbow and upper arm and femoral artery 95, 97. In Japan, more than 90% make use of the brachial artery 98. The advantages of using the brachial artery include: (i) ease of surgery; (ii) fewer complications; and (iii) possibility to perform with the use of local anesthesia.
3. Because superficialization of the femoral artery involves a greater surface area to be detached than the brachial artery, special attention should be taken for frequently seen complications such as lymph fluid retention and necrosis 99.
4. The length of the incision should allow for an adequate area for cannulation 99.
Other points to be aware of are the following: Immediately following superficialization, there is slight adhesion between the vessel and surrounding tissue, and in many cases, hematomas are formed after needle removal. Because of this, it is best to wait until the wound has completely healed before cannulation, 2 weeks or more (3 weeks if possible) 99. Bleeding should be stopped manually, applying as little pressure as necessary so blood does not seep through. Approximately 20–30 mmHg should be sufficient to stop the bleeding in a superficialized artery.
In complications in arterial superficialization, be careful of included infection, aneurysm, stenosis, and occlusion 99.
Chapter 3-5 Catheterization Methods and Perioperative Management
Statements
-
Non-cuffed catheters are mainly used for conditions that require emergent blood purification; short-term use is recommended (O).
-
Cuffed catheters are mainly used for purposes of long-term blood purification extending for periods of 3 months or longer. Indwelled use is recommended (O).
-
Situations where use of a cuffed catheter is applicable: (i) cases where AVF/AVG cannot be constructed; (ii) cases with severe cardiac failure; (iii) cases where the patient's condition makes this the most appropriate VA method, for example, contracture in the limbs, dementia making cannulation difficult, or where there is a high risk of needle removal during dialysis; (iv) pediatric hemodialysis (2-C).
-
Catheterization using a non-cuffed catheter should take place in a private room or an area partitioned off from the rest of the room. It is recommended that the Seldinger method be used in a clean environment (1-C).
-
It is recommended that catheterization using a cuffed catheter should take place under cleanliness conditions as in an operating room with the use of an X-ray device and using the Seldinger method (1-C).
- 5-1.
It is recommended that an X-ray be taken immediately following the operation.
- 5-2.
It is recommended that the catheter from the point of insertion to distal tip be confirmed upon X-ray.
- 5-3.
It is recommended to confirm by X-ray that there are no complications associated with the catheterization method.
- 5-1.
-
It is important to prepare for perioperative complications associated with hemorrhaging. It is desirable to stop administration of anticoagulants and antiplatelet agents at an appropriate time before the operation (1-C).
- 6-1.
Take appropriate measures for hemostasis if hemorrhaging occurs post-operation. Performing observation as required is desirable.
- 6-2.
Poor blood outflow or increased blood inflow pressure can indicate a kinked catheter. It is recommended that immediate measures be performed.
- 6-1.
Commentary
1. To make hemodialysis catheters more understandable and to increase compliance with the medical reimbursement system, the guideline committee has decided to eliminate the original guideline's naming of “short-term vascular access catheter” and “long-term vascular access catheter” and instead use the two categories of “non-cuffed catheter” and “cuffed catheter” 100.
Hemodialysis catheters are labeled with different names in different countries, and there is no worldwide standard; however, in many cases, they are labeled by their shape 19, 101, 102.
2. The role of the non-cuffed catheter in the treatment of chronic hemodialysis patients includes (i) starting emergency hemodialysis in end-stage renal failure patients and (ii) a method to fall back on in the event that other methods of VA are unavailable; one must bear in mind that its usage should be limited to around 1 month. Even though cases of long-term usage exist with adequate infection management, placement of a safer method of VA should be implemented as soon as possible 103, 104.
3. Indications for cuffed catheter use include (i) cases where AVF/AVG cannot be constructed due to damaged vessels in the limbs or hypotension, etc.; (ii) cases with severe cardiac failure 105, 106; (iii) cases with severe limb contraction, inability to bear the pain of cannulation, unexpected body movement, etc., making cannulation dangerous, or a high possibility of the needles being removed during hemodialysis, which make this a highly appropriate method of VA. (iv) This method is one method of VA that is appropriate for pediatric patients 107, 108. When an AVF/AVG cannot be constructed and the choice is between arterial superficialization and this method, the condition of the patient should be taken into careful consideration when making the decision 109.
- The procedure should be performed by an experienced surgeon or under the guidance of an experienced surgeon.
- A private room or partitioned section of a room should be used to obtain as clean an environment as possible. The procedure should take place under maximal precaution.
- Venal puncture should be performed with ultrasound. The procedure should use the Seldinger method as its basis.
- X-ray should be taken immediately following the procedure to confirm the position of the catheter's distal tip and rule out any complications related to the surgery 112, 113.
- Following the surgery, appropriate observation should be performed for hemorrhaging at the point of puncture.
- The surgery should be performed in an operating room or in an environment and manner with a similar degree of cleanliness.
- The surgery should be performed under X-ray guidance using the Seldinger method.
- Venal puncture should be performed with ultrasound guidance for safety and precision. The right internal jugular vein should be the first choice for cannulation but, based upon the patient's condition, the left internal jugular vein, the right femoral vein, and the left femoral vein could also be possible choices. In order to prevent the occurrence of iliac vein occlusion for patients scheduled for renal transplant, insertion of the catheter through the femoral vein should be avoided.
- The distal tip of the catheter should be placed floating in the right atrium. In cases where the tip of the catheter is placed in the superior vena cava or the innominate vein, even though there may be no problems with inflow and outflow immediately following insertion, in some cases inflow and outflow problems may arise in the future. In cases where the tip of the catheter is placed in the inferior or superior vena cava, care should be taken so as the opening at the tip does not come in contact with the vessel wall 114, 16.
- The position of the catheter tip is an important factor in determining catheter function.
5-1. It is recommended that immediately following surgery, X-rays be taken of the appropriate areas to confirm the position of the catheter and to confirm that there are no complications associated with the surgery 110.
5-2. Careful attention should be paid to the following: (i) deviation of the catheter tip from cardiac or venous images; (ii) a kinked portion or severe twisting of the catheter; (iii) hemothorax or pneumothorax 110.
5-3. A check for hemorrhaging and pneumothorax is required.
6. It is recommended that the appropriate measures for the management of perioperative hemorrhaging be taken as well as performing necessary observation to confirm the absence of complications 110, 114.
6-1. Severe complications associated with indwelling of a central venous catheter are mainly bleeding complications related to puncture. Other complications include malpositioning of the catheter tip and (especially in the case of cannulation of the internal jugular vein) poor outflow or increased inflow pressure.
6-2. Poor outflow and increased inflow pressure related to a kinked catheter can be evaluated by postoperative X-ray.
Chapter 4 Daily Management of Vascular Access
Chapter 4-1 Cannulation
Statements
-
Visual inspection of the access limb prior to cannulation is recommended (O).
-
AVF usage:
- 2-1.
An appropriate waiting period following construction until the time of use is preferred (O).
- 2-2.
When cannulation of a new portion of an AVF is performed, it is recommended to verify whether the vessel being cannulated is an enlarged artery or an arterialized vein (O).
- 2-3.
It is recommended that the site of cannulation be chosen so that it avoids the area surrounding the anastomosis and a site chosen so that the needle will not move with movement in the limb containing the VA during dialysis (O).
- 2-4.
To prevent recirculation of blood, the arterial puncture site should be closer to the anastomosis than the venal puncture site and the two needles should be separated as much as possible (O).
- 2-5.
The cannulation site should be changed each time and sites should be chosen evenly over as wide an area as possible (2-D).
- 2-6.
Apply a lidocaine patch or use the buttonhole method for patients who complain of intense cannulation pain (O).
- 2-7.
The angle of cannulation for an AVF should be approximately 25° (O).
- 2-8.
When removing the needle and stopping bleeding, the tape holding the needle should be removed first, the needle entrance should be covered with a sterile gauze, and the needle removed. Once the needle is removed, a hemostasis clamp can be placed over the gauze and pressure applied to stop bleeding (O).
- 2-1.
-
The buttonhole method:
- 3-1.
The buttonhole method of cannulation can be applied to those patients complaining of intense pain during cannulation (O).
- 3-2.
For buttonhole cannulation, remove the scab formed at the entrance of the fixed puncture route, and insert the specialized needle along the established route (O).
- 3-1.
-
AVG usage:
- 4-1.
The AVG requires a longer waiting period from construction to use than the AVF (2-C).
- 4-2.
Avoid repeated cannulation of the same site; spread the sites evenly over the entire graft (2-C).
- 4-3.
A larger angle is recommended for AVG cannulation than that for an AVF (O).
- 4-4.
For needle removal and hemostasis, first withdraw the needle, and after the tip has cleared the skin, compress the skin over the puncture opening of the graft with appropriate pressure (O).
- 4-1.
-
Superficialized artery usage:
- 5-1.
VA constructed with a superficialized artery requires a longer waiting period following construction until usage. Cannulation should be started after the artery has adhered to subcutaneous tissue (2-C).
- 5-2.
The superficialized artery is used for the outflow of blood, but to prevent the recirculation of blood, a superficialized vein, etc., should be obtained for inflow (O).
- 5-1.
Commentary
1. First, examine the limb with the VA for swelling or redness; next, examine by touch for thrill, pulse, and warmth.
2. Cannulation of the AVF should take place more than 10 days after surgery.
As a VA over a long period of time, the artery, continually delivering blood to the vein, has enlarged in diameter and grown in length as well, and as it winds its way directly beneath the skin, its form is palpable 115.
Repeated cannulation of the VA over a narrow area leads to disruption of the peripheral nerves, causing cannulation pain not to be felt. However, repeated cannulation of the VA over a narrow area enlarges the vessel, making it easier to cannulate. Over the long run, however, this can cause pseudoaneurysms or stenosis in the vessel in the area leading up to or away from the site of cannulation 116. A lidocaine patch should be applied 1–2 h prior to cannulation, 30 min before cannulation at the latest. Cannulation pain is extremely lower with the buttonhole method 117 as compared with routine cannulation.
The angle of insertion of the needle will differ based upon the condition of the blood vessel. A vessel close to the surface or narrow will require a sharp angle, while a deep or large vessel will require a more obtuse angle 118.
3. The buttonhole method using a specialized dulled needle inserted into the same cannulation site every session differs from the cannulation method of repeatedly cannulating the VA over a narrow area 116. In cases with damaged veins or cardiac function disorders, cannulation is performed with an arterial access port 119.
If the tip of the needle is reluctant to enter into the VA vein, raise the needle to an angle of 45° and try to insert again. In many cases, this will allow the tip of the needle to be inserted into the VA vein. If insertion is still not possible, a standard sharp needle passed through the fixed cannulation route can be used, but only for that particular session 120.
4. Edema occurs at the site of construction for AVG using ePTFE as a result of serum seeping out through the multiple pores in the wall of the graft. The higher the internal pressure of the graft, the more severe and longer lasting the edema will be. Cannulation can begin after the edema has subsided and the graft has adhered to surrounding tissue after 2 weeks or more 121. On the other hand, edema is hardly ever present in AVGs using PU or PEP, and hemostasis is favorable; cannulation is possible on the day following construction 115, 81.
Repeated cannulation of the graft at the same site leads to pseudoaneurysms and reduces the useful life of the graft 122. Especially for PU grafts, the graft material being soft, if strong pressure is applied to the cannulation site, blood flow will stop and the risk of graft occlusion will be increased 123.
5. Cannulation of a superficialized artery should begin only after edema has subsided following removal of the sutures, and the subcutaneous tissue and artery have sufficiently adhered. It is reported that this will require approximately 3 weeks following surgery 124, 95.
Chapter 4-2 Prevention of Infection (AVF, AVG)
Statements
- It is recommended that intranasal MRSA carriers be identified and the bacteria eliminated prior to surgery (1-C).
- Carefully inspect the arm with the VA for redness, swelling, or tenderness prior to starting dialysis. If signs of infection are present, avoid puncture in that area (O).
- Prior to disinfection for cannulation, the staff should wash their hands and put on gloves. Gloves should be changed for every patient. The patient should wash the area with the VA well with soap prior to cannulation (O).
- Alcohol or povidone-iodine should be used for disinfecting the skin prior to cannulation. Whichever is used, start application from the site of cannulation and swab outward to the surrounding area (O).
- PTA should be performed in an operating room or angiography room. The staff and patient should use sterile gowns and sterile surgical drapes as well (O).
Commentary
1. Bactroban ointment should be applied prior to surgery in MRSA carriers. The implantation of an AVG is considered to be a sterile operation. There is the opinion that prophylactic administration of antibiotics limited to the time of surgery is appropriate; however, there is greater opinion for administration for 3 days following surgery 125.
2. The three signs of infection must not be overlooked. Procedures for the site of suspected infection in an AVG should take priority, and measures should be taken to prevent bloodstream infection 126, 127.
3. Cannulation involves the risk of bacterial infection. Some dialysis centers use sterile gloves, especially in the case of AVGs. Once infection occurs at the cannulation site, it is difficult to heal, and often the graft must be removed 128-130.
4. One reported method is to first disinfect the skin with alcohol; after that, a 10% povidone iodine is swabbed on and left for 2–3 min 131, 132.
Chapter 4-3 Surveillance and Monitoring of VA Function
Statements
- Establishment of an organized program to monitor VA function is recommended (1-B).
- It is recommended that blood flow be measured for the surveillance of AVF (2-C).
- It is recommended that blood flow be measured for the surveillance of AVG (2-C).
- The measurement of venous pressure should be used to monitor AVGs (1-C).
- It is possible to use the measurement of the recirculation rate for the surveillance of AVF/AVG (2-C).
- It is also possible to use ultrasonography for the surveillance of AVF/AVG (2-D).
Commentary
1. Furthermore, the first item the CSN guideline lists is that the establishment of a monitoring system for VA function is required 133.
A monitoring method would include weekly examination of fistula to evaluate thrill, murmur, and palpation of the entire arterialized vein to check for stenosis, evaluation of pillow condition, extended time for hemostasis, and swelling in the fistula limb 134. For example, the flowchart in Figure 2 shows one method of monitoring and surveillance. Ikeda et al. 135 employ shunt trouble scoring to objectively evaluate VA function and form and have reported good success. As one method of improving access patency, shunt trouble scoring as shown in Tables 1 and 2 for the early detection of access failure in function and form can be used. There are also reports that access surveillance with sophisticated equipment did not improve the patency rate 136-139; what is especially important, though, is the establishment of an access monitoring program.
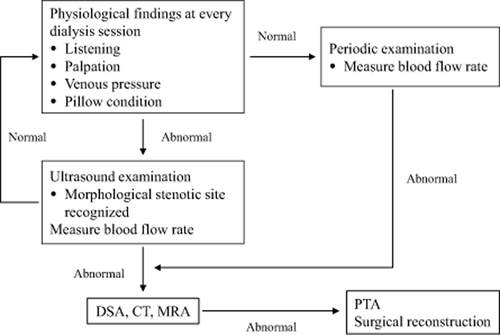
VA Function Monitoring and Surveillance Flowchart
| (Points) | |
|---|---|
| 1) No Abnormalities | 0 |
| 2) Stenosis audible | 1 |
| 3) Stenosis palpable | 3 |
| 4) Increased venous pressure greater than 160 mmHg | (AVF: 1, AVG: 3) |
| 5) Increased hemostasis time | 2 |
| 6) Inadequate outflow (puncture in opposite direction at start) | 5 |
| 7) Insufficient blood flow in last 1 h of dialysis treatment | 1 |
| 8) Shunt sound inaudible | (AVF: 2, AVG: 3) |
| 9) Reduced pillow pressure | 2 |
| 10) Rescued pillow pressure | 1 |
| *3 points or more, DSA or PTA should be considered | |
- (Excerpted from Ref. 135).
| (Main Category) [Categories requiring early PTA] |
|---|
|
- (Excerpted from Ref. 135)
2. The measurement of blood flow is reported to reduce the risk of occlusion by 30% 140-143. Ultrasound dilution, Doppler, Critline, and thermodilution are minimally invasive methods useful for evaluating access blood flow. Measure the access blood flow regularly. If it is less than 500 mL/min or has decreased by 20% or more from a baseline measurement, probable stenosis has been discovered 133.
In Japan, the method most reported for the measurement of access blood flow is the Doppler method: a blood flow of 500–1000 mL/min denoting favorable access function 145-157, with a borderline at 500 mL/min for dysfunctional accesses 148, 153, 157.
3. Ultrasound dilution, Doppler, Critline, and thermodilution are minimally invasive methods useful for evaluating access blood flow.
The Vascular Access Society has not decided on a specific value for the blood flow rate but states that monitoring over time for a trend is effective. The CARI Guidelines have concluded that measuring blood flow did not lower the rate of occlusion for grafts 158. According to reports by Kobayashi et al. 159, who have been using ultrasound blood flow measurement for graft evaluation, comparing the per-patient measurements for 2001, measured 3.4 times/year per patient, and for 2002, measured 4.1 times/year per patient, the rate of graft occlusion was 0.34/year per patient for 2001 and 0.17/year per patient for 2002, showing a reduction and therefore verifying its usefulness.
4. Because the arterial chamber is located closer to the patient's body than the dialysis blood pump in other countries, the arterial pressure can also be measured in the case of grafts. Stenosis of the outflow vein can be determined from the ratio of arterial pressure to venous pressure, but in this guideline, it is shown that static venous pressure is more accurate in diagnosing outflow vein stenosis than dynamic venous pressure, and we look forward to more studies 160, 161.
5. Measure the recirculation rate if possible and use it as a reference. The measurement of the recirculation rate is not by the urea method, but by the dilution method or the method described in Table 3, the urea dilution method.
| Measure after stopping ultrafiltration 30 min after the start of hemodialysis. |
|---|
|
6. Recently, the resistance index (RI) of the brachial artery measured by Doppler ultrasound as an objective index to determine VA failure is being studied. Murakami et al. 162 compared the RI of a group with favorable VA to a group with unfavorable VA; the index was 0.550 ± 0.097 and 0.784 ± 0.089, respectively, with the unfavorable group exhibiting a significantly higher value.
Chapter 4-4 Cardiac Function and Access
Statements
- Vascular access (AVF, AVG) that shunt artery and vein have an effect on cardiac function. If the access shunt volume exceeds cardiac function (cardiac reserve function), then it should be recognized that cardiac failure symptoms will occur (1-B).
- It is recommended to check to make certain that access blood flow does not exceed the patient's cardiac function at each hemodialysis session or when signs or symptoms present the possibility of cardiac failure for dialysis patients with an AVF or AVG (O).
- Clinical manifestations are most important for evaluating cardiac function. Examinations include electrocardiogram and chest X-ray as a basis in conjunction with echocardiogram and Holter electrocardiography (1-C).
- In cases of cardiac failure, closure or banding of the AVF or AVG may bring about improvement in circulation dynamics or echocardiogram findings. However, in cases where cardiac failure is advanced, closure of the access may not provide improvement in the cardiac failure (1-C).
Commentary
1. It is already known that the creation of a shunt affects cardiac function, atrial peptides, and cardiac hypertrophy 20, 163. In the case that shunt flow compared to reserve cardiac function (maximum cardiac output) is relatively large, an appropriate increase in cardiac output is difficult to achieve for an increase in shunt blood flow, systemic circulation is inhibited, and cardiac failure develops 164. As evidence that AVF and AVG alter hemodynamics, closing the shunt in patients without cardiac failure after undergoing renal transplant and in patients who have transferred to indwelled catheters brought about an improvement in hemodynamics after 3–6 months 165-167. It is also known that short-term compression of the shunt relieved the stress on the heart and brought about an improvement in the morphology of the left ventricle 168, 169.
Permanent VA that places no load on cardiac output includes the following:
Arterial superficialization:
Brachial artery, femoral artery
Cuffed catheter
Femoral venous cannulation (extended, flexible indwelled needle) 170
Drop in blood pressure during dialysis: Excessive water removal brings about a decrease in wall motion. This is known to have adverse effects on prognosis 171.
2. To determine whether or not the access blood flow is excessive relative to reserve cardiac function, it is necessary to pay close attention to the patient's clinical characteristics.
3. Shunt blood flow evaluations: Excessive blood flow should be suspected when estimated shunt blood flow exceeds 20% of cardiac output. Cardiac output measured by the thermodilution method (pulmonary artery catheter or peripheral artery catheter) 166, 167.
Until now, use of a pulmonary artery catheter for the thermodilution method was considered the gold standard in the measurement of cardiac output 172, 173. The transpulmonary thermodilution method using central venous or peripheral artery catheters is considered just as accurate but is not generally used 174.
4. Cardiac failure can be improved by closure of the shunt in AVF or AVG patients presenting cardiac failure 175-179. VA will be lost by closure of the shunt, but there are other methods such as banding 180, 181 of a shunt with excessive blood flow or by relocating the shunt to a more distal location from the upper arm to the forearm that can preserve access and improve hemodynamics 182. In the case of banding, if the reduction in blood flow is insufficient, further surgery will be required.
If cardiac failure is advanced, closure of the shunt may not bring about an improvement 168, 179. When considering shunt closure, the amount of improvement should be estimated. For past ischemic heart disease, the more is the time that has passed from shunt construction, the more difficult it may be to achieve improvement 179.
Chapter 4-5 Catheter Management
Statements
- It is best that non-cuffed catheters be left indwelled for no more than 3 weeks. Their use should be limited to hospitalized patients only (2-C). Cuffed catheters compared with non-cuffed catheters are for long-term use (2-D).
- The position of the tip of the catheter should be confirmed after placement. If inserted from the right internal jugular, the preferred position for a non-cuffed catheter is the right superior vena cava; for a cuffed catheter, the outlet is in the right superior vena cava, with the return line near the right atrium (2-C). After being indwelled for a certain period of time, the position of the tip should be rechecked.
- It is recommended that both non-cuffed and cuffed catheters should be filled with an amount of heparin appropriate to their luminal volume at the finish of dialysis. At the start of dialysis, residual heparin and clotting should be removed from the catheter. It is recommended to monitor blood flow rate and arterial pressure to aid in the early detection of complications of occlusion (1-C).
- A sustained infusion of urokinase into the catheter is recommended in the case where blood flow is inadequate or the return of blood is difficult. If this is not effective, in the case of thrombosis in the catheter, replace the catheter on the same side using a guide wire. For external thrombosis, placing a catheter by a different route will obtain blood flow (1-C).
- It is preferred that both non-cuffed and cuffed catheters be connected to and disconnected from the dialysis circuit by two experienced staff members under sterile conditions (2-C) and that the route should not be used for other intravenous infusions (O).
- On the day of dialysis, the catheter exit should be observed for the presence of infection. In addition, it is preferred that each facility establish surveillance for infection and methods of dealing with infection and closing off the routes of infection (2-C).
- Cuffed catheters mainly used for long-term blood purification are indwelled in many cases; for intranasal MRSA carriers, bacteria should be eliminated before placing the catheter (O).
- Catheter infection includes infection of the exit site, tunnel, and interior of the catheter. Slight infection can be treated with antibiotics, but in the case where drainage or unroofing does not bring about improvement or administration of antibiotics is ineffective, early removal is recommended as preferred and safe (1-C).
- Care should be taken to keep water from entering the catheter and steps taken to prevent infection during bathing or showering for patients with cuffed catheters (2.C).
Commentary
1. Although there is no clearly defined limit to the number of days for use of a non-cuffed catheter in Japan, the previous guideline stated 3 weeks. The reason for this was that catheter insertion would cause central venous stenosis 103. For this reason, as short a time as possible is best. K/DOQI 19 however recommends use in hospitalized patients only for a period of 1 week or less.
2. In a person of average build, insertion of a catheter through the left internal jugular vein, which is sized in length for right-side insertion, the tip will fail to reach the right atrium in many cases, as it must curve going from the innominate vein into the right superior vena cava. In this case, a long 19–22 cm catheter should be used 113. With regard to determining the catheter tip position, Vesely et al. 114 have stated that determining catheter position is affected by many clinical factors such as catheter type, insertion location, patient physique, habits, and the purpose of the catheter, and to achieve the best results for dialysis, the tip should be placed in the upper right atrium. They also state that standard chest X-ray is insufficient to determine tip position 114.
The catheter tip is already located within the lumen of the vessel; it is important to keep it from coming in contact with the vessel wall or internal cardiac wall. With regard to adhesion, Miyata et al. 105 have altered the patient's position during dialysis or during insertion, maintaining that it is important that the tip be located properly within the right atrium. It is necessary to reconfirm the position of the tip of catheters that have moved from near the entrance of the right atrium 114.
3. In an effort for early detection of occlusion or inadequate outflow, monitor the blood flow volume and venous pressure during dialysis. For inadequate outflow, sometimes the input and output lines are connected in reverse; this method provides a temporary increase in blood flow but there is the possibility that the recirculation rate will increase 183. In this case, catheter angiography should be performed to determine the cause and treatment provided to correct it.
Agharazii et al. 184 have reported that heparin may leak from a catheter that has been filled with too much heparin, leading to a bleeding tendency. There are numerous reports from Europe and the United States of filling catheters with citrate (citrate-taurolidine) to control infection and thrombosis but there have been no clinical reports in Japan.
The effectiveness of the use of antiplatelet and anticoagulants to prevent thrombosis in catheters is unclear. The dominant opinion is that 1 mg of mini-dose warfarin is not effective 185. On the other hand, there are reports where significant thrombotic events have been reduced with dosages of aspirin showing anticoagulant effects 186 and high-dosage warfarin 187. Ervo et al. 188 suggest anticoagulants for all patients with catheters except when there are contraindications.
4. A high incidence of bacteremia has been reported in cases where frequent urokinase infusion has been used for inadequate outflow 189.
In the United States, the use of balloon pressure to crush the thrombus is common; brushing of the lumen or fibrin sheath stripping are not recommended because of a lack of persuasive data and because of cost and an increased death rate 190.
It is possible to exchange the catheter on the same side if the inadequate blood flow is caused only by thrombosis in the catheter and if washing or suction were ineffective, but insertion by a different route is recommended if stenosis of the vein, thrombus formations along the wall, or a fibrin sheath exist around the catheter 191. IVR techniques are used for stenosis or occlusion of the central vein during catheter insertion or after removal. Because the access surgery may end in failure due to central venous occlusion, it is the opinion of many reports that the catheter should not be inserted unnecessarily into the central vein for access construction after catheter removal 191-193, 25. When exchanging a non-cuffed catheter for a cuffed type, the central vein can be preserved, limiting exchange over a guide wire using the same route 194.
5. After undergoing training, the actual connection and disconnection are best performed by a team of two staff members. Along with equipment operation, roles should be clearly defined, leading to a reduction in infections 195. In an evaluation of central venous catheter (CVC)-related infections in dialysis patients in whom connection and disconnection were performed by two staff members using sterile techniques, the occurrence rate of bacteremia in this group was 0.70/1000 CVC days, low compared with another report of 3/1000 CVC days, thus showing the effectiveness when the procedure is performed by two persons 127. The connector of the catheter uses a closed system (Planecta) and is replaced once a month. Povidone is used as a disinfectant by many facilities 196, 197.
There are some facilities like in Itou et al. 198 that use 0.5% chlorhexidine to disinfect the entire catheter.
6.
- (1)
Insertion site contamination (catheter care—exit Site)
In general, the use of chlorhexidine alcohol (2% chlorhexidine gluconate in the United States, 0.5% chlorhexidine gluconate in Japan) or chlorhexidine gluconate aqueous solution as noted in the CDC Guideline or K/DOQI 2006 184 is more common than povidone-iodine. Povidone-iodine can no longer be used because of an increased use of silicon material in catheters, and forbidden iodine use. Valente et al. 126 compared the wound infection rate with the use of saline solution and tap water in the cleansing of wounds in children. With an infection rate of 2.8% for saline solution and 2.9% for tap water, they found no difference; using tap water to cleanse the exit site maintained cleanliness of the surrounding skin and was considered to be effective in preventing infection, provided that no infection exists beforehand. Observational studies on the use of tap water for cleansing have appeared in Japan 199-201, but because no large-scale comparisons have been conducted, further research is warranted.
- (2)
Dealing with infection of contaminated medicines.
Frequent administration of urokinase alone can be a risk factor 189. Klevens et al. 196 have a surveillance system in which the occurrence rate of infections and a comparison with other outpatient dialysis centers is possible over the Web. The establishment of a surveillance system for infected catheters over the Web is also desired in Japan.
Recently, insertion of cuffed catheters in place of non-cuffed catheters has been used as a “bridge,” but the use of cuffed catheters in place of non-cuffed catheters did not reduce catheter-related bacteremia and resulting infectious endocarditis 128-130.
According to a study on infection risk factors by Jean et al. 189, in a comparison of bacteremic and non-bacteremic catheters, patients with frequent urokinase catheter infusion and exit site infection showed frequent incidence compared with other factors such as diabetes mellitus, arteriosclerosis, previous history of bacteremia, Staphylococcus aureus (SA), long catheter survival time, and higher intravenous iron dose. On the other hand, monovariate survival analysis showed that nasal carriage of SA, previous history of bacteremia, arteriosclerosis, and diabetes mellitus were significant risk factors for bacteremia 189.
Surveillance of indwelled catheter-related bloodstream infections in Japan was begun by Morikane 202. Infection rates were reported by Azuma et al. 194 for 90 catheters in total inserted in 82 long-term patients in an 8.5-year period from June 2000 to the end of December 2008: there were eight (9.8%) examples of catheter-related bloodstream infection and a 1000 catheter/day of 0.28.
7. Jean et al. 189 performed surveillance of the risk factors and outbreak of central venous catheter (CVC)-related bacteremia for the years 1994–1998. SA was suggested to have been the cause of the bacteremia. Thirty-five percent of the patients with inserted catheters were shown to be nasal carriers of SA.
8. For catheter bacteremia, do not wait for the results of the blood culture; start intravenous administration of antibiotics. For catheter bacteremia, it is possible to treat with antibiotics such as vancomycin or ciproxan, but in the case of non-cuffed catheters, the rate of successful treatment without removal of the catheter is only 19% 203. In addition, antibiotic treatment with the catheter in place will require 10–21 days 204. In the case of cuffed catheters, to eradicate the bacteria in the biofilm of the catheter, an antibiotic lock was tested 205; however, the antibiotic dosage, method of administration, etc., have not been standardized. Furthermore, caution is necessary because it is known that approximately 2% of the patients undergoing treatment by catheter lock for catheter-related infectious bacteremia suffer secondary infection by Candida 206.
9. To maintain cleanliness of the catheter insertion site and surrounding area, there are facilities that allow showering with the site uncovered 199 and facilities that cover the site with Lapack, etc., and allow bathing 196. Improving catheter management is not something that can be accomplished in a short period of time. We support the opinion that all members of the team should make concerted efforts for continuous quality improvement 19.
Chapter 4-6 Patient Education
Statements
- It is recommended that the patients themselves understand well that dialysis medical care is a complementary collaboration between the patient, their family, and the dialysis staff (O).
- Even though the patient may have reached understanding and recognition at the start of dialysis, their feelings may waver at any time, and the dialysis staff needs to understand this (O). With this in mind, it is recommended that the dialysis staff take care to be close and provide support (O).
- It is recommended that the dialysis staff, after having defined the needs of each patient, constantly provide them with appropriate guidance and encouragement (O).
- It is recommended that the dialysis staff always show interest in any questions, dissatisfaction, or complaints the patient may have regarding VA and treat them sincerely (O).
- Subjects for VA-related guidance are shown in Table 1. It is recommended that these be repeatedly discussed and the level of patient understanding confirmed (O).
|
Commentary
1. Opinion is above.
2. Ueda 207 defines acceptance periods for disability as 1) period of shock, 2) period of denial, 3) period of confusion, 4) period of solution seeking, and 5) acceptance period.
3. Patients can be categorized into cooperative/uncooperative, optimistic/pessimistic, trusting/distrusting, independent/dependent, etc., but many patients will not fit into only one category, and it must be understood that they will show many different aspects 208.
4. Many patients are apt to have feelings of anxiety or dissatisfaction toward the continuous use of the VA, cannulation, or the pain felt during cannulation 209, 210.
5. The role of the patient in VA care to maintain the VA's function and form is, in fact, very important. It is important to explain the topics listed in Table 1 in easy-to-understand terms.
Chapter 5 Management of Vascular Access Trouble
Chapter 5-1 Stenosis and occlusion
Statements
- Because stenosis of the VA could possibly adversely affect untroubled performance of hemodialysis and the efficiency of dialysis, the proper diagnosis and evaluation of the stenosis are essential (1-C).
- Treatment methods for VA stenosis include interventional methods and surgical methods, but the choice of method not only should be based on diagnosis of the site and degree of stenosis but should also take into account the results of previous treatment and the reoccurrence of stenosis. Conditions for treatment of VA stenosis are a stenosis of 50% or greater and one or more of the following clinical abnormalities 211: decreased blood flow, aneurysm formation 212, elevated venous pressure 213, abnormally high BUN 214, unexplained reduction of dialysis efficiency 215, abnormal physical condition (1-B).
- VA occlusion is not only an obstacle in the performance of hemodialysis, but requires immediate treatment to prevent the spread of complications due to thrombosis (1-C).
- Treatment of VA occlusion is possible by either intervention or surgical methods. First is the performance of procedures for thorough and safe removal of the thrombus or use of thrombolytic therapy, while at the same time giving attention to and treatment for the cause of the occlusion are necessary (1-C).
Commentary
1. [The basics of diagnosing VA stenosis]
- Visual inspection: Stenosis can be viewed clearly with application of a tourniquet, but also inspect for skin color and edema.
- Palpation: The firmness of the stenosis can often be palpated. The neighboring veins dilate with the application of a tourniquet, making it easier to determine the site and degree of stenosis. Proximal to the stenosis, thrill can be felt relatively easily, but distal to the site of stenosis, thrill cannot be felt and becomes pulse-like.
- Auscultation: Auscultation of the site of stenosis reveals a high-frequency sound: more distal from the site reveals an intermittent sound and proximal to the site provides a continual sound.
- Ultrasound: A highly noninvasive method with few complications, showing widespread general use. Sharpness of the image has increased in recent years, and ultrasound equipment with improved image quality geared specifically for dialysis has been developed 213.
- Angiography: The most suitable method to diagnose the degree and site of stenosis as well as obtain an overall view of the access 214.
- (1)
Selection of treatment method: (i) Proximal venous stenosis: balloon PTA intervention therapy is the first choice, but in case the PTA is unsuccessful, preparation for backup surgical treatment is necessary. (ii) Stenosis close to the arteriovenous anastomosis: If a guide wire can pass through, PTA is the preferred choice. (iii) Stenosis at the site of arteriovenous anastomosis: PTA using the exact-size balloon is possible, but in cases where veins have been damaged, surgical reconstruction is preferred 215-218. (iv) Arterial stenosis: Consider PTA using the appropriate-sized balloon. (v) Graft-vein anastomosis stenosis: PTA using a high-pressure balloon catheter can be performed, however, for extremely rigid stenosis; PTA using very high-pressure balloon catheters or a cutting balloon is recommended 219. (vi) Surgical reconstruction should be added to the list of choices for cases that have required two or more PTA treatments for stenosis in a 3-month or shorter time period 220-222. (vii) The usage of stents is indicated for proximal veins from the elbow above, which will not be used for cannulation, but as a rule, their best application is to central venous stenosis in which elastic recoil has occurred 211, 216.
- (2)
Indication for balloon PTA: Examples of indications of PTA for stenosis, in the case of AVF, are the situation where (i) blood outflow is less than 180 mL/min when cannulation is in the direction of the anastomosis; (ii) the stenosis is less than 2.5 mm in the anastomotic site, near the site or in a runoff vein as shown by DSA. There are stenotic sounds; the stenosis is palpable or blood outflow worsens in the latter half of the dialysis session. In the case of AVG, (i) when there are two puncture sites in the graft, recirculation is considered due to increased venous pressure and the recirculation rate is 10% or more, and (ii) DSA is performed because of extended hemostasis time, increased venous pressure, and decreased shunt sounds, and the DSA shows a stenotic site measuring less than 2.5 mm 223.
There are noncompliant and semi-compliant balloons for a choice of compliance but either can be chosen if the pressure they can withstand is high (greater than 15 atm) 224.
- (3)
Indication for stent placement: Absolute indications for stent placement include cases in which recoil is brought about by balloon PTA and cases of obstructed blood flow due to extravascular exclusion in the central vein. In addition, relative indications include frequent stenosis within 3 months after undergoing balloon PTA, obstructed blood flow caused by pressure on the vessels by extravascular hematoma, which is due to damage during dilation, and high possibility of re-occlusion due to residual thrombus that could not be removed 225.
[Explanation of treatment conditions for VA stenosis]
For VA treatment conditions you can refer to these shunt trouble scoring values created based upon more detailed conditions referring to GL-4 213, 226.
3. VA occlusion consists of thrombotic occlusion and non-thrombotic occlusion, and the major cause of thrombosis is stenosis. Other causes include hypotension, dehydration, hyperanticoagulation ability, surgery, pressure to the cannulation site, and infection. Occlusion occurs more readily when stenosis is already present, and one of these secondary factors is added. Treatment of occlusion promptly within 48 h of its occurrence is preferred in order to avoid catheter insertion or to shorten the duration of catheter insertion 227, 228.
4. Surgical treatments: Because the success rate of interventional treatments is lower 222 when treatments are carried out after an extended lapse in time following the onset of occlusion, even in cases of thrombotic occlusion, surgical treatments are indicated. Thrombus removal should be performed with the use of a thrombus removal catheter. If the removal procedure is unsuccessful, re-anastomosis or graft bypass should be performed 227-229.
[Treatment for non-thrombotic occlusion]
Interventional treatment is possible for non-thrombotic occlusion if a guide wire can pass through the occluded site.
Chapter 5-2 Aneurysm
Statements
- It is recommended that the diagnosis of aneurysm be performed by interview for medical history, physiological examination, and, if necessary, ultrasound examination (O).
- Emergency surgery is recommended for cases of impending rupture, aneurysms associated with infection, and aneurysms that rapidly increase in size (O).
- For non-emergency aneurysms, it is preferable to decide whether surgery is indicated and the type of surgery based upon the type of vascular access, the size of the aneurysm, its position, calcification, presence of thrombus on the wall, access blood flow, and the presence of stenosis (2-D).
- It is preferred to prevent aneurysm formation associated with cannulation (2-D).
Commentary
1. Shunt-related aneurysms are categorized as shown in Table 1 by wall structure, site, and cause of formation.
| 1) Categorized by wall structure |
| a. True aneurysm: aneurysm maintains vessel wall structure |
| b. Pseudoaneurysm: aneurysm without vessel wall structure |
| 2) Categorized by access type |
| a. AVF aneurysm |
| b. AVG aneurysm |
| c. Superficialization aneurysm |
| 3) Categorized by site |
| a. Shunt anastomosis aneurysm |
| b. Non-anastomosis aneurysm |
| 4) Categorized by cause of formation |
| a. Cannulation-related aneurysm |
| > Pseudoaneurysm caused by cannulation or hemostasis error |
| Aneurysm caused by repetitious cannulation |
| b. Non-cannulation-related aneurysm |
| > Partial increase of internal pressure due to jet flow |
| Anastomosis site aneurysm of aneurysm following stenosis |
| > Increase in internal pressure |
| Aneurysm due to increased internal venous pressure caused by stenosis |
| > Excessive blood flow |
| In many cases the entire vein is dilated |
The main points to be investigated by ultrasonography are shown in Table 2.
|
2. For aneurysm treatment, the first thing to be determined is whether emergency surgery is indicated or not. Aneurysms at risk for rupture and those associated with infection are indications for emergency surgery.
3. For anastomotic aneurysms with excessive blood flow, banding of the inflow artery, the radial artery, is performed, as it is effective in reducing the blood flow volume and internal pressure. Resection of the aneurysm and reconstruction at a more proximal site is possible, but caution is needed so excessive blood flow does not develop. For aneurysms that are the result of venous stenosis in which excessive blood flow is not involved, PTA is effective for the stenosis while surgical resection of the aneurysm is not always necessary. It is also possible to resect the aneurysm and reconstruct at a site proximal to the venous stenosis 230.
The use of a tourniquet on the upper arm to temporarily interrupt blood flow should be considered for extremely large aneurysms 231.
Pseudoaneurysms formed by cannulation of a graft (in the case of grafts, all are pseudoaneurysms) have a high risk of rupture because there is no vessel wall 232, 233.
4. Aneurysms associated with cannulation include those due to repeated cannulation of the same site causing the wall to become thin and pseudoaneurysms caused by inadequate hemostasis after removal of needles during hemodialysis. Both of these can be prevented 234, 235.
Chapter 5-3 Venous Hypertension
Statements
- Venous hypertension is a condition of venous insufficiency in shunt blood flow affecting the AVF or AVG limb locally or in its entirety, and if symptoms continue, early testing and treatment are desirable (O).
- Angiography is the desired method for diagnosis and evaluation of venous hypertension (2-C).
- Surgical reconstruction methods are available for treatment of venous hypertension as well as less invasive interventional methods such as balloon PTA and stents (2-C).
- Treatment for central venous stenosis only when significant swelling occurs in the shunt limb or when the patient complains of pain. If the edema is slight or there is no effect on dialysis, treatment is unnecessary (O).
- In extremely advanced venous hypertension that is not indicated for interventional or surgical treatment, the VA will have to be closed and a new VA can be constructed on the opposite limb (O).
Commentary
1. Swelling in the shunt limb is the most outstanding characteristic; its form varies from swelling of the entire limb to the shoulder, the forearm from the elbow down, to only the palm of the hand.
2. The level of venous hypertension is determined by shunt blood flow volume and the degree of venous stenosis proximal to it, branching veins, and permeability of the vessels. The cause of the proximal vein stenosis includes internal stenosis due to indwelling of a dialysis catheter into the subclavian vein 236, 27, 28, intimal thickening at the vessel junction (thought to be caused by turbulence), etc., as well as reports of stenosis due to pressure outside the vessel wall. Patients who have undergone surgery for breast cancer or with implanted pacemakers can easily develop venous hypertension. Catheter insertion in the subclavian vein should be avoided because of the high possibility that it can cause venous hypertension 237.
3. 4. 5. Intervention should be the first choice for venous hypertension caused by central venous stenosis 238-243. However, intervention should be avoided in cases where the risk of vessel rupture by intervention is high.
Chapter 5-4 Steal Syndrome
Statements
- Steal syndrome is a peripheral circulatory disorder that develops due to the construction of VA; it is recommended that the condition and cause be examined (1-B).
- It is recommended that the proper diagnosis and evaluation be performed for steal syndrome (1-C).
- Determining the severity of steal syndrome is important, and Fontaine classification can be used to determine the severity (O).
- Improvement in blood flow of a restricted access, ultrasonography, angiography, and digital brachial pressure index (DBI < 0.6) can be useful in the objective evaluation of steal syndrome (1-C).
- It is recommended that the treatment method for steal syndrome be based upon its condition and severity (1-C).
Commentary
1. Steal syndrome has a high rate of occurrence in patients with peripheral circulatory disorders such as the elderly, those with diabetes, SLE, etc., in patients complicated with occlusive arteriosclerosis, and in patients with reduced peripheral arterial blood flow due to frequent access surgery 244-249. According to reports, the frequency of occurrence differs from 1% to 9%, and compared with the 0.35–1.8% of forearm accesses, the more frequently constructed accesses using the brachial artery have a higher rate at 4–9% and the symptoms in many cases are more serious 248, 250-252. In addition, the occurrence rate is higher in the forearm when grafts are utilized compared with native vessels.
In addition, peripheral steal syndrome is not the only possibility to consider. Subclavian steal syndrome must also be kept in mind as VA construction and excessive blood flow can lead to stenosis in the subclavian artery or vertebral artery causing ischemic symptoms (dizziness, headache) in the vertebrobasilar artery 253, 254.
2. The ischemic symptoms of the fingers (pain, numbness, coldness) that develop after VA construction are the main complaints in steal syndrome; however, an objective evaluation is required to obtain a proper diagnosis. In patients who originally have carpal tunnel syndrome (CTS), reduced blood flow to the nerves brought about by the access can cause CTS symptoms to appear. Especially in long-term dialysis patients, in many cases, CTS symptoms appear following the construction of AVF or AVG at the elbow 255.
- Stage I: Reduced DBI with coldness and paleness of the fingers
- Stage II: Pain during dialysis or exertion
- Stage III: Pain at rest
- Stage IV: Ulceration and necrosis of the skin
4. Among these, it has been reported that a DBI of less than 0.6 following VA construction has a sensitivity of 100% and specificity of 76% and is shown to be effective in the diagnosis of steal syndrome 257, 258.
- Digital brachial pressure index (DBI)
- Ultrasonography
- Laser Doppler flowmetry
- Angiography (DSA)
- Plethysmography
- Thermography
5. The onset of ischemic monomeric neuropathy (IMH) within 24 h of access construction is indicated for emergency surgical access closure due to the possibility that the condition will be irreversible 259, 260. When ischemic symptoms advance, such as significant coldness or numbness, follow-up with surgical banding or drug therapy is possible if neuropathic symptoms are absent (Fig. 3). Perform an objective evaluation in a Stage II AVF or Stage III or IV AVF or AVG, necessary to determine if stenosis is in a proximal or peripheral artery. If stenosis is in a proximal artery, PTA is effective (Fig. 4a). Surgical banding of the outflow vein near the arteriovenous anastomosis can be performed in a clear case of excessive blood flow, but for Stage III or IV, in many cases, surgical closure of the access can be necessary 261. Also, for elbow or upper arm access, ligation of the distal brachial artery for forearm ischemia, distal vascularization-interval ligation (DRIL), has been shown to be effective (Fig. 4b) 262-264.
VA function monitoring and surveillance flowchart.
Elective treatment for steal syndrome.
Chapter 5-5 Excessive Blood Flow
Statements
- VA (AVF, AVG) affects hemodynamics and cardiac function. It should be recognized that excessive blood flow is an exacerbating factor (1-B).
- The symptoms of excessive blood flow should be thoroughly checked and a proper evaluation performed (1-C).
- High-output cardiac failure can result from increased VA blood flow, so it is important to verify the clinical symptoms to make a diagnosis (1-B).
- It should be recognized that excessive blood flow is a cause of peripheral steal syndrome and an exacerbating factor (1-D).
- Excessive blood flow can lead to subclavian steal phenomenon (syndrome); caution and observation are required (2-C).
- Treatment methods appropriate to the conditions should be chosen for treatment of excessive blood flow (1-B).
Commentary
- VA is essential for providing hemodialysis but it is non-physiological and artificially short-circuits circulation, and the construction of VA clearly has an effect on hemodynamics and cardiac function 20, 163, 265-270. It is therefore difficult, at present, to precisely define a blood flow volume as excessive blood flow 267. However, when presented with a cardiac function disorder (ischemic heart disease, dilated cardiomyopathy, hypertrophic cardiomyopathy, valvular heart disease, myocarditis, etc.), even though access flow may not be high, it is important to recognize that excessive blood flow may occur and perform evaluation 176, 266, 268-271.
- It can occur soon after construction for upper arm AVF or AVG 176, 272, 273.
2.
-
The symptoms accompanying excessive blood flow are shown in Table 1. Multiple symptoms often exist 274. Question the patient carefully and perform thorough observation, palpation, and auscultation 19.
Excessive blood flow should be suspected and a medical examination should be carried out, especially when the following symptoms are present 272:
-
Methods for dealing with excessive blood flow need to be considered, especially when cardiac failure manifests as described below 274.
Excessive cardiac load (stress) due to impaired cardiac function:
- Left ventricle ejection fraction (EF) < 50%
- Tricuspid regurgitation pressure gradient (TG-PG) > 30 mmHg
- Pulmonary artery (PA) pressure > 40 mmHg (greater than moderate pulmonary hypertension)
- Aortic stenosis: left ventricle-aorta pressure gradient > 75 mmHg
- Mitral regurgitation > III degree
- Sick sinus syndrome
- New York Heart Association classification: Class II or greater
|
[Evaluation of excessive blood flow]
High-output cardiac failure may occur if VA flow (Flow) is greater than 1500–2000 mL/min or if Flow/CO is greater than 30–35% 175, 267, 268, 272, 274, 276.
3. The symptoms of high-output cardiac failure are shown below 265-274: dyspnea during exertion, palpitations, edema in lower limbs, loss of appetite, orthopnea, etc. The NYHA classifications are concise and serve as a reference.
NYHA (New York Heart Association) classification:
- Cardiac disease present but no symptoms shown.
- Slight limitation of physical activity. No symptoms at rest but symptoms appear during normal daily activities.
- Marked limitation of physical activity. No symptoms at rest but symptoms appear during less than normal daily activities.
- Inability to carry on any physical activity without discomfort. Symptoms may be present at rest; symptoms worsened by physical activity.
4. One of the causes of peripheral steal syndrome is excessive blood flow. It must also be considered as one of the factors aggravating the condition 276.
5. In subclavian steal phenomenon (syndrome), steal from the subclavian artery is caused by excessive blood flow, leading to ischemic symptoms of the vertebrobasilar system. The symptoms that develop are transient cerebral ischemic symptoms (dizziness, nausea). Generally, the cause is stenosis or occlusion of the subclavian artery, but in some cases, it may be caused by excessive blood flow 253, 277, 278. If stenosis exists, even though slight, excessive blood flow will become an exacerbating factor 279, leading to more pronounced symptoms.
- In cases diagnosed with excessive flow, first dry weight (DW) and blood pressure control, moderate exercise, and diet and drug therapy can be initiated and then reevaluation performed 272. If improvement is not noted, surgical procedures can be undertaken. Surgical methods include methods to regulate blood flow as well as performing shunt closure for non-shunt access methods 274.
- There are various surgical methods for restricting blood flow 274. These include banding or interposition of a graft, ligation of the feeding artery (distal side, proximal side), placation 274, 280, distal revascularization-interval ligation (DRIL) 281-284, revision using distal inflow (RUDI) 285, 286 as well as construction of a VA on the opposite side. Of these, banding has been associated with recurrence of excessive blood flow in a large number of cases 287. To solve this, there are reports of methods such as banding a 10 cm length of the outflow artery 288, and banding of the feeding artery 289, 290.
- When the access flow is <400 mL/min in an AVF and <600 mL/min in an AVG, there is an increased risk of access occlusion and impaired outflow 291, 292 and there is a limit to what restricting blood flow can do. If excessive blood flow symptoms (especially high-output cardiac failure symptoms) are not improved even after restricting blood flow or if cardiac function shows a remarkable decrease, the access will need to be closed and a non-shunt access (arterial superficialization, long-term indwelled catheter, A-A jump graft, direct puncture of the femoral vein) will need to be considered 267, 274.
Chapter 5-6 Infection
Statements
- It is necessary to give attention to local infection (1-C).
- It is necessary to give attention to systemic infection (1-C).
- The expanse and rate of progression of infection require proper evaluation (1-C).
- It is recommended that surgical procedures be given priority for AVG infection (O).
- The risk of rupture is high for infected aneurysms, thus requiring surgical procedures (O).
- Catheter infection must be distinguished between 1) exit-site infection, 2) tunnel infection, and 3) intraluminal infection, and treatment applied based on this distinction (1-B).
Commentary
1. Observe the puncture site for redness, warmth, pain, purulent drainage, skin ulcers, and induration at every dialysis session. The algorithm presented in Figure 5 can be used for infection at the puncture sites of AVF or AVG 293-305.
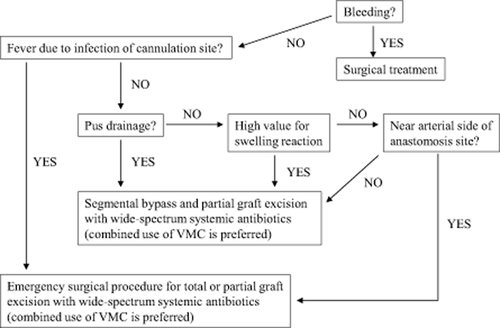
AVG cannulation site infection protocol.
2. For systemic infection, total graft excision along with partial blood vessel resection is necessary. Continual observation of fever, WBC, and CRP as well as blood cultures should be performed. Gallium scintigraphy is useful for grafts 293-305.
3. Evaluation should be performed in a stepwise manner as per the algorithm and the necessary procedures performed.
4. The bacteria causing infection is an indigenous skin bacteria; for septicemia 90% are Staphylococcus aureus, 40% being MRSA; therefore, combined usage of vancomycin and broad-spectrum antibiotics is desired 296, 299-301.
5. The risk of rupture is high, and emergent surgery for drainage and resection of the aneurysm is required.
6. Catheter infection is categorized as exit-site infection, tunnel infection proximal to the cuff, and intraluminal infection. Exit-site infection can be improved by local treatment, oral antibiotics without the use of intravenous antibiotic administration; however, prolonged treatment can cause fungal infection. Unroofing can be used for tunnel infection near the cuff, but if the cuff becomes exposed, the catheter can easily be pulled loose or may be difficult to fix in place. Once improved, change the exit site of the catheter. At this stage, exchanging the catheter over a guide wire is possible 306.
Periodic observation through the dressing is required for subcutaneous tunnel infection.
Intraluminal infection must meet either criteria A or criteria B for laboratory-confirmed bloodstream infection (LCBI) 306. The clinical sepsis criteria are for infants less than 1 year old in the United States, but was added as a criteria for hemodialysis catheters in Japan because the examination is performed in all cases, not just confirmed cases. When there are sources of infection other than the catheter, bacteria can be embedded in the catheter; examples of inserted foreign objects that could be sources of infection are ports for nutrition and pacemakers. Catheter infection can also be the cause of metastatic infection 105, 307-310.
Antibiotic lock 311-317: Because biofilm is a major cause of catheter-related infection 315, it can be the deciding factor for removal or exchange. If bacteremia is present, first remove the catheter and then treat with anticoagulants. If the thrombus is larger than 2 cm, and if infection is present, surgically remove the thrombus and treat with antibiotics and anticoagulants 318-321. Sai et al. reported on two cases in which large thrombus on the tip of an indwelled hemodialysis catheter was removed by open heart surgery; both were removed by thoracic surgery 322-324. A prospective study of central venous catheters in the ICU pointed out that the risk of sepsis increased when thrombus existed 22, 325, 326.
On the other hand, however, it has been reported that catheter-related bacteremia is present but the relationship between bacteremia and intracardial thrombosis is not clear 327.
[Treatment of infection in other countries]
Randomized prospective study: Seventy-six catheters in 58 patients were divided into two groups and compared. There were four cases of catheter-related sepsis in the heparin group and no cases in the group using a citrate-taurolidine lock. It was found that filling the catheter with citrate-taurolidine was effective in preventing catheter-related bloodstream infections 190.
Chapter 5-7 Seroma
Statements
- Seroma is a complication specifically related to ePTFE grafts. It does not develop with blood vessels or other artificial grafts (1-B).
- Seroma is thought to develop due to the structure of ePTFE, but the true mechanism is currently unknown (O).
- The frequency of occurrence of seroma is low (2-C).
- Diagnosis is easy, if it can be confirmed by angiography or ultrasound that there is no blood flow within the mass (O).
- Surgery can include incision drainage, resection of the mass, partial replacement, or complete removal of the graft, but the most certain method is replacement of the graft with resection of the mass (2-C).
Commentary
1. Seroma is a complication that develops in internal shunts constructed with the use of ePTFE grafts 328-333.
2. The mechanism for seroma formation is a lack of endothelial cell formation on the inner wall of the graft and a lack of adhesion to surrounding tissue along the outer wall.
3. The frequency of occurrence for seroma has been reported to be from 1.7% to 4.2% 333-336.
4. The onset of seroma occurs shortly after implantation of the graft. As a method of prevention, when factors are considered to be patient related, the use of ePTFE grafts should be avoided and PU grafts or nonporous grafts should be chosen 76.
5. Surgery includes incision drainage, resection of the mass, partial graft replacement, or removal of the entire graft. The surest method is replacement of the graft in combination with resection of the mass 337. Tissue adhesion has been tested in the treatment of seroma but the results are varied 332, 338. Treatment is not necessary for those that are small and show no signs of growing larger, but surgical treatment will be required for those that show a tendency to enlarge.
Chapter 5-8 Access-Related Pain
Statements
- When vascular pain occurs during dialysis, subjects listed in Table 1 should be considered (1-C).
- When pain is presented at times other than during dialysis, subjects listed in Table 2 should be considered (1-C).
- For pain that is similar to access-related pain, causes to be considered are listed in Table 3 (1-C).
| 1) Cannulation site pain |
| 1-1 Pain during cannulation |
| 1-2 Pain due to suction of vessel wall or valve |
| 2) Pain distal to shunt |
| 2-1 Pain due to outflow from shunt: Worsened peripheral circulation (steal syndrome): Chap. 5, Sec. 4 |
| 2-2 Sore thumb syndrome: Chap. 5, Sec. 3 |
| 3) Pain proximal to shunt |
| 3-1 Pain due to stenosis or occlusion of the proximal outflow vein (venous hypertension): Chap. 5, Sec. 3 |
| 3-2 Pain due to pressure on nerves from increased venous pressure caused by inflow |
| 3-3 Muscular pain due to decreased blood flow in the lateral branch and increased rate of flow in the brachial artery because of outflow |
| 1) Pain distal to shunt |
| 1-1 Steal syndrome: Chap. 5, Sec. 4 |
| 1-2 Sore thumb syndrome: Chap. 5, Sec. 3 |
| 2) Pain proximal to shunt |
| 2-1 Venous hypertension (worsens during dialysis): Chap. 5, Sec. 3 |
| 3) Other |
| 3-1 Shunt occlusion: Chap. 5, Sec. 1 |
| 3-2 Shunt aneurysm: Chap. 5, Sec. 2 |
| 3-3 Seroma: Chap. 5, Sec. 7 |
| 3-4 Shunt or graft infection: Chap. 5, Sec. 6 |
| 3-5 Pain related to shunt surgery |
| 3-6 Pain due to reflex sympathetic dystrophy |
|
Commentary
Access-related pain is a relatively frequent complication and its causes are wide ranging. At times, the symptoms can be intense, and dealing with them, in many cases, is distressful 339-342. In this guideline, we considered treatment and methods as shown in the flowchart in Figure 6 343.
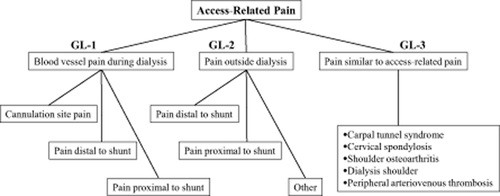
Flowchart for access-related pain.
1. (Table 1) 1-1 can be dealt with by applying lidocaine tape, by using needles for the buttonhole method 341, or by altering the cannulation site. 1–2 can be dealt with by altering the cannulation site or lowering the blood flow rate. For 2-1, the administration of drugs to improve peripheral circulation or treatment for steal syndrome (please refer to other chapters for more detail). 2-2, treatment for sore thumb syndrome (please refer to other chapters for more detail). For 3-1 and 3-2, preference should be given to treatment for venous hypertension (please refer to other chapters for more detail). For 3-3, alter the cannulation site or lower the blood flow rate. Warm the area where pain originates. If the pain cannot be alleviated, consider changing the access (creating an access on the opposite side).
2. (Table 2) For 1-1 and 1–2, perform treatment for steal syndrome and sore thumb syndrome (please refer to other chapters for more detail). For 2-1, perform treatment for venous hypertension (please refer to other chapters for more detail). For conditions listed under 3, please refer to the chapters in which they are noted, for it is important to perform treatment for each condition 344, 345.
3. (Table 3) Use all available means in the treatment of each of the conditions 1–5 345.
Chapter 5-9 Catheter Trouble
Statements
- Catheter trouble is more prevalent in cuffed catheters and includes 1) kinking, 2) breakage, 3) connector damage, and 4) catheter occlusion. It is recommended that each of these be dealt with in the appropriate manner (1-C).
- In the case of catheter kinking, it is recommended that the kink be removed through surgical procedure (1-C).
- It is recommended that ruptured catheters be removed and replaced by a new catheter (O).
- For damaged catheter connectors, it is recommended that the catheter be completely closed off and the connectors be replaced (O).
- In the case of catheter occlusion, it is recommended that main methods of treatment are the use of urokinase to dissolve the thrombus and replacement of the catheter (1-C).
Commentary
1. Presently, many cuffed catheters are constructed of PU or silicon tubing with stainless steel and plastic, and it is preferred that signs and symptoms of trouble be discovered in the early stages during routine observation 346, 347.
2. Generally, PU catheters have a high in vivo stability but kink rather easily. In situations where this symptom is presented during the period of catheter maintenance, in many cases it was caused by a change in the position of the catheter due to movement of the arms, head, and neck. Procedures to deal with it are the same as in the early stage; replacement of the catheter should also be considered 186.
3. Catheter breakage is not limited to long-term use only; therefore, caution is required. Because it can lead to serious complications if the damaged part of the catheter should stray into the right ventricle or pulmonary artery, catheters should be designed taking into account the internal pressure in the area near the tip in an effort to prevent breakage.
4. Even if a crack has occurred, it may not be visible to the naked eye. But it can be confirmed by careful observation in cases where air is being pulled into the system, bleeding or fluid leakage occurs at the connector.
5. Many times, complete differentiation will be difficult because the symptoms are similar. Therefore, first fill the catheter with urokinase or continually inject urokinase, and then evaluate for an improvement in symptoms 105, 114, 348. If urokinase has no effect, diagnostic imaging should be performed for diagnosis and to determine therapy 349. Catheter function can be restored by use of the appropriate guide wire or the use of urokinase, but in most cases, the effect is only temporary. For this reason, replacement of the catheter should be kept in mind while attempting the treatments above. It is possible to perform catheter replacement for infection or other abnormalities using an over-the-wire method 350, 351. Also, at this stage, no conclusion has been reached on the effectiveness of the administration of warfarin or the use of heparin-coated catheters 185-188, 352-355.
Chapter 6 Vascular Access Types, Morbidity, and Mortality Rates
Statements
- The incidence of functional abnormalities of VAs (stenosis, thrombosis or occlusion, infection, etc.) differs significantly among VA types (AVF, AVG, superficialization, intravascular catheterization, external shunt, etc.) (1-B). Consequently, the morbidity and mortality varies with VA type.
- The type of VA affects the 1-year survival rate (1-B).
- When it becomes time to begin hemodialysis therapy, it is extremely important to construct or prepare the best possible AVF, excelling in form and function (O).
Commentary
1. AVFs have the lowest incidence rates for the major VA-related complications such as stenosis, occlusion, and infection and therefore are the best choice for VA at this time 354-357. Approximately 90% of the maintenance hemodialysis patients use an AVF 358, 359. However, in recent years, there has been an increase in elderly and long-term patients and a sharp increase in patients with diabetic nephropathy, increasing examples in which construction and repair of VA have shown difficulty (Table 1). On the other hand, 30% use indwelled catheters at the initialization of hemodialysis because they do not have an AVF 61, 295. The main reason for this is a delayed introduction of the patient to a dialysis physician, while other factors include occlusion immediately following construction, insufficient blood flow, and failed development of venous arterializations 360. While catheter indwelling is a simple and useful method, it is easily accompanied by infection, stenosis, thrombus formation, etc., so it is important to recognize it as a “double-edged sword” 361.
| 1) Expended time on dialysis |
Damage to vessels –> AVG, catheter Reduced cardiac function –> Superficialization Transfer to PD |
| 2) Advanced age of those starting dialysis |
Damage to vessels –> AVG Reduced cardiac function –> Superficialization Choose PD |
| 3) Increase of patients with cardiac dysfunctions |
Correction of excess blood flow Superficialization Intravasucular catheter |
| 4) PTA-related methods, insurance coverage for materials |
PTA or surgery PTA: limit to number of procedures High costs of materials |
| 5) Shortage of VA surgeons (construction/repair carried out by a variety of surgeons) |
Training of VA surgeons Standardization of methods Large-scale clinical study (evidence) |
| 6) Care management of VAs |
Cooperation between physician, nurse, technician, and patient Care management manual |
2. The relative risks affecting 1-year survival by type of VA according to the results of calculation after adjustment of (Kt/V) urea and % creatinine generation rate for each group are as follows: if AVF is considered as 1.00, then arterial superficialization is 1.867 and AVG is 1.908 357. AVF showed a statistically lower risk in 1-year survival when compared with arterial superficialization and AVG 358 (Figure 7). Xue et al. 362 analyzed the 1-year death rate in cases 67 years and older, finding 24.9% for AVF, 28.1% for AVG, and 41.5% for catheters, with the relative risk of death being highest for catheter patients. However, there are also indications that the complications that forced the introduction of catheters are the reason for their worse results 355.
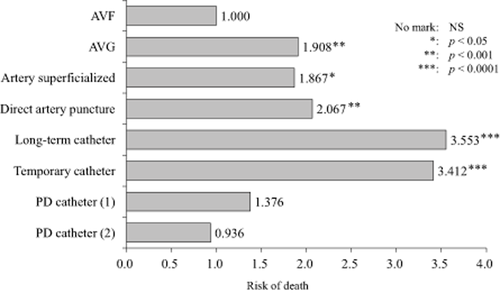
Primary access types and prognoses (adjusted for gender, age, major condition, and eGFR) (Jpn Soc Dial Ther, 2008).
3. It is a self-evident truth that it is important to construct an AVF that is easily cannulated and can provide a predetermined blood flow at the time of the initiation of dialysis 360, 363, 364. Even if an AVF is constructed, there are individual differences in the degree of maturation (dilation) of the arterialized veins, and, moreover, because early-stage AVF malfunction does occur to a certain extent 61, this makes well-thought-out evaluation of overall and local conditions at the time of construction, AVF construction site selection, post-surgery management, cannulation, and post-surgery monitoring important 360, 364.
Chapter 7 Addendum: Patency of Vascular Access
Patency rates for AVF: According to a report by Ohira 15, who followed the patency rates of radiocephalic AVFs for 7 years (Fig. 8) (excluding 9% that initially failed), the younger-aged group (40–50 years old) had a higher patency rate than the older-aged group (65–70 years old). The patency rate tended to be higher for males, but there was no significant difference. In addition, the diabetes group, a combined-age group, had a low patency rate.
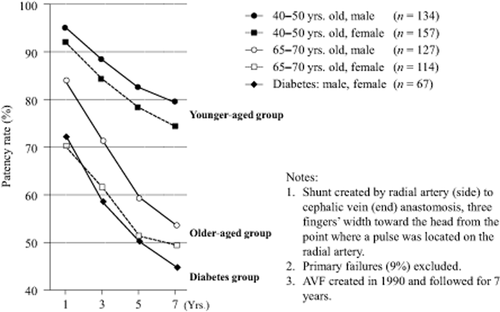
Patency rates for initial shunt in the distal forearm (Ohira S: Angio access problems and methods in diabetic dialysis patients. Kidney and Dialysis (2001 Suppl): 714–720, 2001).
Patency rates for AVG: Sakai 299 reported on the results of 180 AVGs using PTFE grafts and noted a favorable 3-year primary patency rate of greater than 50% (Fig. 9).
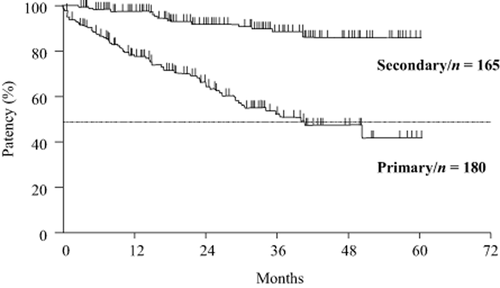
Patency rates for AVG (Sakai S: Graft complications and methods regarding them. Jpn Assoc Dial Phys, Committee of Complications and Treatment Methods 17: 31–40, 2008).
Patency rates for arterial superficialization: Though there is no definition for the patency rate for arterial superficialization, according to a report by Murotani and Haruguchi 99, the patency rate in superficialization where the veins were usable was favorable with 94% for 1 year, 85% for 3 years, and 78% for 5 years (Fig. 10).
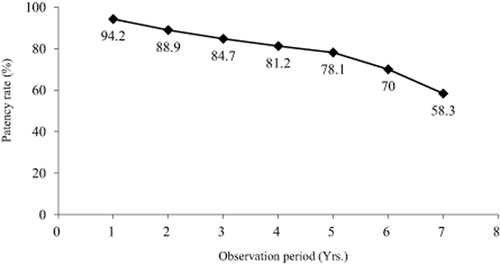
Patency rates for arterial superficialization (Murotani N, Haruguchi H: Arterial superficialization: application, method, management. In Vascular access-construction, maintenance, repair. Ohira S, et al. eds. P.49–57, Chugai Igakusha, Tokyo, 2007).
Conflict of interest: No Conflict of Interest.
Appendix
Members of the guideline working group
Kazutaka Kukita: Chairman of the working group of “Vascular Access Construction and Repair for Chronic Hemodialysis,” Division of Artificial Organ Therapy Center, Hokuyukai Sapporo Hokuyu Hospital, Hokkaido.
Seiji Ohira: Vice-Chairman of the working group of “Vascular Access Construction and Repair for Chronic Hemodialysis,” Tousoukai Sapporo Kita Clinic, Hokkaido.
Izumi Amano: Vice-Chairman of the working group of “Vascular Access Construction and Repair for Chronic Hemodialysis,” Nagoya Vascular Access Amano Memorial Clinic, Aichi.
Hidemune Naito: Naito Medical Research Institute, Hyogo.
Nakanobu Azuma: Shouenkai Tokatsu Clinic Hospital, Chiba.
Kiyoshi Ikeda: Ikeda Vascular Access, Dialysis, Internal Medicine Clinic, Fukuoka.
Yutaka Kanno: Kanno Dialysis and Vascular Access Clinic, Nagano.
Takashi Satou: Kaikoukai Meiko Kyoritu Clinic, Aichi.
Shinji Sakai: Nigata Shinrakuen Hospital, Nigata.
Tokuichiro Sugimoto: Mitsui Memorial Hospital, Nephrology, Tokyo.
Yoshiaki Takemoto: Osaka City University, Department of Urology, Osaka.
Hiroaki Haruguchi: Haruguchi Vascular Access Clinic, Tokyo.
Jun Minakuchi: Kawashima Kai, Kawashima Hospital, Tokushima.
Akira Miyata: Department of Cardiovascular Surgery, Japanese Red Cross Kumamoto Hospital, Kumamoto.
Noriyoshi Murotani: Department of Surgery, Dialysis, Chiba Social Insurance Hospital, Chiba.
Hideki Hirakata: Division of Nephrology and Dialysis Center, Fukuoka Red Cross Hospital, Fukuoka.
Tadashi Tomo: Department of Nephrology, Oita University Hospital, Oita.
Tadao Akizawa: Chairman of the JSDT, Division of Nephrology, Department of Medicine, Showa University School of Medicine, Tokyo, Japan.




