Clinical Practice Guideline for the Management of Chronic Kidney Disease-Mineral and Bone Disorder
The kidney plays an important role in the mineral metabolism 1; in addition to being a target organ for various hormones involved in calcium and phosphorus metabolism, the kidney is the main organ that activates vitamin D 2. Thus, it is quite understandable that kidney dysfunction can result in derangement of mineral metabolism.
Ever since the first report of severe osteitis fibrosa cystica with parathyroid hyperplasia 3, this disorder was considered to be a skeletal/bone disease and was named “renal osteodystrophy”. The clinical management of renal osteodystrophy, therefore, primarily aimed to maintain parathyroid hormone (PTH) levels appropriate for normal bone metabolism 4.
In the last decade, it has become widely accepted that deranged mineral metabolism in patients with chronic kidney disease (CKD) results not only in bone disease, but a higher risk of cardiovascular disease and reduced survival, through the development of vascular calcification. This lead to the proposal of a new concept, “CKD-Mineral and Bone Disorder (CKD-MBD)” 5. CKD-MBD is a systemic condition that manifests as abnormalities in PTH, calcium, phosphorus and vitamin D; bone abnormalities and extraskeletal calcification 5. As a systemic disease, management of these abnormalities should ultimately aim to reduce the risk of cardiovascular events, bone fracture and survival 5.
The Japanese Society for Dialysis Therapy (JSDT) clinical practice guidelines for the management of secondary hyperparathyroidism in chronic dialysis patients was originally published in Japanese in 2006, then in English in 2008 6. This guideline put emphasis on improving patient survival and it was one of the first guidelines in this therapy area, preceding the Kidney Disease: Improving Global Outcomes (KDIGO) guideline 7. In this original guideline, we set target ranges for serum phosphorus, calcium, phosphorus, and PTH levels based on survival data of Japanese dialysis patients, and we set an order of priorities for clinical management: management of serum phosphorus levels, management of serum calcium levels, and then control of parathyroid function. In addition, considering that Japanese dialysis patients tend to have a longer dialysis duration than American and European patients, we proposed that parathyroid intervention is to be done at an earlier stage in this population 6.
During the last 5 years, this first guideline has considerably contributed to a better understanding and control of secondary hyperparathyroidism in CKD patients by physicians, other medical professionals and by patients themselves 8. However, since its publication, several new therapeutic modalities have become available for Japanese patients, which added more evidence to this area. Thus, we revised the guideline to include several new policies, and the new guideline was published in Japanese in 2012 9. This article contains the new guideline text, and footnotes translated into English.
- We have tried to keep the same simple and user-friendly format of the first guideline. Statements are limited to those relevant for the majority of patients. Additional useful supporting information is provided.
- We have verified the validity of the target ranges for serum markers of mineral metabolism and the order of priority for management, using the JSDT patient registry database.
- We have included new drugs that have been introduced into the Japanese market since publication of the first guideline.
- We have added new areas of discussion on CKD-MBD, which were not covered in the first guideline. These include: vascular calcification, amyloid bone disease, peritoneal dialysis, pediatric patients, predialysis CKD, and kidney transplantation. For this purpose, we collaborated with specialists from relevant societies, especially the Japanese Society of Nephrology.
- We have included new evidence from papers published in English during these 5 years. In addition, we also analyzed the JSDT registry database for this purpose as appropriate.
- Evidence levels and strengths of recommendations are defined and presented in combination, based on an evidence-grading system adapted from the KDIGO position paper 10. As shown in Table 1, the strength of the recommendation is graded as either 1 (“strong”: i.e. “we recommend” you do it, for positive recommendations, and “we recommend” you do not do it, for negative recommendations) or 2 (“weak”: “we suggest” you do it, or “we suggest” you do not do it). For the final category, “No grade” (“it is reasonable”), there is insufficient evidence available to give a grade; however, these ungraded statements are based on a consensus of expert opinion, and the expectation is that consideration should be given to follow the statement.
- We have proposed future perspectives and suggestions of study plans for areas where there is currently insufficient evidence to make recommendations.
- Conflicts of interest declarations for JDST guideline working group members have been included for the first time (Appendix I).
| Grade for strength of recommendation | Strength | Wording |
|---|---|---|
| Level 1 | Strong | “We recommend … should” |
| Level 2 | Weak | “We suggest … might” |
| No grade‡ | – | “It is reasonable” |
| Grade for quality of evidence | Quality of evidence | |
|---|---|---|
| A | High | |
| B | Moderate | |
| C | Low | |
| D | Very low | |
- †Each statement is shown as a combination of the grade and level of evidence, such as 1A or 2C. ‡The expectation is that it is reasonable to follow this statement as it is a consensus statement based on expert opinion.
We hope this guideline will be useful for daily practice and will lead to better quality of life and survival in CKD patients.
Chapter 1: Essential Routine Tests and Frequency of Measurements
Statements
- As routine tests measure the following parameters:A
- We suggest the measurement of serum phosphorus, calcium, albumin, PTH, and alkaline phosphate (ALP) levels (2D).B,C
- We recommend that both patient evaluation and determination of therapeutic plans be based on the trends of several measurements, not by a single result of laboratory tests (1C).
- We suggest that treatment plans be changed when test results show successive rises or falls, even within the normal range (2C).
- It is reasonable to use the values obtained at the beginning of the first dialysis session in each week (No grade).
- Frequency of measurements:
- It is reasonable to measure the serum phosphorus and calcium levels at least one or two times per month (No grade).
- In the event of serum phosphorus or calcium values showing a marked deviation or if there is the potential for a deviation from their target range, we suggest to measure them more frequently until the values stabilize (2D).
- Usually measure PTH once every 3 months; however, we suggest measuring it monthly until the values stabilize if the PTH concentration deviates from the target range, if there is a change in therapy, or if the patient begins taking active therapy—intravenous vitamin D receptor activators (VDRA), cinacalcet hydrochloride, or parathyroid interventions—as treatment for secondary hyperparathyroidism (2D).
Supplementary notes
AIn evaluating the results of a laboratory examination, we recommend that information concerning the timing of blood sampling and drug administration, drug adherence, and the measurement method is ascertained (1C).
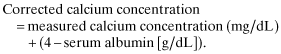
CInitially use the ALP concentration, as a marker of bone metabolism, which is usually measured monthly in regular dialysis practice.
Rationale
Even in this revised guideline, the basic policy of the previous version has been retained; that is, the results of blood examinations measured routinely in regular dialysis practice are utilized, and specific examinations are only performed in accordance with specific situations. The measurements of bone metabolism markers are also limited in daily clinical practice. Because serum ALP levels can substitute for bone ALP levels in patients without hepatobiliary complications and serum ALP is usually measured monthly, it is considered to be reasonable to use serum ALP levels as a first-line test.

However, as in previous versions, Payne's equation was adopted in the present guideline for three reasons. First, this equation has been accepted widely in many fields, including dialysis medical care, for several decades in Japan. Second, this equation is simple because it does not need to multiply 0.8. Third, there are not significant differences in the serum corrected calcium levels calculated by each of the two equations 13. It should be noted that correction by serum albumin is important to avoid overlooking hypercalcemia modified by hypoalbuminemia.
Laboratory test results can be affected by various factors. For example, serum calcium levels change according to timing of blood sampling (because of the circadian rhythm and the effects of hemodialysis such as water removal), and medical treatments can affect blood calcium levels. In particular, because serum calcium and PTH levels decrease from 4 to 8 h after cinacalcet hydrochloride administration 14, 15, it is important to ascertain the timing of blood sampling and drug administration, and drug adherence.
For PTH measurement, intact PTH assays are mainly used, as recommended in the previous guideline (see Chapter 3). There are several commercially available kits that measure intact PTH with second-generation assays, but measurement variability between kits can occur depending on whether the samples are serum or plasma as well as the measurement bias by the kit 16, 17. Thus, the modality of assay, including sample collection, is a significant concern, and we recommend taking this information into account when evaluating intact PTH levels. In addition, adequate dialysis, appropriate protein intake based on the nutrition status, and appropriate phosphorus binder administration should also be confirmed, as these factors are essential for the evaluation and management of serum phosphorus and calcium levels. We recommend that evaluation of a patient's condition and determination of therapeutic plans should be based on the trends of several measurements, not by a single result of a laboratory test.
In evaluating parathyroid function using PTH levels, progression to secondary hyperparathyroidism can be predicted when PTH levels remain at a high level or increase continuously, even within the normal range. Additionally, mortality may increase and complications such as cardiovascular calcification will develop if serum phosphorus and calcium levels persist around the upper limit of the target range 18, 19. In such a case, we suggest that treatment plans are changed, even if the test results are within the normal range.
With regard to the timing of blood sampling for patients who have a typical thrice weekly dialysis prescription; serum phosphorus and calcium levels are higher at the beginning of the week, being affected by food intake and removal by dialysis sessions 20. In particular, serum phosphorus levels are significantly higher when measured 3 days after dialysis than those measured midweek 20. Because it is the convention in Japan that blood sampling is done at the beginning of the week; in other words, from the first dialysis session in each week, it is reasonable to use the blood sampling results obtained at this moment for the evaluation of CKD-MBD.
The frequency of measuring serum phosphorus and calcium levels is described as monthly in the 2003 KDOQI guideline, and every 1–3 months in the 2009 KDIGO guidelines. It is reasonable for the frequency to be set for the purpose of monitoring the efficacy of treatment and/or adverse events, caused by deviation from the target ranges, although there is currently no clinical evidence that the frequency of measurement is associated with patient mortality. However, if the measurement interval is extended, there may be a risk of inadequate monitoring. In particular, serum phosphorus and calcium levels will vary with a meal; thus, we consider that statements that suggest making treatment adjustments, based on a single laboratory measurement value that is measured only once a month, are not acceptable. We consider that it is reasonable to measure serum phosphorus and calcium levels at least 1–2 times a month, considering clinical practice in Japan. However, more frequent measurements of serum phosphorus and calcium are suggested under circumstances when these levels deviate, or are likely to deviate, markedly from target ranges, when initiating therapy, changing the dose and/or discontinuing treatment with intravenous VDRA and/or cinacalcet hydrochloride; or when patients undergo parathyroid interventions, such as PTx or selective percutaneous ethanol injection therapy (PEIT).
The recommended measurement frequency of PTH levels is once every 3 months in this guideline. Although the frequency in the KDIGO guideline is set at every 3–6 months, which is longer than in this guideline, the long-term control of PTH levels and monitoring of parathyroid function over time are essential in Japan where there are many dialysis patients with a long dialysis vintage. Thus, we suggest that intact PTH be usually measured once every 3 months, but be measured monthly until the values stabilize if the PTH values deviate from the target range, there is a change in therapy or the patient is taking active therapy (intravenous VDRA, cinacalcet hydrochloride, or parathyroid interventions) as treatment for secondary hyperparathyroidism.
Chapter 2: Control of Serum Phosphorus and Calcium Levels
Statements
- Target ranges of serum phosphorus and corrected calcium concentrations:
- Target range for serum phosphorus concentrations: 3.5–6.0 mg/dL.
- Target range for corrected serum calcium: 8.4–10.0 mg/dL.
- Therapeutic guidelines based on the target range of serum phosphorus and calcium:
- We recommended that serum concentrations of phosphorus, corrected calcium, and PTH (see Chapter 3) are kept within the target ranges and that control of serum phosphorus should have the highest priority, followed by that of calcium, and then that of PTH (1C; see Fig. 1).
- If serum phosphorus or corrected calcium levels are constantly high, a prompt change in treatment modality is recommended (1B).A,B,C
- In principle, after controlling serum phosphorus and corrected calcium levels, we suggest that attempts might be made to maintain serum PTH concentrations within the target range by adjusting the dose of VDRA or cinacalcet hydrochloride (Fig. 2) (2D).
- When serum PTH levels are high, administration of cinacalcet hydrochloride might be considered as a way to control phosphorus and calcium (2D).D
Supplementary notes
AWhen serum phosphorus levels are high, we suggest to ensure that the dialysis dose is adequate and instruct the patient to reduce intake of dietary phosphorus (2D). If malnutrition is thought to be the cause of hypophosphatemia, we suggest to try to improve patient's nutritional status (2C).
BDecreasing the dose of or discontinuation of calcium carbonate (CaCO3) is suggested if the patient is likely to develop hypercalcemia, when significant vascular calcification is seen, if adynamic bone disease is suspected, or if a low PTH level persists (2C).
CWhen hypercalcemia or hypocalcemia is prolonged, we suggest to consider changing the calcium concentration of dialysate (2D).
DWhen cinacalcet hydrochloride is to be started, we suggest that the corrected serum calcium concentration is ≥9.0 mg/dL (2D).
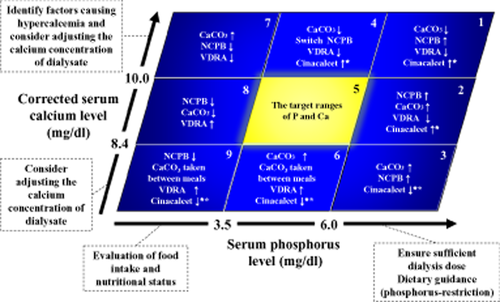
Control of phosphorus and calcium during treatment. Nine example clinical scenarios (1–9) show how serum phosphorus and corrected serum calcium levels can be used to guide the selection of a therapeutic modality.
↑ = start or increase of treatment and ↓ = reduction or suspension of treatment where serum parathyroid hormone (PTH) is high* and low**.
CaCO3, calcium carbonate; Cinacalcet, cinalcalcet hydrochloride; NCPB, non-calcium-containing phosphorus binder (calcium-free phosphorus binder); VDRA, vitamin D receptor activators.
Guidance notes
Classifying the serum levels of phosphorus and calcium.
Classify the serum levels of phosphorus and calcium using the following 9 patterns to select the appropriate treatment:
1. Serum phosphorus levels ≥target range.
Irrespective of the serum calcium levels; a sufficient dialysis dose should be ensured, and dietary advice should be given to limit the intake of phosphorus. Then select the appropriate treatment for hyperphosphatemia based on the serum calcium levels.
High serum calcium levels (1)†
Confirm that CaCO3 is being taken orally during meals or immediately afterward.
Dose reduction or discontinuation of CaCO3 and/or VDRA administration (switch to or increase the dose of a calcium-free phosphorus binder, such as sevelamer hydrochloride or lanthium carbonate).
When serum PTH levels are high, consider starting/increasing the dose of cinacalcet hydrochloride.
Serum calcium within the target range (2)
Confirm that CaCO3 is being taken orally during meals or immediately afterward.
Commence treatment with or increase the dose of a calcium-free phosphorus binder and/or CaCO3 administration.
Reduce the dose of or discontinue VDRA.
When serum PTH level is high, consider starting/increasing the dose of cinacalcet hydrochloride.
Low serum calcium (3)†
Confirm whether oral CaCO3 is actually being taken.
Commence treatment with or increase the dose of CaCO3 and/or a calcium-free phosphorus binder.
When serum PTH level is low, reduce the dose or discontinue cinacalcet hydrochloride.
In addition, ensure that a serum phosphorus binder is administered.
2. Serum phosphorus is within the target range
High serum calcium (4)†
Dose reduction or discontinue CaCO3 (switch to a calcium-free phosphorus binder).
Reduce the dose or discontinue VDRA.
When the serum PTH level is high, commence treatment with or increase the dose of cinacalcet hydrochloride.
Serum calcium within the target range (5)
Continue the current treatment and optimize the PTH level.
Low serum calcium (6)†
Commence treatment with or increase the dose of CaCO3 (administration between meals).
Commence treatment with or increase the dose of VDRA.
When serum PTH level is low, consider reducing the dose or discontinuing cinacalcet hydrochloride.
3. Serum phosphorus ≤target range
Confirm whether food intake is sufficient and whether the nutritional state is poor, irrespective of the serum calcium level. If malnutrition exists, correct this.
High serum calcium (7)†
Reduce the dose or discontinue CaCO3/calcium-free phosphorus binder.
Reduce the dose or discontinue VDRA.
Serum calcium level within the target range (8)
Reduce the dose or discontinue CaCO3/calcium-free phosphorus binder.
Commence or increase the dose of VDRA.
Low serum calcium (9)†
Reducing the dose or discontinue calcium-free phosphorus binder.
Commence treatment with or increase the dose of CaCO3 given between meals, or commence treatment with/increase the dose of VDRA.
When the serum PTH level is low, reduce the dose or discontinue cinacalcet hydrochloride.
†If hypercalcemia or hypocalcemia persists even after the above treatment, identify the cause and consider adjusting the calcium concentration of dialysis fluid.
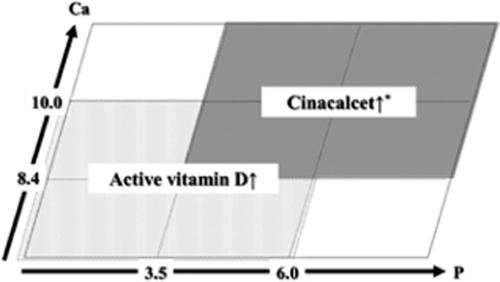
Differentiation between vitamin D receptor activators (VDRA) and cinacalcet hydrochloride when parathyroid hormone (PTH )levels are high. When cinacalcet hydrochloride is to be started, we suggest that the corrected serum calcium concentration is ≥9.0 mg/dL (2D).
Rationale
One of the main characteristics of CKD-MBD, according to the 2003 KDOQI guideline, is that abnormal calcium/phosphorus metabolism in renal patients is not just associated with bone disease. CKD-MBD is also related to clinically significant vascular calcification and an increased risk of mortality 4. In the present JSDT guidelines, the serum phosphorus and calcium target ranges are based on patient prognosis, and recommendations are made regarding the treatment and doses to use for maintaining serum phosphorus/calcium concentrations at appropriate levels.
A number of reports have been published concerning the validation of target ranges for serum phosphorus and calcium concentrations 18, 21-30. These studies, mainly from the Western world have largely used mortality as a primary endpoint. In this 2012 JSDT guideline, we define target serum phosphorus/calcium ranges according to the results of an analysis of data from the JSDT patient registry, where patients were treated in accordance with the previous JSDT guideline 6, 31.
Following publication of the previous 2008 guideline 6, awareness about CKD-MBD has increased in Japan, and new drugs, such as cinacalcet hydrochloride and lanthanum carbonate, have been listed in Japan's National Health Insurance formulary. Accordingly, data from 128 125 dialysis patients, who could be monitored from the end of 2006 to the end of 2009, were analyzed 32. In addition to analysis of the previous baseline model (B) (with a 3-year life expectancy), time-dependent (TD) and time average (TA) models were used to set target levels for serum phosphorus/calcium and PTH, using mortality as an endpoint. Based on this analysis, we have set a target range for serum phosphorus of 3.5–6.0 mg/dL. The dialysis serum phosphorus range was set at 3.5–5.5 mg/dL in the KDOQI guideline, while the new 2009 CKD-MBD KDIGO guidelines 7 recommended that the serum phosphorus level should be lowered if higher than the reference level. We derived the target level by stratifying serum phosphorus levels in our dataset, and this analysis produced a J-shaped curve: mortality being increased with both hyperphosphatemia and hypophosphatemia. When P < 0.01 was used to indicate statistical significance, the recommended target level range was 3.6–5.0 mg/dL for model B, 4.1–6.0 mg/dL for model TD, and 4.1–5.5 mg/dL for model TA. When a hazard ratio (HR) of >1.2 was considered statistically significant, the recommended range was 3.1–6.0 mg/dL for model B, 3.6–6.5 mg/dL for model TD, and 4.1–6.0 mg/dL for model TA. Model TD characteristically reflects a relatively short-term prognosis, whereas model TA reflects a relatively long-term prognosis. Regardless of the differences between models, the results were generally similar, and, therefore, we continue to recommend that the serum phosphorus target range should be between 3.5–6.0 mg/dL, in accordance with the previous guidelines 6.
We recommend that the target range for serum calcium should be between 8.4–10.0 mg/dL. The KDOQI guideline states that serum calcium levels should be from 8.4–9.5 mg/dL, while KDIGO states that the target should be within the normal range. Serum calcium levels were validated in the same way as phosphorus levels; when the significance level was set at P < 0.01, the recommended range was determined to be ≤9.0 mg/dL for model B, ≤9.0 mg/dL for model TD, and 8.6–9.5 mg/dL for model TA. When a HR of >1.2 was considered statistically significant, the recommended range was ≤10.0 mg/dL for model B, ≤9.0 mg/dL for model TD, and 8.1–10.0 mg/dL for model TA. Unlike with serum phosphorus concentrations, the risk of mortality increased in a linear pattern for both models B and TD. There is still scope for discussion concerning the elimination of the lower limit, and based on the J-shaped curve from modeled TA results and the reference level for healthy people, we consider that 8.4–10.0 mg/dL should be used as the target level, in accordance with the previous guidelines 6. Nevertheless, this statistical validation using data from the JSDT patient database indicate that serum calcium concentrations in patients on dialysis should be maintained at the lowest possible.
This guideline clearly recommends that control of serum phosphorus should have the highest priority, followed by that of calcium, and then that of PTH. Previous reports showed that appropriate control of serum phosphorus and calcium levels resulted in a better prognosis than control of PTH alone 26, 33, 34. However, it was not clear whether serum phosphorus or calcium levels should have a higher priority. We projected the 3-year prognosis for different combinations of serum phosphorus, calcium, and PTH that reached the target levels specified in the earlier guidelines 6, and found that a good prognosis would be obtained using the following parameters: [attainment of target for serum phosphorus, calcium, and PTH] > [serum phosphorus and calcium] > [serum phosphorus only] > [calcium only] > [PTH only] > [target not attained for any for the three variables]. Therefore, based on these observations, we recommend that the target priorities should be: serum phosphorus, then calcium, and then PTH. We also suggest that the serum levels of serum phosphorus and corrected calcium should be controlled first, and then the VDRA or cinacalcet hydrochloride doses should be adjusted to keep serum PTH levels within the target range.
From the previous guidelines, the 9-section chart (Fig. 1) has been adopted as a treatment tool for keeping serum levels of serum phosphorus and calcium optimal. In each of the nine categories shown in Figure 1, the method for adjusting the dose to keep serum phosphorus/calcium levels in the target range is provided. To validate the recommendations published in the previous guideline, we used the same JSDT patient registry dataset to project the 3-year prognosis for patients in each of the nine categories. The results showed that the risk of mortality decreased in the group with normal serum calcium/phosphorus levels and in the group with normal serum phosphorus plus low calcium levels. These findings indicate that the prognosis improves when both serum phosphorus and calcium are kept within the target range. When we examined the relationship between the frequency of attaining target serum phosphorus/calcium levels and prognosis from 2006 to the end of 2008, we found that the more frequently the target level is attained, the lower is the mortality risk. This suggests that constant maintenance of serum phosphorus/calcium levels within the target ranges leads to improvement in life expectancy. Based on these findings, we recommend prompt treatment change when the serum phosphorus or corrected calcium levels are constantly high.
In accordance with Figure 1, and as discussed earlier in Chapter 1, when serum phosphorus levels are high we suggest that a sufficient dialysis dose is ensured and that patients are instructed regarding dietary phosphorus restriction. Also, it is important to assess a patient's nutritional state, including the amount of food eaten, when serum phosphorus levels are low. However, caution is advised with regard to excessive phosphorus restriction. As the intake of phosphorus strongly correlates with the amount of protein intake, excessive phosphorus restriction may induce malnutrition and exacerbate the mortality risk. In this regard, it is important to reduce the consumption of significant amounts of phosphorus-rich food, including dairy products, small fish, as well as products with phosphorus-containing additives/preservatives, such as processed food, instant food, confectionary, and pre-packaged convenience store lunches. Once these precautions are taken, we suggest that pharmacologic therapy should be started to control the mineral parameters in the following order of priority: serum phosphorus, calcium, and PTH. In cases with high serum phosphorus levels, the start/increase of a phosphorus binder should be considered, and VDRA should be reduced/suspended depending upon the patient.
When a phosphorus binder is prescribed, patient compliance must be confirmed. Furthermore, it is important to bear in mind that certain drugs are more effective when taken at specific times, as shown in Tables 2 and 3. For instance, according to their package inserts, sevelamer hydrochloride should be taken before a meal, and CaCO3 and lanthanum carbonate should be taken immediately after a meal. As the efficacy of CaCO3 is influenced by gastric pH, co-administration of a gastric secretion inhibitor may weaken the drug's efficacy 35. Lanthanum carbonate, a chewable tablet, should always be chewed, and if an elderly person cannot chew the tablet, it should be crushed and administered orally. If the serum phosphorus level is low, reduction/suspension of phosphorus binder should be considered, and starting/increasing VDRA should be considered in some cases.
| Drug | Administration | Side-effects, contraindications and precautions |
|---|---|---|
| CaCO3 | Immediately after a meal |
Likely to cause hypercalcemia with appetite loss Efficacy is weakened by co-administration of a gastric acid secretion inhibitor Less GI adverse reactions than other drugs Relatively inexpensive |
| Sevelamer hydrochloride | Just before meal |
Does not contain calcium Expected to inhibit progression of vascular calcification Has LDL-cholesterol-lowering effects Frequently induces GI symptoms, including constipation and flatulence |
| Lanthanum carbonate | Chew after a meal |
Does not contain calcium Good phosphorus-absorbing capacity Induces vomiting, nausea, and other GI symptoms. Insufficient evidence for long-term administration |
- CaCO3, calcium carbonate; GI, gastrointestinal; LDL, low-density lipoprotein.
| Drug | Main characteristics and precautions |
|---|---|
| Cinacalcet hydrochloride |
Drug must be taken at the same time each day Assessment is recommended, taking into account that the PTH level is lowest at 4 to 8 h after administration and that the calcium level is lowest at 8 to 12 h after administration Start the drug when the calcium level is >9.0 mg/dL |
- PTH, parathyroid hormone.
When serum calcium levels are high, dose reduction/discontinuation of VDRA and/or CaCO3 are considered. When a concurrently high measured PTH level is observed, starting or increasing the dose of cinacalcet hydrochloride (Table 3) should be considered. If hypercalcemia persists, the reason for the lack of improvement, including low physical activity, should be sought, and a change in the calcium concentration in the dialysate should be considered. When the serum calcium level is low, starting treatment with/increasing the dose of VDRA and/or CaCO3 should be contemplated, and if cinacalcet hydrochloride is being administered, the dose may need to be reduced/discontinued. Administration of CaCO3 between meals is also effective, because it increases the serum calcium level 36.
The addition of cinacalcet hydrochloride to this 9-section chart is a change from the previous guideline. Although cinacalcet hydrochloride is mainly used to suppress PTH, it is included in the cases in which the PTH level is high (or low), because cinacalcet hydrochloride simultaneously lowers serum phosphorus/calcium levels 37, 38, and PTH control and serum phosphorus/calcium control are closely linked. In other words, administering cinacalcet hydrochloride as a method to control the serum calcium or phosphorus level is advisable for patients with high serum PTH levels. Nevertheless, there are several precautions to be taken when administering cinacalcet hydrochloride. First, when the drug is going to be administered, it is reasonable for the corrected calcium level to be maintained ≥9.0 mg/dL in order to avoid excessive hypocalcemia. Second, any assessment should take into account that the PTH level is lowest 4 to 8 h after cinacalcet administration, and the calcium level is lowest 8 to 12 h after cinacalcet administration 14, 39.
Figure 2 shows how to differentiate the use of VDRA and cinacalcet hydrochloride for treating secondary hyperparathyroidism on the 9-section chart. When the PTH level is high and serum phosphorus or calcium is normal to high, administration of cinacalcet is to be considered, and administration of VDRA is to be contemplated when serum phosphorus or calcium is normal to low.
Chronic kidney disease-MBD-related drugs should be considered not only as a tool for serum phosphorus/calcium control, but also from a prognosis perspective. A number of recent observational cohort studies of dialysis patients indicate that administering VDRA is associated with lower total/cardiovascular mortality risk, independently from attainment of serum calcium/phosphorus/PTH levels 29, 40-45. Furthermore, a low level of 25-hydroxyvitamin D (25(OH)D), a precursor hormone of active vitamin D, is a significant mortality risk factor even in healthy people 46-48. Most dialysis patients undergoing hemodialysis have insufficient 25(OH)D and the active form of vitamin D, 1,25-dihydroxyvitamin D (1,25(OH)2D) 49-52. VDRA may be given, so long as the serum phosphorus/calcium levels do not show a significant deviation. As shown in Figures 1 and 2, VDRA may be used regardless of PTH levels.
Cinacalcet hydrochloride is expected to prevent progression of vascular calcification and improve prognosis because it can simultaneously lower serum calcium/phosphorus/PTH levels in the blood. Analysis of the combined result of four safety survey studies showed that the risk for vascular disease-related hospitalization was reduced in cinacalcet hydrochloride groups 53. Furthermore, a large-scale observational study revealed that cinacalcet hydrochloride was associated with a low risk for overall/cardiovascular mortality 54. Currently, the EValuation Of Cinacalcet HCl Therapy to Lower cardioVascular Events (EVOLVE) study, a double-blind randomized controlled trial, is ongoing 55. The primary endpoint is the time to occurrence of composite events such as all-cause mortality or nonfatal cardiovascular event.
As for studies on serum phosphorus binders, an observational cohort study of patients started on hemodialysis showed that the risk of mortality was lower in patients treated with phosphorus binders than in those not treated with phosphorus binders 56. In accordance with the previous guidelines, it is reasonable to consider the appropriate upper limit for CaCO3 administration to be around 3 g/day, which allows for the importance of avoiding excessive calcium load 57. Compared to the number of reports on calcium-containing phosphorus binders (such as CaCO3), there are more publications reporting that sevelamer hydrochloride inhibits progression of vascular calcification 58-65; therefore, we suggest to restrict the dose of CaCO3 in some patients. Specifically, reduction/suspension of CaCO3 may be considered when hypercalcemia is likely to occur, when there is marked vascular calcification, dynamic bone disease is suspected, or when the blood PTH level is constantly low. In addition, switching to a calcium-free phosphorus binder is reasonable. Very few reports on lanthanum carbonate are available with regard to prognosis and vascular calcification 66, and therefore, long-term results and safety data need to be acquired. While each phosphorus binder has different properties, there is no consensus regarding which phosphorus binder is best for improving prognosis 67-69.
It is not yet known which calcium concentration in dialysate should be selected, 2.5 mEq/L or 3.0 mEq/L. KDIGO states that a calcium level between 2.5 and 3.0 mEg/L is reasonable 7. We suggest to adjust the dialysate calcium level as a method for controlling the serum calcium level should be done in patients who have prolonged hypercalcemia or hypocalcemia. With a 2.5 mEq/L dialysate dose, the serum calcium concentration can be kept at a relatively low level, making administration of VDRA and/or CaCO3 relatively easy, although the PTH level is likely to increase and blood pressure during dialysis may become unstable 70-72. Meanwhile, using 3.0 mEq/L dialysate may help to control PTH by loading calcium; although, co-administration with CaCO3 or VDRA may readily induce hypercalcemia. The calcium balance during dialysis is influenced by the predialysis serum calcium level; therefore, whether the total body calcium level is excessive or deficient varies according to the individual 73-75. It is reasonable to consider the characteristics of each dialysate before adjusting the dose of phosphorus binder, VDRA, or cinacalcet hydrochloride in order to properly control serum phosphorus, calcium, and PTH levels.
In this chapter, we have described how the target ranges of serum phosphorus and calcium have been validated using statistical survey data from the JSDT patient registry. We also described how pharmacologic regimens can be used to attain target range and the relationship between different medications and prognosis. A randomized controlled study is needed to identify the effects of drugs on important clinical outcomes (i.e. mortality and cardiovascular diseases), and we expect to obtain further evidence from Japan.
Chapter 3: Assessment and Management of Parathyroid Function
Statements
- Guidelines for managing PTH:
- We suggest the target range of intact PTH is set between 60 and 240 pg/mL (2D).A,B
- We recommend that control of serum phosphorus and calcium levels is achieved prior to PTH (1D).
- Treatment strategy when PTH levels exceed the target range:
- When intact PTH levels constantly exceed the upper limit of the management target range, it is reasonable to first decrease the level of intact PTH by medical therapy, including serum phosphorus/calcium management and the administration of VDRA and/or cinacalcet hydrochloride (2; No grade).C,D
- Consideration of parathyroid intervention therapy is recommended when circulating phosphorus, calcium, and intact PTH levels cannot be maintained within the target ranges by medical treatments (1B).
Supplementary notes
AOtherwise, we suggest maintaining whole PTH levels between 35 and 150 pg/mL.
BFor patients who have had parathyroidectomy (PTx), intact PTH levels are allowed to be below the lower limit of the target range.
CAn effective treatment strategy for patients whose intact PTH levels are persistently below the target range has not yet been established.
DFor patients taking cinacalcet hydrochloride, parathyroid function should be assessed by serum PTH levels at least 8 h after taking the drug.
Rationale
Parathyroid function generally increases along with the decline in kidney function. Although the normal range of intact PTH is supposed to be 10–65 mg/mL, the levels in patients with CKD stage 5 generally exceed it 76. However, since the end-organ action of PTH (i.e. on the kidney) is blunted in CKD, even a PTH level within the normal range can actually mean a hyperparathyroid status 77.
In the physiological state, the main target organs of PTH are the kidney and bone; however, in patients with CKD stage 5, the kidney is practically no longer a functional target. On the other hand, PTH possibly affects the development of cardiovascular disease and impacts upon survival by its indirect actions: altering bone and mineral metabolism and/or unknown actions independent of bone and mineral metabolism 34, 42, 78.
Bone histomorphometric studies have indicated that circulating PTH levels should be two to three times more than the upper limit of normal (ULN) to sustain the maintenance of normal bone metabolism 79. Based on this, the 2003 KDOQI guideline set the target range in dialysis patients as intact PTH between 150 and 300 pg/mL, and this range was also generally accepted as the target in Japan until 2006. However, in 2006, the JSDT established its own clinical practice guideline and introduced a new concept—with the aim of improving survival, stricter control of parathyroid function is needed. Analysis of the materials from the JSDT statistics survey showed a very gentle J-curve relationship between intact PTH levels and the risk of mortality. It is suggested that patients with intact PTH < 180 pg/mL had good 1- and 3-year prognosis, almost without any exception 31. Thus, the previous JSDT guideline set the recommended range of intact PTH between 60 and 180 pg/mL, in order to drive the parathyroid function towards a more suppressive direction. This concept of the previous guideline has been well-accepted worldwide and was rapidly adopted in clinical settings; however, the criticism was sometimes made that it was difficult to maintain parathyroid function according to the guideline because of the narrow target range.
Until the mid-2000s, many clinical studies had reported that better survival was associated with suppressed parathyroid function, similar to the aforementioned analysis 34, 78, 80. However, since the late 2000s, several studies reported that the relationship between parathyroid function and mortality draws a steep J- or U-shaped curve 29, 30, 81, indicating that excessive suppression of parathyroid function is also associated with poor survival. Based on these findings, the latest data from the JSDT registry were carefully reanalyzed, and it was confirmed that circulating intact PTH levels and mortality produces a U-shaped curve, and patients with intact PTH < 60 pg/mL have a significantly increased risk of poor survival. These results support introducing a lower limit to the target PTH range. However, why very low PTH levels are associated with poor prognosis has not yet been identified.
On the other hand, this re-analysis revealed that PTH levels higher than what has been conventionally thought to be acceptable, can be acceptable, which is supported by many clinical studies 29, 30, 81. In respect of these findings, we recommend that the upper limit of the target range should be raised from that of the previous guideline 6. This action, at the same time, would cope with any criticism of the PTH target range being too narrow.
However, since all the clinical studies mentioned above only analyzed a relatively short-term survival, it is uncertain whether applying these results would be beneficial to improve long-term survival. The long-term treatment policy that advocates “parathyroid function should be controlled to avoid the development of hyperparathyroidism” has been adopted in Japan, and the studies mentioned above do not deny its validity. Increasing the upper limit of the PTH target range could give clinicians an unintended message that it is acceptable for parathyroid function to be hyperactive. Furthermore, high PTH levels are reported to make the control of serum phosphorus/calcium difficult 82, which indirectly disturbs the achievement of therapeutic goals recommended by this guideline.
Taking all of the above into account, the JSDT committee decided in principle to conserve the policy proposed by the previous guideline 6, but to widen the target range slightly towards a higher direction. Thus, in this revised JSDT clinical practice guideline, the new standard target range for intact PTH levels is between 60 and 240 pg/mL.
The KDIGO guideline set the target range for parathyroid function among dialysis patients as intact PTH levels between two and nine times higher than the ULN in the general population 7. The lower limit of the target range proposed by the KDIGO practice guideline for CKD-MBD is less than the lower limit of the normal range. Similar to this guideline, the purpose of the KDIGO guideline is also to improve survival. Studies supporting the use of a sub-physiological lower limit of the intact PTH target range have now been published 11, 12, and therefore, this guideline recommends a target range even lower than the range set out by the KDIGO guideline.
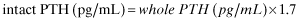
However, although intact PTH and whole PTH levels show linear correlation in groups of patients, whole/intact PTH ratios fluctuate widely between individuals 86, 87, and moreover, the ratio is reported to be affected by treatments for secondary hyperparathyroidism 88. For these reasons, this revised guideline abandoned the conversion formula, and added a target range for whole PTH, which was obtained by target intact PTH range approximately divided by 1.7. Whether this measurement is preferable to assess parathyroid function in dialysis patients needs further investigation.
Currently available medical strategies for suppressing parathyroid function include: (i) using therapies that control serum phosphorus/calcium levels; (ii) VDRA, and (iii) cinacalcet.
Management of serum phosphorus/calcium metabolism seems to contribute more to the improvement of survival than that of PTH control does 18, 78. However, clinical studies supported the contention that better parathyroid function control enables better control of serum phosphorus/calcium 82. Therefore, flexible interpretation is sometimes required in clinical practice. There is no established consensus as to whether VDRA or cinacalcet hydrochloride are superior tools for the treatment of secondary hyperparathyroidism. Multiple bioactive actions other than a parathyroid suppression effect are reported for VDRA 40, 41, 89, and paying attention to this advantage, there is an opinion that VDRA should be part of the management of all dialysis patients; even those do not need parathyroid suppression. On the other hand, cinacalcet hydrochloride not only has a strong effect in suppressing parathyroid function by itself, but also appears to increase the dose of VDRA by lowering circulating phosphorus/calcium levels.
Since cinacalcet hydrochloride is a drug with short biological half-life, the circulating PTH levels measured in patients taking this treatment show a nonnegligible circadian variation 14. Therefore, to standardize the assessment of parathyroid function in patients taking cinacalcet hydrochloride, this revised clinical guideline recommends obtaining blood samples at least 8 h after taking the drug to determine circulating PTH levels. However, this recommendation does not guarantee that the PTH level at this time-point is the best to reflect the parathyroid function in all patients. The most reliable method to assess parathyroid function in patients taking cinacalcet hydrochloride has not yet been established, and this evidence gap definitely requires further investigation.
Chapter 4: Indications and Methods of Parathyroid Intervention
Statements
- We recommend PTx for severe secondary hyperparathyroidism refractory to medical treatment (1B).A
- It is reasonable to consider selective PEIT if only one parathyroid gland is enlarged, and it is located at a puncturable site (No grade).
Supplementary notes
ASevere secondary hyperparathyroidism is defined as intact PTH levels >500 pg/mL or whole PTH levels >300 pg/mL. It is also reasonable to consider surgical PTx even at lower PTH levels if hyperphosphatemia or hypercalcemia is difficult to manage with medical treatment.
Rationale
Severe secondary hyperparathyroidism not only causes symptoms such as joint and bone pain, muscle weakness, and itching, but also affects survival adversely, mediated through vascular calcification 18, 25, 26, 29, 34. Indications for PTx should thus be considered in the context of its impact on survival and secondary hyperparathyroidism-related symptoms. PTx for patients with secondary hyperparathyroidism results in dramatically reduced PTH levels, improved control of serum phosphorus and calcium levels, amelioration of secondary hyperparathyroidism-related symptoms 90-93, histological improvement in high-turnover bone disease 94, 95, and increased bone mineral density 96-98. Furthermore, observational studies suggest the potential of PTx to reduce the risk of bone fracture 99 and mortality 100-103. Although further investigations are required, these data form a basis for the recommendation of surgical PTx for patients with severe secondary hyperparathyroidism refractory to medical treatment.
In dialysis patients with secondary hyperparathyroidism, persistent PTH hypersecretion is associated with progression of parathyroid hyperplasia, from diffuse to nodular hyperplasia. The presence of nodular hyperplasia can be suggested by the size of parathyroid gland, and it is suggested that parathyroid enlargement with an estimated volume of ≥500 mm3 or ≥1 cm in diameter is a useful indicator of nodular hyperplasia 104. Nodular hyperplasia is associated with more marked proliferation 105-107 and reduced expression of vitamin D receptors 108 and calcium-sensing receptors 109, 110, and patients with parathyroid hyperplasia in excess of the abovementioned size often develop resistance to medical therapies including VDRA 111-114. Therefore, the presence of nodular hyperplasia or its suggestive parathyroid enlargement is an important factor to consider indications for PTx.
On the basis of these rationales, the former JSDT guideline recommended surgical PTx for patients with intact PTH levels higher than 500 pg/mL, in whom the presence of enlarged parathyroid glands is strongly suggested 6. It is, however, noteworthy that this threshold is lower than the target range for intact PTH recommended in the KDIGO guideline (two to nine times the upper limits of normal, corresponding to 130 to 600 pg/mL) 7, providing a relatively early indication for PTx compared to other countries. Although it is still unclear whether such an early indication for PTx provides a survival benefit in patients with secondary hyperparathyroidism, observational studies have shown increased risks of mortality associated with intact PTH levels higher than 400 to 600 pg/mL 18, 25, 26, 29, 34. A recent observational study in Japan also reported a lower likelihood of achieving the JSDT targets for serum calcium and phosphorus in patients with intact PTH levels higher than 500 pg/mL and a higher likelihood of achieving these targets in patients with a history of PTx 82. Furthermore, the better survival of patients receiving maintenance dialysis in Japan compared to other countries 115 raises the possibility that prolonged medical treatment for moderate to severe secondary hyperparathyroidism may lead to further progression of the disease. Accordingly, the current JSDT guideline followed the threshold for PTx recommended in the former guideline. When whole PTH assays are used, patients with PTH levels higher than 300 pg/mL should be considered for PTx, based on the reported correlation between intact PTH and whole PTH 116, 117. It is reasonable to consider surgical PTx even at lower PTH levels if hyperphosphatemia or hypercalcemia is difficult to manage with medical treatment.
In addition to the indications mentioned above, PTx should be considered more readily in the presence of the following features: (i) symptoms of severe secondary hyperparathyroidism, (i) increased serum markers of bone turnover (such as ALP), (iii) radiological signs of bone lesions (such as salt and pepper skull, rugger jersey spine, and subperiosteal resorption in the phalanges), and (iv) progressive ectopic calcifications (vascular calcification, cardiac valve calcification, and tumoral calcinosis). Several studies reported attenuation of progressive vascular calcification 118, regression of tumoral calcinosis 119, 120, and amelioration of anemia 121, 122, hypertension 121, 123 and cardiac function 124-126 in patients who underwent PTx for severe secondary hyperparathyroidism. Surgical series also reported clinical improvement of calciphylaxis following PTx 127-129.
One of the major changes since the release of the previous JSDT guideline is the introduction of cinacalcet hydrochloride to the Japanese market 37. Several studies have shown the efficacy of cinacalcet for patients with secondary hyperparathyroidism refractory to VDRA treatment and those with marked parathyroid hyperplasia, where surgical PTx can be the treatment of choice 130, 131. Indeed, a post-hoc analysis of randomized clinical trials reported significant reductions in PTx in patients treated with cinacalcet compared with the placebo group 53. Thus, indications for PTx and cinacalcet treatment overlap significantly. Given the absence of evidence comparing these two treatment approaches, we suggest that therapeutic decision be made on a case-by-case basis, considering the patient's wishes and general condition. Patients who are refractory to cinacalcet treatment, and those who discontinued treatment due to adverse effects should be considered for PTx.
There are different variations on the procedure of PTx, including subtotal PTx, total PTx with autotransplantation, and total PTx without autotransplantation. Studies from other countries reported no significant differences regarding the efficacy and recurrence rate between subtotal PTx and total PTx with autotransplantation 132, 133. However, the risk of recurrent secondary hyperparathyroidism after subtotal PTx should not be negligible, particularly in Japanese dialysis patients who would require long-term dialysis after surgery. Total PTx is frequently performed as the initial operation in Japan, because adhesion to surrounding tissue after subtotal PTx causes difficulties in the procedure of reoperation for recurrent secondary hyperparathyroidism. Because total PTx without autotransplantation may cause extremely low PTH levels and its long-term impact on outcomes is unclear, total PTx with autotransplantation is the current standard procedure in Japan. It is important, however, to note that the impact of autotransplantation on patient-level outcomes has not been examined sufficiently and the best surgical approach is still a matter of debate. The PTx procedure should be performed by skilled operators anyway.
For preventing persistent or recurrent secondary hyperparathyroidism after PTx, it is important to search for ectopic parathyroid glands, such as thymic and mediastinal glands, at the initial operation 134, 135. We thus suggest that 99mTc-MIBI scintigraphy 136-138, in addition to neck ultrasonography, be performed as a preoperative imaging. Application of other imaging modalities such as CT and MRI might also be considered, if necessary.
After successful PTx, patients usually develop a marked net increase in bone uptake of calcium (i.e. hungry bone syndrome) 139 and require calcium supplementation. For patients with severe hungry bone syndrome developing a rapid and progressive reduction in serum calcium, administration of intravenous calcium through a central venous line is often required. Long-term management of serum phosphorus and calcium levels after PTx should be considered similarly to that of general dialysis population (see Chapter 2).
If the enlarged parathyroid is accessible to puncture, control of secondary hyperparathyroidism can be achieved by PEIT 140-142; it constitutes an effective therapeutic approach, especially for patients with refractory secondary hyperparathyroidism for which PTx is not yet indicated (intact PTH levels between 400 and 500 pg/mL) and for those who cannot undergo surgery. The indication for PEIT should, however, be restricted to those with one enlarged gland, as long-term control of PTH with PEIT is difficult if two or more enlarged glands are involved 143. Following PEIT, it is important to manage remaining hyperplastic glands with medical therapy, including intravenous VDRA. The indications and techniques for PEIT should follow the guideline by the Japanese Society for Parathyroid Intervention 144. It must be noted that PEIT often causes adhesion to surrounding tissue, which makes it difficult to identify the recurrent laryngeal nerve and increases the risk of nerve damage during subsequent PTx 145. As in the case of PTx, the PEIT procedure should be performed by skilled operators.
Chapter 5: The Assessment and Control of Bone Metabolism
Statements
- It is reasonable to maintain PTH levels within the target range as set out in Chapter 3 (2; No grade).A,B
- It is reasonable to maintain the levels of bone metabolic markers, including ALP, within the standard range of each medical institute (2; No grade).C
- It is reasonable to consider bone biopsy if bone pain, a recurrent pathological fracture, delayed healing of a bone fracture, any other bone symptoms requiring treatment, or bone conditions of unknown etiology occurs (2; No grade).
Supplementary notes
APTH levels could also be regarded as a bone metabolic marker
BSevere hyperparathyroidism potentially promotes increased bone fracture risk.
CALP levels may be regarded as a bone metabolic marker in patients without significant hepatobiliary disease.
Rationale
Variable forms of systemic bone metabolic diseases are found in patients with CKD. Such abnormal bone metabolism used to be called renal osteodystrophy. However, the term renal osteodystrophy purely indicates histomorphological changes in bone tissue found in CKD patients 7, without reference to the systemic nature of bone metabolism changes, and so it has now been largely replaced by the term CKD-MBD.
There have been reported few clinical studies that could be used as the base to consider bone metabolism from the perspective of improved survival. Therefore, bone fracture, which is an important outcome associated with bone metabolism, is also taken into consideration to establish the assessment and treatment strategy in this revised guideline. However, like in the general population, it is already established that a bone fracture event has a strong association with survival and mortality among dialysis patients 146, 147.
The prevalence of hip fracture among dialysis patients is considerably higher than that in the general population 148; however, the reason remains obscure. It is sometimes explained that an increased risk of fall, due to muscle weakness, is the main cause of hip fracture 149, yet the possibility that the uremic condition itself causes bone fragility has not been negated either. On the other hand, it remains unconfirmed whether the risk of spinal bone fracture is high among CKD patients 150.
Circulating PTH levels reflect bone turnover, showing an even better correlation with it than other bone metabolic markers do among dialysis patients 79, 151. Because PTH action on bone is blunted in the CKD condition, maintaining a bone turnover level comparable to the normal population in dialysis patients requires two- to three-fold more PTH than the upper limits of normal (ULN) of the PTH range in the general population. Note that this means bone turnover is likely to become comparable to that in the normal healthy population, but does not mean that bone and mineral metabolism will reach a normal level in patients with CKD.
In fact, the relationship between parathyroid function and fracture risk is not so clear-cut. However, it is a common bedside clinical observation, derived from extensive clinical experience, that severely overactive parathyroid function promotes increased fracture risk, and many clinical studies support this 152, 153. Thus, this revised guideline advises that severe hyperparathyroidism could increase fracture risk in patients with CKD. On the other hand, it is still controversial as to whether extremely suppressed parathyroid function increases fracture risk among dialysis patients 153-155.
Bone metabolic markers other than PTH mainly indicate the level of bone turnover, rather than bone metabolism. Aside from ALP, markers such as bone ALP 156, the cross-linked N-telopeptide acid of type I collagen 157, and TRACP5b 158 have been reported to predict bone turnover 159. Bone turnover is one of the factors affecting bone strength, and therefore, levels of these markers possibly contribute to the maintenance of bone quality; however, it remains unclear whether these markers could predict the risk of fracture solely by themselves. On the other hand, circulating levels of some bone metabolic markers demonstrate associations with cardiovascular disease and mortality 160, 161. The tightness of the relationship between circulating ALP levels and survival deserves particular attention 162, 163.
Bone mass is generally slightly decreased among patients with CKD and the result of bone mineral densitometry cannot accurately predict the risk of future bone fractures among these patients 164. However, because bone mass is a considerable, if not the most considerable, factor determining bone strength even among CKD patients, the significance of bone mineral densitometry is not fully negated.
KDIGO proposed a new histomorphometric classification of renal osteodystrophy termed the “Turnover-Mineralization-Volume” criteria, which designates bone turnover, bone mineralization rate and cancellous bone volume per unit on three assessment axes 165. However, the JSDT clinical practice guideline does not yet recommend introducing this new classification because the method of measurement has not been fully established and moreover, its clinical utility has not been fully validated. On the other hand, diagnostic terms based on conventional histomorphometric classification, such as “osteitis fibrosa”, “osteomalacia”, and “adynamic bone”, are still being used. These terms are based on morphological findings, which cannot be obtained by non-invasive diagnostic methods. Therefore, it is inappropriate to use these terms for patients who have not undergone bone biopsy examination. Bone biopsy is the only method to diagnose bone disease in patients with CKD according to the bone histopathological classification. Importantly, no other diagnostic test can detect disorders in bone mineralization. However, in daily clinical practice, the indications for bone biopsy are limited.
Chapter 6: Diagnosis and Treatment of Bone Complications Associated with Dialysis-related Amyloidosis
Statements
- The diagnosis of bone complications associated with dialysis-related amyloidosis is recommended to be made using radiological images (1B).A
- Properly modified blood purification therapy might be helpful to retard the development of bone complications associated with dialysis-related amyloidosis (2C).B
Supplementary notes
AMagnetic resonance imaging (MRI) is useful for a diagnosis of spinal bone and/or cord lesions.
BA combination of direct hemoperfusion therapy using a β2-microglobulin adsorption column with ordinary blood purification therapy ameliorates both subjective and objective symptoms.
Rationale
Dialysis-related amyloidosis, which is also referred to as β2-microglobulin associated amyloidosis, is a systemic amyloidosis that is specifically found among CKD patients 166. Although this condition can be found in the predialysis stage, it tends to be recognized as a specific complication for HD patients with a vintage of more than 10 years. Although dialysis-related amyloidosis is classified as one category of systemic amyloidosis, bone and larger joints are most frequently involved, forming its specific clinical features.
Aseptic inflammation is frequently present in tissues surrounding β2-microglobulin amyloid depositions, which cause arthralgia and/or joint contractions. Specifically, inflammatory osteolytic lesions such as bone cysts are often documented nearby those affected joints 167, which can become the point of mechanical weakness in large weight-bearing joints. Joint contractions also promote the risk of fall. For the above reasons, dialysis-related amyloidosis is believed to increase fracture risk in long-term dialysis patients 168, 169. Thus, dialysis-related amyloidosis and its associated bone and joint complications seem to share clinical needs with CKD-MBD from the perspective that they both increase the risk of causing motor impairment. However, the consensus is not yet obtained as to whether this disease condition should be regarded as a component of CKD-MBD, even among experts.
Osteolytic lesions associated with dialysis-related amyloidosis are primarily diagnosed by radiological investigation. MRI is recommended to guide treatment strategies for spinal lesions that can cause severe clinical outcomes 170. MRI sometimes detects spinal cord lesions that are not detectable by simple X-ray images. Spinal/spinal cord lesions can precede the development of carpal tunnel syndrome. An adequate screening schedule to detect severe cases has not yet been established. However, it seems not to be efficient to perform screening examinations on all patients under maintenance HD therapy, since bone and joint lesions associated with dialysis-related amyloidosis are seldom observed in those patients under maintenance HD therapy for less than 10 years. It is difficult to evaluate the disease condition of bone complications associated with dialysis-related amyloidosis and to predict its future development by blood biochemical examinations. Deposition of β2-microglobulin amyloid fibrils around the affected lesions must be confirmed by histological examinations in order to make a definite diagnosis of dialysis-related amyloidosis.
Properly modified blood purification therapy, such as ultra-purification of dialysate, the use of type IV/type V dialysis membranes, hemodiafiltration, combined use of direct hemoperfusion using a β2-microblobuin adsorption column, may retard the progression of bone complications associated with dialysis related amyloidosis, and ameliorate clinical symptoms such as bone/joint pains and/or limitation in joint motility 171, 172. A recent study reported the effect of the direct hemoperfusion therapy on osteolytic lesions 173.
The prevalence of dialysis-related amyloidosis may be decreasing these days, and if this is true, the improvement of blood purification therapy during later years would have played an important role in this favorable trend. Nevertheless, the mechanism as to how a properly modified blood purification method suppresses the development of dialysis-related amyloidosis, and its associated bone complications, remains unknown. Use of a small amount of glucocorticoid agents also ameliorates the subjective symptoms of bone and joint complications associated with dialysis-related amyloidosis; however, some experts believe that the over-use of glucocorticoids has a potential risk to promote further bone destruction. Even if initial beneficial effects are successfully obtained, symptoms generally recur immediately after the discontinuation of these therapies mentioned. The development of bone and joint complications associated with dialysis-related amyloidosis stops after receiving kidney transplantation; however, it is uncertain whether the bone lesions heal thereafter 171.
In contrast with diffuse bone lesions associated with abnormal systemic mineral metabolism, those associated with dialysis-related amyloidosis tend to be multiple well circumscribed focal lesions where the border between the affected area and disease-free area is clearly distinguishable. Therefore, severe lesions often become amenable to orthopedic surgery. Surgical therapy can be indicated for lesions associated with existing pathological fractures, and also for spinal osteolytic lesions that have a considerable risk for causing future severe spinal cord injury. There is no consensus for the surgical management strategy for osteolytic lesions not associated with fractures, apart from those in the vertebrae.
Chapter 7: Management of Vascular Calcification
Statements
- Since vascular calcification is frequently observed in dialysis patients and is an important factor of their prognosis, we suggest that it be evaluated whenever clinically necessary (2A).
- We suggest that calcification of the aorta and femoral artery should be confirmed by plain radiography of the chest, abdomen, pelvis, or lateral view of the lumbar vertebrae (2B).A
- We suggest that aortic calcification be further evaluated using abdominal plain computed tomography (CT) scans and for coronary arteries, using the calcification score on coronary artery CT if necessary (2A).B
- To prevent the progression of vascular calcification, control of calcium and phosphorus metabolism and, particularly, the control of the serum phosphorus levels, are important. We recommend using calcium-free phosphorus binders if possible (1B).
Supplementary notes
AParticularly suited for the detection of atherosclerotic calcification.
BCT scan is useful for the detection and quantification of vascular calcification and provides prognostic information.
Rationale
In dialysis patients, the prevalence of death due to cardiovascular disease accounts for more than 40% of all-cause mortality. In addition, the number of patients with vascular calcification is markedly greater in dialysis patients than that in non-dialysis patients. Therefore, arteriosclerosis with vascular calcification may be an important pathophysiological mechanism in the development of cardiovascular disease. Vascular calcification is known to be an important risk factor influencing mortality in dialysis patients. Though vascular calcification has been considered a passive phenomenon occurring because of cell degeneration, recent studies have described it as an active process 174.
To consider the prevention and treatment of vascular calcification, its detailed pathophysiological mechanisms must be understood 175. Vascular calcification is divided into two major types based on the mechanism of atherosclerotic formation: one mechanism involves atherosclerotic calcification occurring in the intimal layer of the artery, and the other mechanism involves Monckeberg's arteriosclerosis occurring in the medial layer of the artery. The former type of calcification, which tends to progress around a lipid core lesion, involves cellular apoptosis and inflammation. The latter type of calcification is often observed in patients with advanced age, diabetes mellitus, and CKD. The supposed mechanisms of Monckeberg's arteriosclerosis involve transformation of vascular smooth muscle cells into osteoblast-like cells by the uptake of phosphorus into cells through sodium-dependent phosphorus co-transporters and decrease of inhibitors against vascular calcification. These two types of vascular calcification are closely related to the uremic state and accelerators of vascular calcification, including hypercalcemia and hyperphosphatemia. Even in young dialysis patients, vascular calcification is frequently observed. Progression of vascular calcification is related to diabetes, surplus intake of a calcium-containing serum phosphorus binder, and increased calcium-phosphorus products 57. Table 4 shows important risk factors for vascular calcification. As already described in earlier chapters, CKD-MBD is a term used to describe a broader clinical syndrome that includes a systemic disorder of mineral and bone metabolism and vascular calcification related to CKD 5.
| Age |
| Dialysis vintage |
| Diabetes mellitus |
| Severe hypertension |
| Hyperphosphatemia |
| Calcium × serum phosphorus product |
| Excess intake of calcium-containing phosphorus binder |
Several methods are used to evaluate vascular calcification in the clinical setting, including chest and abdominal X-ray (Fig. 3: arrows) and CT scanning (Figs 4 and 5: arrows). Chest and abdominal X-rays are a simple and non-invasive method to evaluate vascular calcification. Some reports have shown that the presence of vascular calcification was a significant and independent determinant for cardiovascular mortality using this method 176, 177. CT is also a useful method for detection and quantification of vascular calcification. Electron beam CT was explicitly developed to provide better images of heart structures, which never stop moving. One complete cycle of movement is performed with each heartbeat. The coronary artery calcification score is a measure of calcium generally included in the results from a CT test for coronary calcification. The score is calculated using a weighted value assigned to the highest density of calcification in a given coronary artery (the Agatston method). A correlation between this score and cardiovascular events has been reported in HD patients 178. With the advent of subsecond rotation combined with multi-detector CT, high resolution, and high speed can be obtained simultaneously, allowing excellent imaging of the coronary arteries. In one published study of aortic calcification in CKD-MBD, the aortic calcification index was assessed using abdominal CT. In brief; 10 slices were examined at 1-cm intervals above the bifurcation of the common iliac vessels. A cross-section of the abdominal aorta on each slice was divided into 12 sectors and aortic calcification index was obtained by counting the number of calcified sectors 179. Other recent studies have reported a compositional plaque pattern in the coronary artery using intravascular ultrasound (Fig. 6) 180. An association between pulse wave velocity and degree of vascular calcification has also been reported as an important predictor for cardiovascular events 181, 182. These evaluation methods are useful for prediction of patient prognosis and review of treatment.
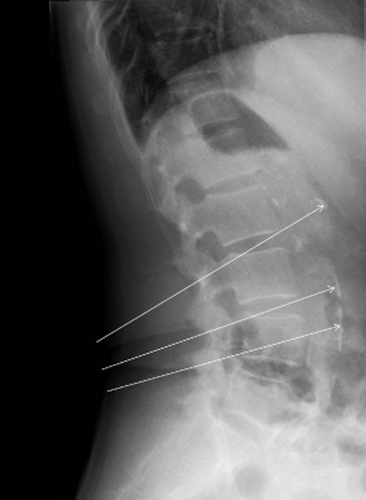
Plain X-ray. The arrows show calcification.
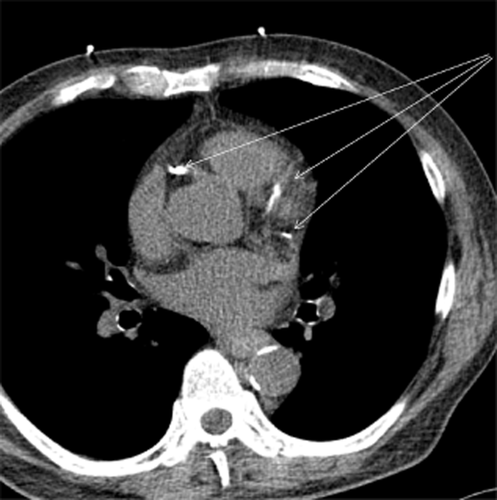
Thoracic computed tomography (CT). The arrows show coronary artery calcification.
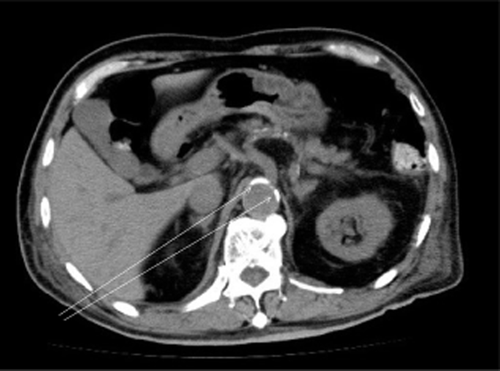
Abdominal computed tomography (CT). The arrows show aortic calcification.
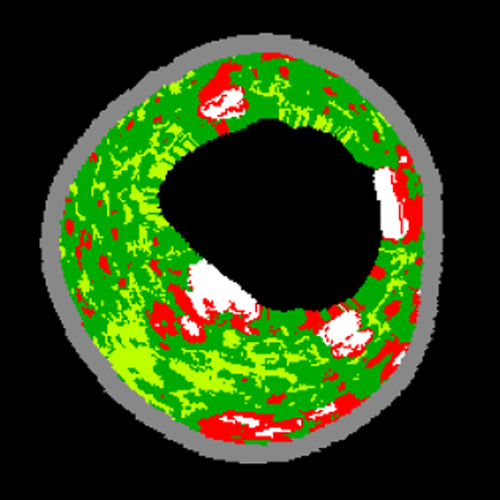
A coronary artery VH-IVUS image. Plaque images are reconstructed as coronary tissue maps that were color-coded by four major plaque components (fibrous, fibro-fatty, necrotic core and dense calcium). VH-IVUS, virtual histology intravascular ultrasound.
In the prevention and treatment of vascular calcification, control of mineral disorders, including abnormal serum phosphorus and calcium levels, is most important (see Chapter 1) 4, 6, 18, 34.
For appropriate control of serum phosphorus levels, restriction of phosphorus intake (≤700 mg/day) and sufficient dialysis to eliminate excess serum phosphorus are essential elements of basic treatment. However, as elimination of phosphorus by dialysis is incomplete; administration of phosphorus binders is necessary in most patients. Calcium-containing serum phosphorus binders are commonly used in the clinical setting (Table 2). These should be used cautiously as they can cause hypercalcemia. In Japan, two types of non-calcium-containing phosphorus binders are available—sevelamer hydrochloride and lanthanum carbonate. Several studies have reported that sevelamer hydrochloride prevents the progression of coronary and aortic calcification 58, 61, 183, 184. The anti-calcific effect of sevelamer hydrochloride may have some pathophysiological mechanisms. Decreased serum low-density lipoprotein (LDL) cholesterol levels and increased serum high-density lipoprotein (HDL) cholesterol levels have been observed as effects of sevelamer hydrochloride. In addition, sevelamer hydrochloride decreases advanced glycation end products. Such effects may be associated with prevention of the progression of vascular calcification. An in vivo study showed that lanthanum carbonate restricted the development of vascular calcification in rats 185, and another clinical study demonstrated that control of serum phosphorus levels using lanthanum carbonate was associated with reduced progression of vascular calcification compared with CaCO3 treatment in hemodialysis patients 186.
Dialysis patients are likely to develop hypercalcemia when taking high doses of calcium-containing phosphorus binders and concomitant VDRA administration. In order to prevent hypercalcemia and excessive calcium load, restriction of calcium in meals, administration of appropriate doses of calcium-containing phosphorus binders and VDRA, and the use of low calcium dialysate levels should be considered. VDRA are frequently administered for medical control of hyperparathyroidism. While the direct effect of VDRA on vascular calcification has been demonstrated in a number of experiments, very little clinical data are available in this regard, and some reports have even suggested that VDRA has no such effect 187. Nevertheless, to avoid vascular calcification, when VDRA is prescribed in dialysis patients, serum calcium and phosphorus levels should be controlled within the target range.
This guideline recommends a target level of serum intact PTH of 60–240 pg/mL. Since survival is the priority in this guideline, the adjustment of serum phosphorus and calcium levels is prioritized over that of PTH levels. However, elevated PTH levels can also be associated with prognosis. A previous report suggested that PTH levels increased with an increase in the severity of coronary calcification 188. Therefore, in patients continuously showing high PTH levels after attaining the target range for serum phosphorus control, it is important to attempt to control PTH levels with VDRA and cinacalcet. Cinacalcet hydrochloride has been reported to inhibit calcification 189. In one study, the number of deaths due to cardiovascular disease and overall mortality among patients treated with cinacalcet and VDRA were lower than the corresponding values in patients treated with VDRA alone 54. Parathyroid interventions such as PEIT or PTx are indicated in patients with medication-resistant parathyroid enlargement. PTx was reported to improve coronary calcification or inhibit progression of coronary calcification 118.
Impaired lipid or glucose metabolism may be another factor affecting vascular calcification. According to the guidelines for evaluation and treatment of cardiovascular complications in hemodialysis patients, LDL cholesterol levels <120 mg/dL or non-high density lipoprotein cholesterol levels <150 mg/dL have been reported to be responsible for the primary prevention of ischemic heart diseases 190. In addition, 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins) for treating hyperlipidemia have attracted attention for their anti-arteriosclerotic action and lipid-lowering effect. In non-dialysis patients, statin treatment does not inhibit calcification, according to one report 191. However, some reports have stated that the reduction of serum LDL cholesterol levels by statins inhibited coronary calcification 192, 193. The frequency and severity of aortic or coronary calcification significantly increased in patients with diabetes 194, 195. Therefore, adequate control of these metabolic impairments is imperative. Warfarin and vitamin K treatment may affect calcification via the following mechanism: warfarin may interfere with the activity of matrix Gla protein, a calcification-inhibiting factor, and vitamin K may promote this interference by warfarin 196, 197. Accordingly, adjustment of the dosages of warfarin and vitamin K may contribute to inhibition of vascular calcification.
Chapter 8: CKD-MBD in Peritoneal Dialysis Patients
Statements
- The target serum phosphorus, calcium, and PTH levels for PD patients are the same as those for HD patients. It is reasonable to start correcting these values in PD patients when the values increase, even if they are below the upper limit for HD patients (No grade).A
- In order to maintain appropriate serum phosphorus levels, we recommend dietary phosphorus restriction, maintenance of residual kidney function to assure phosphorus excretion and a timely prescription of phosphorus binders (1B).B
- Use of a low-calcium (2.5 mEq/L) dialysate reduces the occurrence of hypercalcemia and corrects low-turnover of bone. On the other hand, it has been reported that such dialysates might promote the progression of secondary hyperparathyroidism. We recommend keeping this possibility in mind when prescribing these dialysates (1C).C,D
Supplementary notes
ABecause PD is a continuous purification technique, serum calcium, phosphorus, and PTH levels are relatively unchanged regardless of the timing of treatment. In this respect, PD differs markedly from hemodialysis, in which their concentrations in the blood change at each treatment.
BSevelamer hydrochloride, lanthanum carbonate, and cinacalcet have been confirmed to be just as effective in PD patients as in HD patients.
CPD dialysates are divided into two types from the viewpoint of the transperitoneal calcium balance. One is standard-calcium dialysates (3.5 mEq/L), which transperitoneally load calcium into the body, and the other type is low-calcium dialysates (2.5 mEq/L), which eliminate calcium from the body.
DCaution is necessary not to decrease serum calcium levels during the introduction period. Particularly, the use of low-calcium dialysates in patients with sustained residual kidney function may further reduce serum calcium levels and exacerbate secondary hyperparathyroidism; therefore, the aggressive use of standard-calcium dialysates should be considered. In general, the use of 4.0 mEq/L calcium dialysates is not recommended.
Rationale
Abnormal serum levels of calcium and/or phosphorus are independent risk factors for poor survival in patients undergoing PD. A prospective study from the Netherlands reported that the risk of cardiovascular-related death was significantly increased in patients with serum phosphorus levels ≥5.5 mg/dL or a serum calcium–phosphorus product ≥55 mg2/dL2 198. In PD, blood is continuously purified; therefore, the calcium/phosphorus blood levels remain relatively stable independent of the schedule of PD bag exchange. In patients on HD, serum calcium/phosphorus levels present cyclic changes due to the nature of intermittent dialysis. In HD patients, the reference value is obtained at the beginning of the first dialysis session of the week. Therefore, any PD patient who presents with fluctuations of serum calcium or phosphorus values outside of the target levels should certainly be treated without delay.
In patients undergoing PD, about 200–300 mg of phosphorus can be removed daily by the trans-peritoneal pathway. In light of the daily phosphorus intakes by meals (up to 700–800 mg daily), it is not easy for most patients to maintain adequate serum phosphorus levels by PD therapy alone. Therefore, to achieve target serum phosphorus levels in PD patients, dietary restriction of phosphorus in meals, preservation of residual kidney function for phosphorus excretion, and the use of an appropriate phosphorus binder, are crucially needed, which needs patient education. In terms of the clinical aspects of phosphorus control, it is important to take the following points into consideration: adequacy of PD dialysis dose, and changes of kidney and peritoneal functions.
The dialysis dose of a PD patient is evaluated by urea Kt/V per week (weekly Kt/V), with consideration given to the residual renal function. It is recommended to achieve a Kt/V of 1.7 as a minimum value, and ideal if a value ≥1.8 is achieved 199-203. Over time, an eventual decline in residual kidney function decreases the excretion of phosphorus in the urine. Furthermore, changes in peritoneal membrane function occurring with time could influence the efficiency of trans-membrane excretion of phosphorus. According to the changes of those two pathways over time, dietary phosphorus restriction should be properly tailored.
Regarding the use of phosphorus binders, there are several points to be emphasized. Excessive calcium dosing with a calcium–phosphorus binder may cause vascular calcification or calciphylaxis, and patients should be carefully monitored to avoid hypercalcemia. Further, in patients with impaired bowel motility/history of bowel obstruction, close monitoring is required for the use of sevelamer hydrochloride, lanthanum carbonate, or cinacalcet hydrochloride. These agents are known to induce gastrointestinal symptoms, although the incidence in PD patients is reportedly equivalent to those on HD. Peritoneal calcification could be a unique sign of hyperparathyroidism. But peritoneal calcification in PD patients may indicate the presence of severe peritoneal sclerosis, and it is not always necessarily related to the local vascular calcification or impaired mineral metabolism 204-212.
A low PTH level, coupled with a low bone turnover, used to be considered to be a characteristic of PD patients. In reviewing the historical background of this issue; during the 1980s, a dialysate with high normal calcium levels (3.5 mEq/L) (normal calcium dialysate) was the only available dialysate. And it is now considered that the use of this solution was connected with the pathology of a low turn-over bone state, because it overloaded patients with excess calcium, resulting in excessive PTH suppression.
In the 1990s, a dialysate with low calcium levels (2.5 mEq/L) (low calcium dialysate), was introduced to avoid hypercalcemia and to fully utilize CaCO3 and VDRA. At present, a low-calcium dialysate is used from the initial phase of PD. Low calcium dialysate decreased the incidence of hypercalcemia, and; furthermore, it stimulated low PTH to increase 213, 214. In a cross-sectional observational study conducted in Japan 215, PTH levels were compared between a calcium 3.5 mEq/L-dialysate group and a calcium 2.5 mEq/L-dialysate group, according to PD vintage. Although the two groups showed no differences in serum calcium and phosphorus levels, the usage of CaCO3 and VDRA was higher in the latter group. Moreover, to note, PTH concentrations were relatively higher in the latter group 215. These findings indicate that a negative calcium balance in PD could play a crucial role in stimulating the release of PTH, and, therefore, use of a high-normal calcium dialysate should be considered as first choice to avoid the hypocalcemic state, especially during the introduction phase. Unlike HD therapy using a central dialysis fluid delivery system, tailor-made prescription of dialysate calcium concentration is primarily needed to control adequate PTH levels, and the pharmacological therapy for secondary hyperparathyroidism needs to be based on this strategy (e.g. the efficacy of cinacalcet has been confirmed in PD patients) 216.
Chapter 9: Predialysis CKD-MBD
Statements
- Parameters to be measured and frequency of their measurements:
- Measurements of serum phosphorus, calcium, PTH, and ALP levels should be made once patients reach CKD stage 3 (1C).
- It is reasonable to measure serum phosphorus, calcium, and ALP levels every 6–12 months in patients with CKD stage 3, every 3–6 months in stage 4, and every 1–3 months in stage 5 (No grade).
- It is reasonable to measure PTH levels in patients with CKD stage 3 (to establish a baseline value) and, thereafter, every 6–12 months in CKD stage 4, and every 3–6 months in stage 5 (No grade).A
- We suggest that measurements of bone mineral density (BMD) be considered in patients with CKD stage 1–2 and CKD stage 3 patients without biochemical abnormalities as in the general population (2B).B
- We suggest that measurements of bone markers be considered in patients with CKD stage 1–2 and CKD stage 3 patients without biochemical abnormalities as in the general population (2C).B,C
- Indications for bone biopsy in predialysis CKD patients are the same as those in patients receiving maintenance dialysis (No grade).
- Target ranges for biochemical parameters and treatment approaches to achieve these targets:
- Management of serum phosphorus and calcium levels:
- We suggest that serum phosphorus and calcium levels be maintained within the reference ranges of each facility (2C).
- It is reasonable to control serum phosphorus levels by dietary phosphorus restriction and/or the administration of phosphorus binders (No grade).D
- It is reasonable to control serum calcium levels by the administration of a calcium-containing phosphorus binder and/or oral VDRA and adjustment of their doses (not graded).E
- Management of PTH levels:
- If PTH levels exceed the upper limit of the target range, it is reasonable to consider treatment to lower these levels (No grade).
- It is reasonable to control PTH levels by dietary phosphorus restriction, the use of a calcium-containing phosphorus binder, and/or the administration of oral VDRA (No grade).
- We recommend avoiding causing abnormalities in serum phosphorus or calcium levels and deterioration of renal function when controlling PTH levels (1C).F
- If patients with CKD stage 1–2 fulfill the criteria for the initiation of medications to prevent fragility fractures,B we recommend treatments for osteoporosis similar to those for the general population (1A). For patients with CKD stage 3 without biochemical abnormalities of CKD-MBD, also, we suggest therapeutic strategies similar to those for the general population (2B).G
- Management of serum phosphorus and calcium levels:
Supplementary notes
AIn the face of abnormal laboratory values, treatment changes, and rapid progression of CKD, more frequent measurements should be considered.
BWe suggest that the Japanese Guideline for the Prevention and Treatment of Osteoporosis (2011 edition) are followed.
CWhile bone-specific ALP and tartrate-resistant acid phosphatase 5b (TRACP5b) are unlikely to be affected by renal function, their predictive values for bone diseases in predialysis CKD patients are poor, and there is insufficient evidence as to whether these biomarkers predict the risk of fractures in these patients.
DIn Japan, CaCO3 is the only serum phosphorus binder available for the management of predialysis CKD patients.
EIn Japan, alphacalcidol and calcitriol are the only VDRA available for the management of predialysis CKD patients.
FCaution should be exerted not to allow the occurrence of hypercalcemia or exacerbation of the renal function by overdosing of CaCO3 or oral VDRA. Adverse effects of alphacalcidol and calcitriol on renal function have been reported to be rare up to a dose of 0.5 μg/day and 0.25 μg/day, respectively.
GNo treatment for low BMD has been established for patients with CKD stage 3 with biochemical abnormalities of CKD-MBD, and those with CKD stage 4–5.
Rationale
Abnormalities in bone and mineral metabolism begin to develop early during the course of CKD 217-221. In the early stage of CKD, serum levels of PTH and fibroblast growth factor 23 (FGF23) 222, 223, an osteocyte-derived phosphaturic hormone, increases to maintain normal serum phosphorus levels by enhancing urinary phosphorus excretion 224-226. However, in the advanced stages of renal failure, decreased nephron mass leads to overt hyperphosphatemia, decreased production of 1,25(OH)2D, and resultant hypocalcemia—all of which further stimulate PTH secretion 227, 228. Management of these abnormalities is critically important in patients with CKD, because disordered mineral metabolism not only causes abnormal bone metabolism 151, 159, 229-236, but is also associated with the progression of CKD 237, 238, and elevated risks of cardiovascular events 162, 239-241 and mortality 162, 239-248. Despite an absence of evidence from interventional trials, based on expert consensus opinion, it would be reasonable that the biochemical parameters in CKD-MBD are managed in an attempt to prevent or ameliorate bone and mineral metabolism abnormalities, to prevent cardiovascular events resulting from ectopic calcification and to reduce mortality.
Because the patient's profile of CKD-MBD can alter over time—presumably due to progression of CKD and variations in medications administered, it is suggested to regularly monitor biochemical parameters of CKD-MBD in order to appropriately interpret the condition of CKD-MBD and to guide therapeutic decision making. In general, elevations in PTH occur as early as CKD stages 2–3 and hyperphosphatemia and hypocalcemia begin to occur in CKD stage 4 217-221. Therefore, assessments for these parameters should begin at CKD stage 3. It is reasonable to base the frequency of biochemical monitoring in Japan on the KDIGO guideline 7, but it should be recognized that the frequency of assessment needs to be adjusted according to the presence and magnitude of identified abnormalities, the change of medications, and the rate of progression of CKD.
In addition to the biochemical parameters described above, recent observational data identified serum ALP as a potential biomarker of CKD-MBD. In predialysis CKD patients, elevated serum ALP levels have been associated with types of bone disease 159, 232, 234-236 and adverse clinical outcomes including bone fractures 162, cardiovascular events 162, 239, 240, and mortality 162, 239, 240, 247. Because serum ALP has been reported to be lowered by VDRA 249-255, these levels can be controlled in a specific range with therapeutic interventions. Based on these facts, this guideline included serum ALP as a routine clinical measurement for the diagnosis and assessment of CKD-MBD. It is, however, still unclear whether controlling serum alkaline phosphatase within a specific range actually provides survival benefits in patients with CKD; thus, it seems currently reasonable to use serum ALP as a subsidiary test for the management of CKD-MBD. It is reasonable to set the frequency of monitoring of serum ALP as the same for serum calcium and phosphorus, because testing for ALP is relatively inexpensive and is widely available in clinical laboratories.
Hyperphosphatemia has been shown to contribute to the development of secondary hyperparathyroidism in experimental studies 256-258, while recent data from observational studies indicate that recent hyperphosphatemia is associated with vascular calcification 259, 260, arteriosclerosis 261, progression of CKD 237, and risk of mortality 162, 239, 241-247 in patients with predialysis CKD. Indeed, one observational study reported that treatment with phosphorus binders was associated with improved survival among predialysis CKD patients 262. Another randomized controlled trial also demonstrated a slower progression of coronary artery calcification in patients treated with phosphorus binders, particularly those with non-calcium-containing phosphorus binder, compared with the control group 263. Although more studies are needed, these data may provide the rationale for controlling serum phosphorus levels within a specific range. Because there are insufficient data to determine the optimal range of serum phosphorus in predialysis CKD patients, this present JSDT guideline established the target range of serum phosphorus to be the laboratory reference level of each facility.
Treatment for hyperphosphatemia in predialysis CKD patients includes dietary phosphorus restriction and phosphorus binders. Dietary phosphorus restriction may have reno-protective effects by reducing dietary protein intake in some patients, but for patients with high risk of malnutrition, it is suggested to restrict the intake of food with phosphorus-containing additives rather than reducing dietary protein intake 264, 265. Prescription of phosphorus binders is to be considered for patients in whom hyperphosphatemia cannot be managed by dietary phosphorus restriction alone 266. CaCO3 is the only phosphorus binding agent that is approved for the treatment of hyperphosphatemia in predialysis CKD under the National Health Insurance system in Japan.
Although progression of vascular calcification might be associated with excess calcium load, it was reported that the progression of coronary artery calcification tends to be attenuated in predialysis CKD patients treated with CaCO3 compared with those not treated 263. This study also showed that treatment with sevelamer hydrochloride, a non-calcium-containing phosphorus binder, slowed the progression of coronary artery calcification; however, use of this agent is not approved for predialysis CKD patients in Japan. Lanthanum carbonate 267-269 and sevelamer carbonate 270 are used to treat hyperphosphatemia in predialysis CKD patients in other countries, but the former is unavailable in Japan and the latter is not approved for predialysis CKD patients under Japan's National Health Insurance scheme.
Although the prevalence of hypocalcemia increases as the stage of CKD progresses in predialysis CKD patients 217-221, 243-248, no studies have demonstrated a significant association of hypocalcemia with bone fractures, cardiovascular events, progression of CKD, and mortality 162, 237-242. However, because hypocalcemia can initiate or aggravate secondary hyperparathyroidism 271-273, it is suggested to control serum calcium level within the reference range of each facility. Calcium-containing phosphorus binders and/or VDRA can be used to control serum calcium level. If hypercalcemia exists without the use of these agents in patients with predialysis CKD, other causes such as malignancy or primary hyperparathyroidism should be considered and ruled out. Native vitamin D is not available as a prescription medicine in Japan.
It is known that secondary hyperparathyroidism develops in the early stages of predialysis CKD 217-221. Although its pathophysiology has not been fully elucidated, recent studies have suggested the possibility that FGF23, whose level increases in the early stage of CKD, reduces the production of 1,25(OH)2D and thereby enhances the secretion of PTH 224-228. In addition, it is known that the prevalence of vitamin D insufficiency or deficiency, evaluated by the level of 25(OH)D, is high 50, 52, 217, 219, 220, 274, which can in turn increase the secretion of PTH 275, 276. Moreover, as the stage of CKD progresses, a reduction in renal mass and hyperphosphatemia-induced inactivation of 25-hydroxyvitamin D-1α-hydroxylase further reduces the production of 1,25(OH)2D, resulting in overt hypocalcemia and subsequent hypersecretion of PTH 271-273.
Since secondary hyperparathyroidism can cause high turnover bone diseases in predialysis CKD 151, 159, 229-236, it is reasonable to manage PTH levels to control bone metabolism. As mentioned above, because hyperphosphatemia, vitamin D insufficiency or deficiency, reduced production of 1,25(OH)2D in the kidney, and resultant hypocalcemia, increase PTH secretion in CKD, it is reasonable to control these factors in patients with increased PTH levels. At least in patients with hyperphosphatemia, therefore, it is reasonable to restrict dietary phosphorus and to prescribe phosphorus binders in order to lower the level of PTH. In fact, it has been shown that control of phosphorus levels in predialysis CKD leads to amelioration of secondary hyperparathyroidism 267, 270, 277-279. On the other hand, it is difficult to treat vitamin D insufficiency or deficiency at the moment in Japan, because the measurement of 25(OH)D, which is necessary for the diagnosis of these conditions, is not covered by health insurance, and native vitamin D is not available as a prescription medicine in Japan. Thus, if high levels of PTH persist despite adequate phosphorus control, it is reasonable to use oral VDRA such as alfacalcidol or calcitriol 249-255, 280-283. Although more data are needed, some observational studies have shown an association of the use of VDRA with survival 44, 284; thus, it is reasonable to use these agents at least in patients with high levels of PTH.
The KDOQI guideline has set a target level of PTH according to the stage of CKD, based on expert opinion 4. On the other hand, the KDIGO guideline suggests to treat predialysis CKD patients whose PTH level is above the reference range, without setting any specific target range of PTH 7. Indeed, it is notable that inconsistent results have been reported regarding the associations of PTH levels with survival, and studies have failed to show significant associations between PTH levels and renal prognosis or bone fracture 162, 237-248. Given the absence of sufficient evidence, this current guideline does not set the specific target range, but states that it is reasonable to consider treating patients if the PTH level exceeds its reference range in the same way as the KDIGO guideline.
It is important to give priority to control of serum phosphorus and calcium over PTH so as not to induce secondary derangements of serum calcium or phosphorus levels. In particular, since excess administration of VDRA can increase urinary excretion of calcium and can cause hypercalcemia or acute kidney injury, it is suggested to pay careful attention to these adverse events when clinicians start treatment with VDRA or change the dose. Reports from other countries showed that alfacalcidol up to 0.5 μg/day or calcitriol up to 0.25 μg/day did not significantly affect kidney function in patients with predialysis CKD 249-255, 280-283. Although the appropriate range of urinary calcium excretion is not known in CKD, the Investigation Committee on Abnormalities in Hormone Receptor Mechanisms, designated by the Ministry of Health, Labor and Welfare of Japan (MHLW), recommended, in their criteria for treating hypoparathyroidism with VDRA, that the target ratio of calcium:creatinine in fasting morning urine should be ≤0.3 285.
It is extremely rare that secondary hyperparathyroidism progresses into severe parathyroid hyperplasia in predialysis CKD. Thus, evidence is insufficient for interventional treatments of secondary hyperparathyroidism in patients with predialysis CKD. Uses of falecalcitriol, intravenous maxacalcitol, or intravenous calcitriol are not covered by health insurance in Japan in predialysis CKD.
Measurement of bone mineral density (BMD) is widely used and is useful for the assessment of fracture risk and the efficacy of treatments for osteoporosis in general population 286. It is known that bone mineral density decreases 287, 288 and fracture risk increases 289-294 with the advancement of the stage of CKD. It is not clear whether the reduction of BMD correctly reflects the increased risk of fracture in patients with predialysis CKD. However, considering that most patients with postmenopausal or senile osteoporosis have stage 1–3 CKD, it is reasonable to measure and evaluate BMD in patients with stage 1–2 CKD and stage 3 CKD without biochemical abnormalities of mineral metabolism, according to the 2011 Japanese guideline for the prevention and treatment of osteoporosis 295. On the other hand, it is necessary to consider the possible presence of renal osteodystrophy in patients with stage 3 CKD with biochemical abnormality of mineral metabolism or in stage 4–5 CKD patients. It is not well understood whether BMD measurement is useful for the differential diagnosis of various types of renal osteodystrophy 296 and for the assessment of fracture risk in these patients. Therefore, routine measurement of BMD in patients with stage 3 CKD with biochemical abnormality of mineral metabolism and stage 4–5 CKD is not a strong recommendation.
The assessment of bone turnover, as measured by various bone metabolism markers, is reported to be associated with the risk of fracture 297-299 and useful for estimation of the efficacy of treatment for osteoporosis 300, 301 in the general population. Considering that many patients with osteoporosis have early stage CKD as mentioned above, it is reasonable to evaluate bone metabolism markers in patients with stage 1–2 CKD and stage 3 CKD without biochemical abnormality of mineral metabolism, according to the 2011 Japanese guideline for the prevention and treatment of osteoporosis 295. In contrast, the clinical utility of bone metabolism markers has not been established in patients with advanced CKD (≥ stage 3) who possibly have renal osteodystrophy. Because renal dysfunction has little effect on serum levels of bone ALP and TRAP5b, these markers are expected to be useful in patients with CKD. These markers are actually shown to correlate with bone turnover evaluated by bone biopsy or types of renal osteodystrophy 159, 235, 236. However, the diagnostic power of these markers is not very high, and it is possible that the measured values of these markers are discrepant to the findings of bone biopsy 159. In addition, it has not been established how the measured levels of bone metabolic markers should be used for the treatment of CKD-MBD. Therefore, it is not reasonable to recommend regular measurement of metabolic bone markers in patients with predialysis CKD-MBD.
Bone histomorphometry is useful for the diagnosis of renal osteodystrophy even in predialysis CKD patients 302. However, bone biopsy is an invasive maneuver, and it is not practical to repeat bone biopsies in the same patient. Therefore, bone biopsy should be restricted to patients in whom some uncommon conditions are expected, and in whom non-invasive examinations are inadequate for proper evaluation of bone metabolism and to guide treatment decisions, as in patients with end-stage renal disease.
Drugs used in patients with osteoporosis such as bisphosphonates, raloxifene hydrochloride and teriparatide are reported to be effective in reducing the risk of fractures in the general population. These drugs seem to be effective in patients with stage 1–2 CKD as most patients with osteoporosis have early stage CKD as mentioned above. In addition, it is reported that risedronate 303, alendronate 304, raloxifene hydrochloride 305 and teriparatide 306 reduce the risk of fractures in patients with stage 3 CKD without biochemical abnormality of mineral metabolism. However, the efficacy and long-term safety of these drugs in patients with stage 3 CKD with biochemical abnormality of mineral metabolism and among patients with stage 4–5 CKD have not been evaluated sufficiently. Therefore, it is not recommended to use these drugs in such patients. VDRA are reported to suppress high turnover of bone 250, 252, 281 and increase BMD in predialysis CKD patients 251, 253, 254. While it has not been established whether these changes by VDRA lead to reduced fracture risk, it is reasonable to consider the administration of VDRA at least to patients with secondary hyperparathyroidism.
Chapter 10: CKD-MBD in Kidney Transplant Recipients
Statements
- Before transplantation:
- We recommend that bone and mineral metabolism should be adequately managed before kidney transplantation in order to achieve good control of mineral metabolism after transplantation (1C).
- We suggest to measure serum phosphorus, calcium, and PTH levels at least once during pre-transplant evaluation (2C).
- We suggest that parathyroid intervention be performed before transplantation in case it is indicated (2C).
- Shortly after transplantation:
- During the acute phase after transplantation, especially the first 2 months after transplantation, we recommend measuring serum phosphorus and calcium levels at least once a week until they are stabilized. (1C).A
- It is reasonable to reassess serum PTH levels at least once before discharge after transplantation (No grade).
- We suggest that sequential measurements of bone mineral density (at the interval of 6 to 12 months) by using dual-energy X-ray absorptiometry may be of some help to assess rapid bone loss occurring within the first year after transplantation (2D).
- Chronic phase of transplantation:
- During the chronic phase of kidney transplantation (≥1 year), it is reasonable that serum phosphorus, calcium, and PTH levels are maintained within certain target ranges corresponding to CKD stages in the same manner as that of predialysis CKD patients (No grade).
- For patients with hypercalcemia (corrected calcium ≥10.5 mg/dL) and/or those with elevated PTH (greater than the upper limit of the reference range at the facility) ≥1 year after transplantation, we suggest considering parathyroid intervention (2C).
- We suggest minimizing the dose of glucocorticoid in order to avoid drug-induced fragility of the bone (2C).
Supplementary notes
AIt is reasonable to administer calcium supplements and/or VDRA to the kidney recipients who have undergone PTx in case they have overt hypocalcemia.
Rationale
This chapter discusses the management of CKD-MBD in kidney transplant recipients (KTRs). The management of organ transplant recipients, other than KTRs, is not discussed.
Chronic kidney disease-MBD in KTRs is a very complicated pathophysiologic state which consists of (i) carry-over of CKD-MBD from the dialysis period, (ii) drug-induced osteoporosis, mainly due to glucocorticoids and/or immunosuppressants, and (iii) secondary hyperparathyroidism due to persistent enlargement of the parathyroid gland and/or to graft dysfunction after transplantation. Unlike secondary hyperparathyroidism in the dialysis period, the main pathophysiologic features of CKD-MBD in KTRs are a persistent relative hyperparathyroidism and hypercalcemia, high PTH level, and hypophosphatemia. It has been elucidated by large-scale observational clinical studies that the risk of bone fracture in KTRs is much higher than what is seen in populations of healthy people or even in dialysis patients. However, there is scanty evidence that would help form the basis for a clinical guideline that aims to improve patient survival. Therefore, in this guideline, the management of CKD-MBD in KTRs during the chronic phase after transplantation is modeled on that of CKD-MBD in predialysis CKD patients. Moreover, since there is no evidence-based efficacious treatment against CKD-MBD after transplantation, we mention prophylactic measures that might prevent carry-over of CKD-MBD from the dialysis period.
In Japan, median waiting time to kidney transplantation is longer than that of other countries. Many KTRs present with secondary hyperparathyroidism at the time of transplantation, which is more common in recipients of cadaveric kidney transplant. Generally, secondary hyperparathyroidism ameliorates after kidney transplantation as the kidney function improves. However, it is reported that 17%–50% of KTRs experience persistent hyperparathyroidism at one year after transplantation 307, 308. One case-control study from Belgium showed that the risk factors for persistent secondary hyperparathyroidism (defined as a PTH level more than 2.5-times higher than ULN or requirement for post-transplant PTx) were: longer duration of dialysis and high serum PTH, calcium and phosphorus levels at the time of transplantation 308 and a similar result was reported in a prospective observational study from Japan 309. The management of CKD-MBD from the pre-transplantation stage is therefore, very important without doubt. Especially in cadaveric kidney transplant, because there is no time margin before transplantation for detailed evaluation of CKD-MBD, effective daily pre-transplant management at each dialysis center, is most important. The statements on the pre-transplant period of this guideline have been established to draw attention to laboratory abnormalities of CKD-MBD, which might occur during the post-transplant period.
Bone abnormalities during the dialysis period include adynamic bone and amyloid osteopathy, as well as osteitis fibrosa caused by hyperparathyroidism. According to some reports, adynamic bone disease ameliorates after transplantation 310, but there is no evidence on the effect of transplantation on amyloid osteopathy.
Besides bone disease, vascular calcification can be a big problem among KTRs with a long dialysis vintage. It causes difficulty in vascular anastomosis at surgery, impaired renal perfusion after transplantation, and insufficient management of post-transplant hypertension, all of which possibly lead to early graft dysfunction. Recently, in Japan, there have been an increasing number of kidney transplants from spousal and living unrelated donors. As a result, vascular calcification associated with aging both in donors and recipients has become an issue of increasing importance. We, however, have no proven method to treat it effectively, but aim to prevent it by taking any possible measures.
Although parathyroid intervention before transplantation is raised here, there is no sufficient evidence from any direct comparison between PTx done before and after transplantation to suggest that one strategy is more beneficial for prognosis. Evenepoel et al. reported in 2005 that GFR declined after PTx in KTRs 311, yet in 2007, they revised their opinion that the decline in GFR was temporary, and that it had nothing to do with long-term prognosis 312. Considering our clinical experience, however, it would be rationally better to perform PTx during the dialysis period before transplantation, where the graft function cannot be affected by the operation, than to undertake the risk of a potential decline in post-transplant GFR, even if that might be reversible in some cases. A case-control study from overseas revealed that female gender, higher pre-transplant PTH and hypercalcemia were significant predictors of post-transplant PTx 311, and a prospective observational study from Japan demonstrated that a higher pre-transplant PTH level was significantly correlated with persistent hypercalcemia and hypophosphatemia, independent of longer dialysis vintage 309. Still, a definite cut-off value remains unknown. It is thus reasonable to apply the same indication for parathyroid intervention (see Chapter 4) to the dialysis patients planning kidney transplantation as to the general maintenance dialysis patients. As for surgical procedure, total PTx without auto-transplantation of the parathyroid gland is not recommended because of potential difficulties in the maintenance of mineral and bone metabolism after transplantation 313.
As the kidney function improves after transplantation, the negative feedback for PTH resumes with the activation of vitamin D and phosphorus excretion in the kidney graft, usually followed by a rapid reduction in PTH levels 314. In the patients with remarkable hyperparathyroidism at the time of transplantation, the enlarged parathyroid glands, which mainly consist of diffuse hyperplasia, often regress after transplantation with apoptotic change in the tissue. But, on the other hand, the enlarged glands, predominantly containing nodular hyperplasia, show significantly less apoptotic change than that of diffuse hyperplasia 315, which may bring about a persistent decline in serum phosphorus and a remarkable elevation of PTH and serum calcium. Since serum calcium/phosphorus levels during the first 1–2 months post-transplant can fluctuate widely, the monitoring of serum levels is necessary at least once a week during this time.
As a result of disappearance of skeletal resistance to PTH by uremia after transplantation, while the PTH level gradually decreases, increased bone turnover significantly reduces BMD within a year 316, 317. Although the reduction rate of BMD slows down later on, the risk of fracture is comparatively high in KTRs due partially to long-term administration of glucocorticoid and/or immunosuppressants 318. In general, BMD is supposed not to be a good marker for predicting absolute fracture risk in KTRs, while in some reports, low BMD in the hip region (<0.9 g/cm2) was associated with high fracture risk in the future 319. BMD is at least of some help when evaluating temporal changes in bone mass within each individual. It is reasonable to think that a decrease in BMD may increase the risk of fracture. From that point of view, the evidence that the prophylactic use of VDRA 320 and bisphosphonates 321-323 at transplantation reduces the post-transplant rapid bone loss may be promising. This was, however, not included in this guideline because of the lack of robust evidence on the reduction in the fracture risk and/or mortality by these agents. As for administration of VDRA, studies show that the risk of hypercalcemia is reported to be slightly higher in treated groups than in control groups. Since an overuse of this agent may induce deterioration of the graft function via increasing calcium excretion, we suggest being careful when starting and changing the dose of VDRA. According to the previous report, the safety doses (i.e. without causing deterioration in kidney function in predialysis CKD patients) are up to 0.5 and 0.25 μg/day for alfacalcidol and calcitriol, respectively 324. Meanwhile, there is no particular safety standard against hypercalciuria among KTRs. According to the standard treatment for hypoparathyroidism with VDRA, which is proposed by the MHLW Investigation Committee on Abnormalities in Hormone Receptor Mechanisms, the morning fasting urinary calcium:creatinine ratio is recommended to be kept ≤0.3. There is no evidence that supports administration of VDRA and bisphosphonates to KTRs in the chronic phase of transplantation.
Most KTRs are classified as having “CKD” because of imperfect recovery in their kidney function. Thus, the management of CKD-MBD in KTRs during the chronic phase after transplantation is usually a necessary consideration. Although no study has evaluated the optimal frequency of the measurements, since fluctuations in the serum levels of calcium and phosphorus in this phase become as small as that of predialysis CKD patients, it seems reasonable to arrange the frequency in the same way as it is done in the predialysis period (see Chapter 9).
A study from the USA reported that 24% of KTRs had PTH > 130 pg/mL and 11–25% had abnormal corrected serum calcium levels or calcium × phosphorus product at one year post-transplant, and that the patients with corrected calcium ≥10.5 mg/dL at one year after transplantation had a significantly higher risk of graft loss compared to those without 325. Thus, leaving hypercalcemia untreated can be harmful and the application of parathyroid intervention should be aggressively considered in case medical treatment (e.g. bisphosphonates) fails. Regarding PTx, as written above, a total PTx without auto-transplantation of the parathyroid gland is not recommended. Because of a possible recurrence of nodular hyperplasia or adenoma, which requires re-operation, total PTx with auto-transplantation in the forearm would be the first choice 326. Besides, a subtotal resection could be an option in patients with a single or double adenoma, which has been demonstrated to offer similar efficacy in several long-term observational studies 327, 328. Further investigation by randomized controlled trials is needed to clarify the difference in survival benefits between the two regimens. Although there are only a few small studies on PEIT for tertiary hyperparathyroidism, it has been reported that it successfully reduces elevated PTH levels 329. Therefore, PEIT can be another option for high-risk patients who are not candidates for PTx.
It has been suggested that post-transplant hypophosphatemia is mainly associated with PTH and FGF-23 309, 330. Other causes of hypophosphatemia include renal tubular damage, resulting from immunological mechanisms. Hypophosphatemia not only induces bone abnormalities by disturbing mineralization, but also causes impaired muscular function and disruption of cellular metabolism, including glycolysis and oxidative phosphorylation. Supplementation of phosphorus by disodium phosphate (Na2HPO4) has been reported to improve hypophosphatemia as well as adenosine triphosphate in the muscles and the acid excretion capacity of the kidney 331. However, in Japan, it is difficult to improve hypophosphatemia by prescription medicines, because oral Na2HPO4 is not available at present. Moreover, it is uncertain until when and to what extent we should prescribe phosphorus supplements, because an evidence gap exists.
Although the measurement of FGF-23 is not yet available in clinical practice, the serum level of FGF-23 at the time of transplantation has been shown to be closely associated with a post-transplant rapid reduction of BMD 332, graft survival, or even with mortality 333. It will possibly make a useful prognostic marker.
On the other hand, in the chronic phase after transplantation, glucocorticoids negatively affect bone mass and bone formation evaluated by bone biopsy 334. Thus, as well as re-evaluating the whole scheme of a patient's immunosuppressive therapy, early tapering or withdrawal of glucocorticoid should also be considered as much as possible. Administration of bisphosphonates or VDRA should also be considered in line with the Japanese Society for Bone and Mineral Research guideline for the management and treatment of glucocorticoid-induced osteoporosis 335; however, we declined to recommend it for KTRs since it is reported in some publications that it improves BMD but not the incidence of bone fracture 336 and some bone biopsy studies showed that it might even aggravate low-turnover bone disease 337.
Although, cinacalcet is not covered by health insurance in Japan for recipients of kidney transplants, it was shown in a prospective observational study to be effective for persistent post-transplant hyperparathyroidism accompanied by hypercalcemia 338, as well as by some small studies from Japan. In cases where cinacalcet had been administered after transplantation, a prospective survey 339 reported that it was safe to continue cinacalcet after transplantation and that its effect to reduce PTH was lost if cinacalcet was stopped at the time of transplant 340, indicating that its carry-over effect cannot be expected.
Subcutaneously injectable PTH analog formulation became available in the Japanese market as of 2010 as a novel drug for osteoporosis and attracted considerable expectations as it is the only licensed drug that promotes bone formation 341-343. However, it has since been reported to be ineffective for rapid reduction of BMD after transplantation 344 and given that most post-transplant patients are classified as >CKD-3T–5T, its indication in this patient group would presumably be very limited.
Chapter 11: CKD-MBD in Pediatric Patients
Statements
- Examination items and frequency of their measurements:
- We suggest that monitoring of serum phosphorus, calcium, albumin, PTH, ALP, and bicarbonate ion concentration be started in stage 2 of CKD (2D).A
- We recommend that the height be measured at least every 3 months in children aged <3 years and every 6 months in those aged ≥3 years and above to evaluate whether there is growth disturbance and its severity (1B).
- Management of serum phosphorus and calcium levels:
- We suggest that the serum phosphorus level be maintained in the normal range corresponding to the patient's age (2C).B
- We suggest that the corrected serum calcium level be maintained in the normal range corresponding to the patient's age (2B).B,C
- Management of parathyroid function:
- We suggest that the serum intact PTH level be managed within the normal range until stage 2 and 3 of CKD; to no more than 1.5-times the ULN (<100 pg/mL) in stage 4, and to no more than 1.5–4.5 times the ULN range (100–300 pg/mL) in stages 5 and 5D (2C).
- Indication of parathyroid intervention.
- If marked secondary hyperparathyroidism refractory to medical treatment persists, we suggest that parathyroid intervention should be considered (2C).
- Growth hormone therapy:
- For children with CKD showing growth disturbance (short stature), growth hormone therapy is recommended (1A).
Supplementary notes
AThe serum phosphorus, calcium, PTH, ALP, and bicarbonate ion concentrations should be measured periodically according to the stage of CKD (Table 5). However, more frequent measurement is necessary in infants and small children, patients undergoing medications related to CKD-MBD or growth hormone therapy, those suspected to be noncompliant, etc.
BIt must be noted that normal serum phosphorus and calcium levels vary with age (See Table 6).

| Stage of CKD | GFR (mL/min × 1.73 m2) | Phosphorus, calcium, PTH, bicarbonate, and ALP |
|---|---|---|
| 2 | 60–89 | At least yearly |
| 3 | 30–59 | At least every 6 months |
| 4 | 15–29 | At least every 3 months |
| 5 | <15 or dialysis | At least every month |
- ALP, alkaline phosphatase; GFR, glomerular filtration rate; PTH, parathyroid hormone.
| Age | Serum phosphorus (mg/dL) | Serum total calcium (mg/dL) |
|---|---|---|
| 0–1 months | 5.00–7.70 | 9.00–11.02 |
| 1–2 months | 4.80–7.50 | 9.00–11.01 |
| 2–3 months | 4.60–7.30 | 8.99–11.00 |
| 3–4 months | 4.48–7.10 | 8.98–10.99 |
| 4–5 months | 4.38–6.95 | 8.98–10.98 |
| 5–6 months | 4.27–6.80 | 8.98–10.97 |
| 6–7 months | 4.18–6.70 | 8.98–10.97 |
| 7–8 months | 4.10–6.63 | 8.97–10.95 |
| 8–9 months | 4.01–6.58 | 8.95–10.93 |
| 9–10 months | 3.95–6.50 | 8.93–10.90 |
| 10–11 months | 3.90–6.41 | 8.91–10.89 |
| 11–12 months | 3.90–6.40 | 8.87–10.84 |
| 1 year | 3.86–6.23 | 8.81–10.64 |
| 2 years | 3.80–6.00 | 8.79–10.45 |
| 3 years | 3.80–5.90 | 8.77–10.32 |
| 4 years | 3.85–5.80 | 8.75–10.28 |
| 5 years | 3.90–5.80 | 8.74–10.24 |
| 6 years | 3.90–5.80 | 8.73–10.23 |
| 7 years | 3.90–5.80 | 8.73–10.20 |
| 8 years | 3.85–5.80 | 8.73–10.18 |
| 9 years | 3.80–5.80 | 8.73–10.14 |
| 10 years | 3.75–5.80 | 8.73–10.13 |
| 11 years | 3.70–5.80 | 8.72–10.10 |
| 12 years | 3.60–5.80 | 8.72–10.08 |
| 13 years | 3.50–5.80 | 8.72–10.05 |
| 14 years | 3.33–5.70 | 8.72–10.05 |
| 15 years | 3.20–5.50 | 8.72–10.03 |
| 16 years | 3.08–5.30 | 8.72–10.03 |
| 17 years | 2.90–5.10 | 8.72–10.03 |
| 18 years | 2.80–4.90 | 8.70–10.03 |
| 19 years | 2.80–4.80 | 8.70–10.03 |
| 20 years | 2.80–4.70 | 8.70–10.03 |
Rationale
The concept of CKD-MBD—that the abnormal calcium/phosphorus levels observed in CKD patients should not simply be considered to be associated with specific abnormalities of skeletal homeostasis (commonly called renal osteodystrophy), but as a pathological condition associated with vascular calcification and mortality—applies to children as well as to adults. In pediatric fields, hypercalcemia, hyperphosphatemia, and secondary hyperparathyroidism are potentially modifiable risk factors of cardiovascular calcification: their proper control is important for the prevention and treatment of vascular disease, renal osteodystrophy, and correlates with better long-term prognosis 57, 345.
There are several characteristics and particular aspects in the diagnosis and treatment of CKD-MBD in children. One of the issues specific to children is growth retardation (short stature). Growth retardation is the hallmark of CKD in children, and its pathogenesis is multifactorial, involving various factors, such as the presence of underlying disease, the age at the onset of renal failure, insufficient calorie intake, abnormal protein/amino acid metabolism, metabolic acidosis, electrolyte disturbance, anemia, and abnormalities in the endocrine system, especially in the growth hormone–growth factor axis. Proper diagnosis and specific management of the above factors that can lead to growth retardation are therefore required 346. However, growth retardation in pediatric CKD-MBD is difficult to prevent for several reasons: children are prone to hyperphosphatemia due to increased requirement of protein per body weight compared to adults; VDRA pulse therapy has been shown to be effective in improving bone lesions due to secondary hyperparathyroidism, but may lead to low bone turnover and subsequent aggravation of growth retardation 347; and growth hormone therapy has deleterious effects on bone metabolism through the worsening of secondary hyperparathyroidism 348.
Another issue is that new drugs proven to be effective and safe in adult patients with CKD-MBD, such as sevelamer hydrochloride, lanthanum carbonate, maxacalcitol, and cinacalcet hydrochloride, have not been widely used in children, and none have been approved for use in children in Japan or in Western countries. Thus, only limited treatment options are currently available for children with CKD-MBD.
As there have only been few studies on the diagnosis and treatment of CKD-MBD in children to date, it is impossible to establish diagnostic and treatment guidelines for pediatric CKD-MBD that are based on reliable evidence. When drafting this chapter of the guideline, we reviewed a number of sources, including the most recent (2009) KDIGO guidelines 7, the 2006 guideline proposed by the European Pediatric Dialysis Working Group (EPDWG) 349, the 2005 KDOQI guideline 4, 350, recent textbooks 351-353, review articles published in and after 2010 354-357, and as many recent original papers as possible to provide diagnostic/treatment guidelines adapted to the circumstances in Japan. However, since it is impossible to encompass all aspects of pediatric CKD-MBD in this single chapter, those who will apply the contents of this chapter in clinical practice should also read through and understand other chapters of this guideline concerning adult CKD-MBD.
Decreasing levels of serum calcitriol occur in an early stage of CKD stage 2, during which serum calcium and phosphorus levels are still within the normal ranges, and this decrease is followed by an increase in the level of PTH level 352. Metabolic acidosis usually becomes noticeable when GFR falls to <25 mL/min per 1.73 m2 357. Given that increased PTH level and subsequent secondary hyperparathyroidism have been observed in children with CKD stage 2 358 and that metabolic acidosis is associated with impaired bone metabolism and with longitudinal growth 357, we suggest to start monitoring of serum phosphorus, calcium, PTH, ALP, and bicarbonate ion concentrations during CKD stage 2. Table 5 shows the suggested frequency for measuring serum phosphorus, calcium, PTH, ALP, and bicarbonate ion concentrations in the different stages of CKD. Growth retardation in children is linked to a GFR < 60 mL/min per 1.73 m2 346.
Growth retardation can be diagnosed using a growth curve and a growth velocity curve. The cross-sectional standardized height is determined based on the average and standard deviation of the height data for children of a particular year; the latest version of the growth curve was developed based on the data reported by the Infant Physical Development Survey (MHLW) and School Health Statistical Survey (Ministry of Education, Culture, Sports, Science and Technology), which were conducted in 2000. The longitudinal standardized height is determined based on the data for children with available growth records from infancy up to 17 years of age and also provides data on a target growth rate (growth in height per year). Evaluation of height should be based on (i) comparison with the standardized height, (ii) comparison with the standardized growth rate, and (iii) comparison with the standardized growth curve. Comparison with the standardized value is usually performed based on standard deviation (standard deviation scores method). As the determination of growth rate requires a certain length of time, the growth rate should be evaluated carefully in the short term (<6 months). If a child's current height is within the normal range, but his or her growth rate is lower than the standardized value for his or her age, the child is expected to be of short stature in the future. Evaluation of growth rate is, therefore, important for the early detection of growth retardation. A growth rate of ≤−1.5 SD lasting for more than 2 years indicates the presence of a pathological condition. Plotting the time course of growth in height of individual children on the standardized growth curve, helps to understand each child's growth history and detecting growth retardation.
Finally, careful examination should be performed to detect skeletal deformity, a characteristic finding in children with CKD-MBD 351, 352. Radiological evaluation should be done for children with CKD stage 5 or 5D 349. In the early stage of infancy, when infants put weight on their limbs, extreme care must be taken because skeletal deformities can rapidly develop during this period. The most common form of bone deformity is genu valgum resulting from the bending of bones exposed to weight bearing loads. Other forms of deformity include genu varum, coxa vara, ulnar deviation, and ankle deformity. Infants show symptoms similar to those due to vitamin d-deficient rickets. Slipped epiphysis can occur in children with severe hyperparathyroidism, especially when insufficiently controlled for an extended period, and this most commonly affects the proximal femur, followed by the radius, distal ulna, tibia, and fibula. As slipped epiphysis can lead to osteonecrosis, degenerative joint disease, or severe deformity, attention must be paid to the occurrence of characteristic symptoms such as pain, limping, waddling gait, and limited range of motion.
The normal ranges for serum phosphorus and calcium levels by age are shown in Table 6 359. Serum calcium level is maintained in the normal range even if GFR falls to around 15 mL/min per 1.73 m2, while the serum phosphorus level begins to rise when GFR falls to <30 mL/min per 1.73 m2 352. Therefore, dietary phosphorus intake would be restricted after the CKD stage has progressed, and the serum phosphorus level has exceeded the age-appropriate ULN. However, as described in the standard diet therapy proposed for children with CKD 360, excessive restriction of protein intake should be avoided, considering the effects on growth. Appropriate nutritional guidance is important, such as reducing the intake of foods with high phosphorus content. For infants, therapeutic special milk with reduced phosphorus content can be used. It should, however, be remembered that giving low-phosphorus milk alone can lead to hypophosphatemia and subsequent rickets. After the CKD stage has progressed further to require dialysis, restriction of dietary phosphorus intake is no longer sufficient to control the serum phosphorus level. While providing a sufficient amount of dialysis is a prerequisite, it is difficult to maintain a serum phosphorus level within the normal range through current dialysis methods; PD removes around 240–400 mg of phosphorus per day, and HD removes around 600 mg of phosphorus every 4 h 352. The use of phosphorus binders is therefore required.
CaCO3 is one of the calcium-containing phosphorus binders widely used worldwide, and it has been shown to be effective in reducing serum phosphorus levels in children as well as in adults 353. The major complication associated with the long-term use of high-dose CaCO3 is hypercalcemia, particularly when coadministered with VDRA therapy 352.
Monitoring serum PTH should be started in an early stage of CKD, given a case report reported that children with secondary hyperparathyroidism showed increased PTH as early as CKD stage 2 358. In most cases, an increase of serum PTH level is observed when GFR falls to <40 mL/min per 1.73 m2 352.
In children, a target level of PTH should be determined, considering the effects on growth as well as the development of bone lesions and extraskeletal calcification. The KDOQI guideline 350 proposes a target PTH level of 1.7 × ULN of the reference range (70–110 pg/mL) for children with CKD stage 4, whereas the European EPDWG guideline 349 does not define any target level. With several reports showing that growth retardation does not occur at a PTH level of <2 × ULN 356; we consider it reasonable, for this guideline, to control PTH level at ≤1.5 × ULN (100 pg/mL as intact PTH) for children with CKD stage 4.
A consensus has not been established regarding the recommended target range of PTH for children with CDK stage 5 or 5D 356, with the KDOQI guidelines proposing 200–300 pg/mL (350), the EPDWG guideline proposing 120–180 pg/mL 349, and the KDIGO guideline proposing 120–500 pg/mL 7. A recent international observational study of pediatric peritoneal dialysis patients 361 showed a significantly increased prevalence of clinical symptoms or radiological CKD-MBD lesions in patients with a PTH level of ≥300 pg/mL and profound growth retardation in patients with a PTH level of ≥500 pg/mL, while a PTH level of <100 pg/mL was associated with a high incidence of low-turnover bone diseases. Based on these findings, we consider it reasonable to control serum PTH within the range of 100–300 pg/mL. Further investigations are needed to address this issue.
The control of serum calcium and phosphorus levels is essential for the appropriate management of PTH levels. Both serum phosphorus and calcium levels are suggested to be maintained within the age-appropriate normal ranges. The serum calcium × phosphorus product are also to be controlled within the suggested range (<65 mg2/dL2 for children <12 years and <55 mg2/dL2 for children ≥12 years) 351, 352. When serum PTH level is >300 pg/mL, despite proper control of serum phosphorus and calcium levels, administration of VDRA might be started. Administration of VDRA has been shown to decrease serum PTH levels in children as well as in adults 353, but is associated with major complications of hypercalcemia and extraskeletal calcification when coadministered with CaCO3 352. High expectations are therefore placed on sevelamer hydrochloride, a calcium-free phosphorus binder, for use with children and adults. The efficacy and safety of sevelamer hydrochloride in children has also been demonstrated in clinical studies 353, in case reports 362, 363 and in small controlled studies using a calcium-containing phosphorus binder as a comparator 199, 364. A limitation of this therapy is that sevelamer hydrochloride is currently available only in uncrushable tablets, and it is difficult to give the tablets to infants. Since lanthanum carbonate has been shown to accumulate in bone and other tissues, careful consideration should be given, such as avoiding long-term treatment, when applying the therapy with lanthanum carbonate in children 351, 352, 354. Overdosage of VDRA, including VDRA pulse therapy, is associated with the development of low-turnover bone disease 355. Although a definitive conclusion has not been reached about the relationship between the state of low bone turnover and growth retardation 347, 355, 356, excessive suppression of parathyroid function should be avoided 355 because a state of low bone turnover can lead to extraskeletal calcification 352.
The use of cinacalcet hydrochloride may be considered for patients with poorly controlled PTH and high serum levels of calcium and phosphorus despite VDRA therapy. However, the use of cinacalcet hydrochloride in children is potentially associated with adverse effects on longitudinal growth and puberty development 351, 352, given the fact that calcium-sensitive receptors are expressed in epiphyseal cartilage cells and that a phase III study involving adult patients undergoing hemodialysis showed that 6-month treatment with cinacalcet hydrochloride resulted in a 30% decrease in blood testosterone concentration. On the other hand, several studies of short- to mid-term treatment with cinacalcet hydrochloride in a small number of patients have demonstrated its efficacy without any significant side-effects 365-367. Additionally, some animal studies have shown no adverse effects on growth 368. Cinacalcet hydrochloride therapy is also referred to as chemical parathyroidectomy and can be an option for parathyroid intervention in children. Further studies are needed to determine the efficacy and safety of this agent in children.
Parathyroid intervention should be considered for patients with persistent severe secondary hyperparathyroidism unresponsive to medical therapy. Parathyroid intervention is particularly indicated for patients with persistent hypercalcemia with intolerable itching, progressive extraskeletal calcification, bone pain, or multiple fractures 351, 352. The cut-off value of serum PTH for considering parathyroid intervention proposed by the KDOQI guideline is ≥1000 pg/mL (500 pg/mL in the presence of calcinosis) 350. However, as mentioned earlier, an international observational study of pediatric peritoneal dialysis patients showed profound growth retardation in patients whose PTH level remained >500 pg/mL, suggesting that a serum PTH level of 500 pg/mL may be the optimal cut-off value for considering parathyroid intervention 361. Chemical parathyroidectomy with cinacalcet hydrochloride, as mentioned above, is another potential option, in view of the technical difficulty in performing parathyroid intervention in children 367. Further investigations are also needed to address this issue.
Growth disorder is a major symptom of CKD-MBD in children that becomes apparent when GFR falls to <60 mL/min per 1.73 m2 346. The pathogenesis of growth retardation involves not only MBD but also various other factors, such as inadequate calorie intake, abnormal protein/amino acid metabolism, metabolic acidosis, electrolyte disturbance, anemia, and abnormalities in the endocrine system, especially in the somatotropic hormone axis. In particular, the pathological importance of the somatotropic hormone axis has been demonstrated, and the efficacy and safety of therapy has been demonstrated in the clinical application of recombinant human growth hormone, which started in the late 1980s 346. Considering the risk of slipped capital femoral epiphysis, a known complication of growth hormone therapy 369, pre-treatment assessment of existing bone conditions is required. Furthermore, since growth hormone therapy will increase serum PTH levels 348, serum PTH and phosphorus levels should be controlled appropriately and carefully monitored over the treatment period. Growth hormone therapy should be temporarily discontinued if secondary hyperparathyroidism worsens 352.
Acknowledgments:
This 2012 guideline was developed by the JSDT working group members, in collaboration with members from the Japanese Society of Nephrology, through working group meetings as well as email discussions. Additionally, this guideline has been approved by the Academic Affairs Committee of the Japanese Society of Nephrology. We would like to thank many other individuals for their advice and support, including members of the Statistics Survey Committee who made a huge contribution in the JSDT database analysis.
Appendix I
Members of the guideline working group
Tadao Akizawa, MD, PhD; Chairman of the JSDT, Division of Nephrology, Department of Medicine, Showa University School of Medicine
Hideki Hirakata, MD, PhD; Chairman of the academic committee, Fukuoka Red Cross Hospital
Tadashi Tomo; Chairman of the subcommittee of guideline development, Department of Nephrology, Oita University Hospital
Masafumi Fukagawa, MD, PhD; Chairman of the working group, Division of Nephrology, Endocrinology and Metabolism, Tokai University School of Medicine
Keitaro Yokoyama, MD, PhD; Deputy chairman of the working group, Division of Nephrology and Hypertension/Haemodialysis Center, Department of Internal Medicine, Jikei University School of Medicine
Ryoichi Ando, MD, PhD; Department of Nephrology, Musashino Red Cross Hospital
Takatoshi Kakuta, MD, PhD; Division of Nephrology, Endocrinology and Metabolism, Tokai University School of Medicine
Masatomo Taniguchi, MD, PhD; Department of Medicine and Clinical Science, Graduate School of Medical Sciences, Kyushu University
Naohiko Fujii, MD, PhD; Department of Internal Medicine, Hyogo Prefectural Nishinomiya Hospital
Hideki Fujii, MD, PhD; Division of Nephrology and Kidney Center, Kobe University Graduate School of Medicine
Junichiro James Kazama, MD, PhD; Division of Blood Purification Therapy, Niigata University Medical and Dental Hospital
Hirotaka Komaba, MD, PhD; Division of Nephrology, Endocrinology and Metabolism, Tokai University School of Medicine
Tetsuo Shoji, MD, PhD; Department of Geriatrics and Vascular Medicine, Osaka City University Graduate School of Medicine
Motoshi Hattori, MD, PhD; Department of Pediatric Nephrology, Tokyo Women's Medical University, School of Medicine
Akira Ashida, MD, PhD; Department of Pediatrics, Osaka Medical College
Masaaki Nakayama, MD, PhD; Department of Nephrology, Hypertension, Diabetology, Endocrinology and Metabolism, Division of Dialysis Center, Fukushima Medical University, School of Medicine
Fumihiko Koiwa, MD, PhD; Division of Nephrology, Department of Internal Medicine, Showa University Fujigaoka Hospital
Yugo Shibagaki, MD, PhD; Kidney Disease Center, St. Marianna University
Seiji Fukumoto, MD, PhD; (Japanese Society of Nephrology); Division of Nephrology and Endocrinology, Department of Medicine, University of Tokyo Hospital
Working group supervisors
Kunitoshi Iseki, MD PhD; Dialysis Unit, University Hospital of the Ryukyus
Takashi Shigematsu, MD, PhD; Division of Nephrology, Department of Internal Medicine, Wakayama Medical University
Yusuke Tsukamoto, MD, PhD; Department of Nephrology, Itabashi Chuo Medical Center
Yoshiharu Tsubakihara, MD, PhD; Department of Kidney Disease and Hypertension, Osaka General Medical Center
Working group meetings
First meeting; 4 December 2009
Second meeting; 26 February 2010
Third meeting; 10 April 2010
Fourth meeting; 18 June 2010
Fifth meeting; 3 September 2010
Sixth meeting; 2 October 2010
Seventh meeting; 21 January 2011
Eighth meeting; 5 March 2011
Ninth meeting; 27 May 2011
Tenth meeting; 22 July 2011
Appendix II
Conflicts of interest
The JSDT has been making the best effort to avoid any actual and potential conflicts of interest for there to be a neutral and fair process of guideline development. In 2010, the JSDT developed a new system for working group members to declare any potential conflicts of interest. All members of JSDT guideline development groups are now required to provide signed declaration forms to state any actual or potential conflicts of interest. These forms are updated yearly, or sooner if an individual member's status changes. This present guideline is the first JSDT guideline to include conflict of interest declarations of individual working group members. Further information is available at: http://www.jsdt.or.jp/jsdt/1236.html (Japanese).
Conflict of interest declarations
TA: has received research funds and honoraria from Kyowa Hakko Kirin Co., Ltd. (a company producing and dealing prescription drugs), Chugai Pharmaceutical Co., Ltd. (a company producing, dealing, importing and exporting prescription drugs), Bayer Yakuhin, Ltd. (a company developing, importing, producing and dealing prescription drugs, medical devices and veterinary drugs), Daiichi Sankyo Co., Ltd. (a company researching, developing, producing and dealing prescription drugs), Tomita Pharmaceutical Co., Ltd. (a company producing and dealing prescription drugs, quasi drugs, cosmetics, industrial drugs, food additives and other related products), and Abbott Japan Co., Ltd. (a company developing, producing and dealing prescription drugs, nutrients, medical devices, diagnostic drugs, and diagnostic devices).
HH: has received honoraria from Chugai Pharmaceutical Co., Ltd. (a company producing, dealing, importing and exporting prescription drugs), Kyowa Hakko Kirin Co., Ltd. (a company producing and dealing prescription drugs), and Japan Tobacco Inc. (a company producing and dealing tobacco, drugs, food, and beverages).
MF: has received research funds and honoraria from Chugai Pharmaceutical Co., Ltd. (a company producing, dealing, importing and exporting prescription drugs), Kyowa Hakko Kirin Co., Ltd. (a company producing and dealing prescription drugs), and Bayer Yakuhin, Ltd. (a company developing, importing, producing and dealing prescription drugs, medical devices and veterinary drugs).
KY: has received research funds and honoraria from Kyowa Hakko Kirin Co., Ltd. (a company producing and dealing prescription drugs), Chugai Pharmaceutical Co., Ltd. (a company producing, dealing, importing and exporting prescription drugs), and Japan Tobacco Inc. (a company producing and dealing tobacco, drugs, food, and beverage).
TK: has received honoraria from Kyowa Hakko Kirin Co., Ltd. (a company producing and dealing prescription drugs) and Chugai Pharmaceutical Co., Ltd. (a company producing, dealing, importing and exporting prescription drugs).
MT: has received research funds and honoraria from Chugai Pharmaceutical Co., Ltd. (a company producing, dealing, importing and exporting prescription drugs), Kyowa Hakko Kirin Co., Ltd. (a company producing and dealing prescription drugs), and MSD Co., Ltd. (former Banyu Pharmaceutical Co., Ltd, a company developing, importing, producing and dealing prescription drugs and medical devices).
TS: has received honoraria from Astellas Pharma Inc. (a company producing, dealing, importing and exporting drugs).
AA: has received honoraria from Alexion Pharmaceuticals Inc. (a company producing, dealing and importing drugs in Japan and other countries).
YS: has received honoraria from Astellas Pharma Inc. (a company producing, dealing, importing and exporting drugs).
KI: has received research funds from Daiichi Sankyo Co., Ltd. (a company researching, developing, producing and dealing prescription drugs) and Chugai Pharmaceutical Co., Ltd. (a company producing, dealing, importing and exporting prescription drugs).
TS: has received research funds from Chugai Pharmaceutical Co., Ltd. (a company producing, dealing, importing and exporting prescription drugs).
YT: has received honoraria from Kyowa Hakko Kirin Co., Ltd. (a company producing and dealing prescription drugs) and Chugai Pharmaceutical Co., Ltd. (a company producing, dealing, importing and exporting prescription drugs).
(No other members declare the existence of any conflicts of interest.)




