The odorant-binding protein genes obp67 and obp56d-like encode products that guide oviposition site selection in the Asian tiger mosquito, Aedes albopictus
Abstract
Aedes albopictus is an important vector of arboviruses and prefers small containers of stagnant water as oviposition sites. One of the mechanisms mosquitoes use to search for suitable oviposition sites is relying on odor cues from prospective sites and their surroundings. The genetic and molecular bases of this behavior are not known for Ae. albopictus. Oviposition site-searching behavior can be separated into 2 stages: container location and water detection. We applied a glue compound to the antennae and the maxillary palps of adult females to mask their ability to detect molecules that may guide them to preferred oviposition sites. Treatment of the antennae significantly reduces the location index (P < 0.001), indicating a decreased ability to find oviposition sites, whereas no significant difference was observed in mosquitoes with maxillary palps treated with the same glue compound (P > 0.05). The detection time, measured as the duration from contact with the water surface to the deposition of the first egg, was extended in mosquitoes with treated antennae or maxillary palps, supporting the conclusion that olfaction is involved in the detection of oviposition site. Transcriptomic analysis identified differentially expressed olfactory-related genes, including obp67, obp56d-like, obp19d-like and obp67-like. RNA interference (RNAi)-mediated knockdown of obp67 and obp56d-like significantly affected the location index and detection time, respectively. Cas9/guide RNA-mediated knockout of obp56d-like resulted in a prolonged detection time, compared with the wild type (P < 0.05). These findings help to elucidate aspects of the olfactory mechanisms involved in Ae. albopictus oviposition site selection, and provide a basis for the development of mosquito surveillance and control strategies.
Introduction
Aedes albopictus is an important vector of arboviruses and can transmit dengue, Zika and chikungunya disease-causing viruses (Mondet et al., 1996; Kauffman & Kramer, 2017; Liu et al., 2018). Aedes albopictus is also one of the world's most invasive species, having spread from its original habitat in Asia to all parts of the world, except Antarctica, in less than 50 years (Benedict et al., 2007; Di Luca et al., 2017). Different mosquito species prefer different oviposition sites (Agyapong et al., 2014; Yee & Skiff, 2014; Allgood & Yee, 2017; Parker et al., 2020). Aedes albopictus mosquitoes prefer to deposit their eggs in small containers of standing water (tree holes, pots, cans, tires, etc.). Three-quarters of the mosquito life cycle, including embryonic development in the egg, and larval and pupal stages, are completed in the water of the oviposition site, and it is only as adults that they disperse through flight (Bentley & Day, 1989). Therefore, oviposition sites are key targets for mosquito control.
Odorant detection is an important sensory system in mosquitoes, playing an important role in the physiological processes of foraging, mating, host selection and finding oviposition sites (Potter, 2014; Coutinho-Abreu et al., 2022; Gupta et al., 2023). Mosquitoes search for oviposition sites mainly by using olfactory cues from the site and its surroundings. Once mosquitoes have had a blood meal and have progressed through oogenesis, they use their sensitive olfactory system to find suitable sites for egg deposition. Suitable oviposition sites contain water that emits an odor that attracts gravid females. The odor can be caused by algae, bacteria or other biological sources (Wondwosen et al., 2017; Bokore et al., 2021; Mosquera et al., 2023a; Mosquera et al., 2023b). Chemical cues, including plant volatiles, attract females, and the composition and concentration of these cues can vary among potential oviposition sites. For example, volatile organic compounds released by Klebsiella attract Aedes aegypti to lay eggs and gravid females have a strong preference for farmlands in areas where grasses, maize and sugar cane are grown (Asmare et al., 2017; Wondwosen et al., 2018). Mosquitoes have a strong preference for water containing bamboo extracts, and the presence of metabolically attractive substances produced by the larvae of the same species also attracts mosquitoes (Khan et al., 2023). Gravid mosquitoes can detect these odors and follow them in search of water. Aedes albopictus prefers small containers of stagnant water, but the olfactory organs and molecular mechanisms involved have yet to be described.
Odor-binding proteins (OBPs) are important for the initial detection and binding of volatile molecules that influence subsequent behavioral responses in many animals. Research on mosquito OBPs has focused on identifying the individual odorants that bind to specific OBPs (Leal, 2013). For example, CquiOBP1, a major OBP in the antennae of the Southern house mosquito Culex quinquefasciatus, was determined to be involved in the detection of some oviposition attractants (Pelletier et al., 2010). It was subsequently demonstrated that CquiOBP1 might discriminate the mosquito oviposition pheromone (MOP) from nonanal/picaridin, on the basis of the midpoint transition of a pH-dependent conformational change, and that MOP is accommodated better in the binding cavity than the other 2 ligands (Yin et al., 2015). Humidity-detecting sensilla in the antennae of the vinegar fly, Drosophila melanogaster, express a small protein, OBP59a, that facilitates the detection of water (hygrosensation) and affects desiccation resistance, a process critical to insect survival (Sun et al., 2018).
In this study, we established a laboratory model of Ae. albopictus oviposition site searching behavior using small containers of water as a lure and tested the effects of small-molecule detection in the search for oviposition sites by blocking the olfactory organs. Transcriptome analysis performed with treated and control gravid mosquitoes identified 2 genes, obp67 and obp56d-like, and further RNA interference (RNAi) and/or experiments using clustered regularly interspaced palindromic repeats (CRISPR)/Cas9 (CRISPR associated protein 9) technology revealed OBPs with roles in oviposition site detection.
Materials and methods
Mosquito strains and breeding
The Ae. albopictus Foshan strain was donated by the Guangdong Provincial Centre for Disease Control and Prevention and was reared in the Chen laboratory under the following conditions: temperature, 27 ± 1 °C; relative humidity (RH), 65% ± 10%; photoperiod, 14 h light/10 h dark. After blood feeding, female mosquitoes lay eggs on filter paper and the hatched larvae were fed with turtle food, whereas the adult mosquitoes were fed with 10% glucose water.
Olfaction blocking methods
Using experimental methods adapted from D. melanogaster and applied previously to Ae. aegypti, we blocked the antennal and maxillary palp pores that allow access to the olfactory cells of these organs (Dennis et al., 2019; Hsu et al., 2021). A female mosquito was placed under a microscope (SMZ1000; Nikon, Tokyo, Japan) and the antennae and/or maxillary palps were coated with glue (K-303 shadowless glue; Kraft, Chicago, IL, USA) using a brush (0000# drawing pen; Shettdown, Beijing, China), ensuring that these sensory organs are covered completely with glue. An ultraviolet (UV) lamp (395 nm ultraviolet flashlight, Alonefire™ SV005; Shenzhen Shiwang Technology Co., Ltd., Guangzhou, China) was used to irradiate and fix the glue. The exposure time was 30 s, and the untreated control group also was exposed to the UV light for 30 s. Mosquitoes were allowed to recover for 24 h after application, with an observed survival rate of approximately 80%.
Oviposition site location index assay
Three-hole ovitraps were placed diagonally inside a 1 m3 mosquito net. The 3-hole ovitraps consist of a clear cylindrical plastic jar with a recessed bottom and a black top cap with 3 conical holes (Fig. S1). The clear jar is approximately 10 cm in height and has a diameter of approximately 5.5 cm at the base (Lin et al., 2005). The black cap has 3 small holes, with diameters varying from 7 mm at the top to 5 mm at the bottom. This design reduces the ability of mosquitoes to exit once they have entered the chamber. Deionized water was used to exclude interference from other factors and 100 mL was added to 1 chamber, whereas the other chamber was left dry.
Twenty gravid mosquitoes were placed in the nets at 3 d post blood meal (PBM) and the numbers of gravid mosquitoes in each of the 3-hole ovitraps were recorded after 6 h. The temperature ranged from 28 °C to 30 °C and the humidity ranged from 40% to 50% during the experimental trial. A location index was determined by dividing the number of mosquitoes that searched the 3-hole ovitraps containing water by 20 (representing total number of mosquitoes used). The location index quantifies the efficiency of the mosquito in sensing the characteristics of the oviposition site, with a larger value reflecting a better ability to search for an oviposition site. Six experimental replicates were performed for both treated and control mosquitoes.
Detection time index assay
Video recordings (Stream Cam, 1080 p, 60 fps; Logitech, Lausanne, Switzerland) were used to quantify the time (detection times) required for a mosquito to identify a preferred oviposition site. Detection times measure the response of mosquitoes when close to a potential preferred oviposition site, based on the presence of water, and include smell, sight, taste and so forth. The shorter the detection time, the more suitable the water is for egg deposition, as perceived by the mosquito.
Time zero is set as a mosquito resting on the surface of the water. The behavior of the mosquito was observed (curved abdomen above the water) until the first un-melanized (white) eggs appeared on the water surface. The time (measured in seconds) from contact with the water to egg deposition was set as the probing time. An acrylic board placed inside a 7 cm × 11 cm × 12 cm customized mosquito cage facilitated recording (Fig. S1). Ten gravid mosquitoes at 3 d PBM were put into the cage along with a small water cup holding 30 mL of water and a piece of black cloth, to assist in the detection of the white mosquito eggs. Video recordings were made from 3:00 to 7:00 p.m. and 2 or 3 replicates were performed for each treatment.
Sampling methods for transcriptome sequencing
Total RNA was isolated from glue-treated and control mosquitoes to screen for genes that may vary in transcription product abundance under the experimental conditions. Initially, treated female mosquitoes aged 3–5 d post-emergence were provided with 10% sugar water. They were then subjected to a 2-h fasting period prior to being given access to a blood meal (rat blood). Fully engorged mosquitoes were identified and subsequently fed on 10% sugar water for an additional 3 d. Mosquitoes were transferred on the day 4 into a mosquito net cage with a volume of 1.5 m3. Two 3-hole ovitraps were positioned inside the net: 1 contained 100 mL of deionized water and the other was empty (dry). Gravid mosquitoes entering the ovitraps were promptly immobilized in liquid nitrogen for sample collection. The mosquitoes were dissected subsequently into head, legs and body samples, for further analyses. Control mosquitoes were fed similarly, except that no water was placed in either ovitrap.
The separate tissue samples were homogenized and stored in TRIZOL reagent (Ambion, Life Technologies, Carlsbad, CA, USA), for preservation. Subsequently, the samples were sent to BGI Genomics Co., Ltd. (Yantian, Shenzhen, China) for transcriptome sequencing. Initial (raw) sequencing reads were filtered to eliminate low-quality reads. The reads eliminated include those with adapter sequences and undetermined base information exceeding 10% of the total sequence, and those with a sequencing error rate (Qphred) of ≤20 base numbers accounting for over 50% of their total length. This filtration procedure resulted in clean reads suitable for subsequent bioinformatics analysis (https://ftp.ncbi.nlm.nih.gov/genomes/all/GCF/006/496/715/GCF_006496715.2_Aalbo_primary.1/). Olfactory receptor genes were identified among the differentially accumulated transcripts based on their functional annotation and gene description. The NCBI Blastn tool (National Center for Biotechnology Information, Bethesda, MD, USA) was used to validate the accuracy of gene sequencing and naming. A number of differentially expressed OBP-encoding genes were identified in leg (obp67, LOC109412825; obp67-like, LOC109408075; obp19d-like, LOC109400861) and head (obp56d-like, LOC115257664) samples. We defined genes with a fold change (FC) of more than 2-fold (log2FC ≥ 1) and a Q-value of ≤0.05 as significantly differentially expressed genes, and the remaining list of differentially expressed genes was included in Table S4.
Reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR) detection of differentially expressed genes
Both temporal and tissue-specific expression profiles were determined for obp67, obp67-like, obp19d-like and obp56d-like. Temporal expression profile samples were collected on days 1–5 PBM using 10 mosquitoes each, with a total of 3 replicates. Tissue expression profile samples were collected from female mosquitoes at 3 d PBM, with the pooled dissected parts (heads, legs and bodies) of 10 mosquitoes used in each of the 3 replicates.
Mosquito samples were also collected based on their behavior in the ovitraps. For the experimental group, 10 gravid female mosquitoes flying into the water-containing ovitrap were dissected into pooled head, leg and body samples for 3 replicates. The control group conditions were the same, but the gravid females pooled had not come into contact with the water.
All mosquito tissues collected were homogenized and whole RNA was extracted using TRIZOL reagent. The RNA samples were diluted and dissolved in a 1.5-mL enzyme-free Eppendorf tube using 20 µL of RNase-free water. The cDNA strand synthesis protocol begins with a genome removal step followed by reverse transcription using oligo-(dT) primers and a reverse transcription kit (Promega, Madison WI, USA). RT-qPCR was performed as follows: 95 °C for 10 s, followed by 40 cycles of 60 °C for 15 s and 72 °C for 20 s. The mRNA accumulation levels of obp67, obp67-like, obp19d-like and obp56d-like were compared with an internal reference gene (actin-5C, LOC109405344) using the 2−ΔΔCt method. Oligonucleotide primer information is provided in Table S1.
RNAi of genes with differential transcript accumulation profiles
Oligonucleotide primers were designed to bind to complementary target gene sequences to yield amplified fragments of approximately 300–400 bp. A T7 viral promoter was added to the 5′ end of both upstream and downstream primers (Table S1). Double-strand RNA (dsRNA) synthesis was conducted in accordance with the instructions for the T7 RiboMAX™ Express RNAi System kit (Promega), and dsGFP was synthesized as a control.
Gravid females were aspirated from the feeding cage at 24 h PBM and cold-anesthetized. A dissecting microscope was used to identify a triangular target area of the mosquito thoracic cavity as the injection site and approximately 0.5 µL of dsRNA was injected using a microinjection needle (Fig. S2). The experimental and control groups comprised 50 mosquitoes each, with the experimental group receiving dsRNA targeting the gene of interest and the control group receiving a GFP plasmid fragment. The injection concentration was set at 2000 ng/µL, with adjustments made over time based on the efficiency of the interference. Injected adults were housed individually in disposable medium paper dishes and provided with a 10% glucose solution for moisture and nutrition.
RNAi efficiency validation
Temporal expression spectrum sampling was conducted at 1, 2 and 3 d post-injection. A total of 10 females from each group were pooled. Both experimental and control groups were collected synchronously, with 3 biological replicates in total. Total RNA was extracted using TRIZOL and after achieving the requisite concentration and quality, RNA was reverse-transcribed and cDNA synthesized for RT-qPCR analyses.
Cas9/guide RNA obp56d-like and obp67 gene-editing experiments
Protocols for Cas9/small guide RNA (sgRNA)-based gene editing were adapted from previous studies (Liu et al., 2019; Liu et al., 2020; Guo et al., 2022). A dual gRNA knockout strategy was used to produce large deletions in the genome. An online website (http://crispr.mit.edu) was used to design a suitable guide RNA and following synthesis, the primers were annealed (sgRNA-F and sgRNA-R) (Table S1). The Q5 high-fidelity DNA polymerase was used to generate the sgRNA transcription template in vitro. A DNA purification kit (MiniBEST DNA Fragment Purification Kit Ver.4.0; TaKaRa, Kusatsu, Shiga, Japan) was used for fragment purification and final concentrations were determined using the NanoDrop™ 2000 nucleic acid concentration analyzer (Thermo Fisher Scientific, Waltham, MA, USA). The purified DNA fragments were used as templates for transcription in vitro using the T7 Transcription Kit T7RiboMAX™ Express Large Scale RNA Production System (Promega). Injections into phenotypically wild-type embryos of the Ae. albopictus Foshan (Guangzhou colony) strain were performed.
The injection mixes contained 300 ng/µL of Cas9 protein (Thermo Fisher Scientific), 100 ng/µL purified sgRNA1 and 100 ng/µL purified sgRNA2 added to RNase-free water. The injection mixtures were incubated for 15 min at 37 °C to reconstitute active ribonucleoproteins (RNPs), and these then were injected into embryos with suitable needles. The embryo microinjections used procedures described previously for mosquitoes (Liu et al., 2019; Liu et al., 2020; Guo et al., 2022).
Statistical analysis
Statistical analyses were conducted utilizing SPSS 21 (IBM, Armonk, NY, USA). The normality of all data was assessed using the Shapiro–Wilk test (α = 0.05). The location index and detection time were subjected to independent-sample t-tests, whereas one-way analysis of variance (ANOVA) was employed to compare the location index, detection time and temporal expression spectra across multiple groups from day 1 to day 5. A significance level of P < 0.05 denoted statistical significance. Distinct letters in the data images are used to denote statistically significant differences, with the same letters indicating no statistical significance.
Results
Treating antennae and maxillary palps with glue affects oviposition site-searching behavior
We divided the Ae. albopictus female site-searching behavior into container-location and water-detection stages (Fig. 1A). The location index refers to the proportion of released mosquitoes searching for small water containers, with a higher location index indicating a stronger ability of the mosquitoes to locate oviposition sites. The detection time refers to the time from when the mosquito first touches the water surface to when the first egg is deposited. Here, the shorter the time, the more suitable the water is for egg laying. Glue-treated and control mosquitoes were observed with light microscopy to score these indices (Fig. 1B). Antenna-treated and control mosquitoes exhibited significantly different location indices of 0.48 ± 0.03 and 0.70 ± 0.02, respectively (Fig. 1C, P < 0.001). Detection times also differed significantly, with treated mosquitoes taking 37.58 ± 4.5 s and controls taking 19.01 ± 0.7 s (Fig. 1D, P < 0.001). These data support the conclusion that the antennae have a role in locating oviposition containers and in the subsequent detection of water.
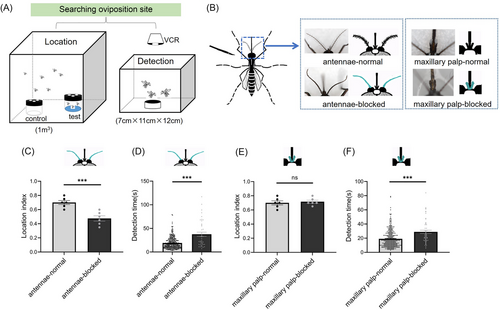
The location index, 0.72 ± 0.03, of mosquitoes with treated maxillary palps did not differ significantly from that of the controls (0.70 ± 0.02) (Fig. 1E, P > 0.05). In contrast, the detection times of the treated and control mosquitoes, 28.94 ± 2.3 s and 19.01 ± 0.7 s, respectively, were significantly different (Fig. 1F, P < 0.001). These data support the conclusion that the odorant receptors of the maxillary palps have a role only in the perception of water at close range during oviposition site selection behavior.
Transcriptome analyses of gravid mosquitoes reveal genes with modulated transcript levels during oviposition site-searching behavior
The results of blocking odorant reception in the antennae and maxillary palps focused our initial attention on the heads of gravid females. However, mosquito legs are also rich in olfaction receptor cells and we included them in our sample collection to explore differentially expressed genes during oviposition site selection (Fig. 2A). The remaining tissues, from the “body”, served as controls. Gravid mosquitoes recovered from the 3-hole ovitraps containing water were collected over a 5-min interval following introduction into the trap, and were designated the experimental group. Mosquitoes in the release cohort that did not select the water chamber were used as controls. Transcriptome sequencing analysis revealed a number of olfactory-related genes with tissue-specific differential transcript accumulation profiles (Figs. 2B and S4). A total of 84 genes with increased transcript abundance were upregulated, and of the 72 annotated gene IDs, obp56d-like was among those related to olfaction. Nineteen of the 25 genes were found within the leg samples, and these included the olfaction-related genes obp19d-like, obp67 and obp67-like. A total of 32 genes showed an increased accumulation of transcripts in the body and no olfaction-related genes were found in the set of 28 annotated genes.
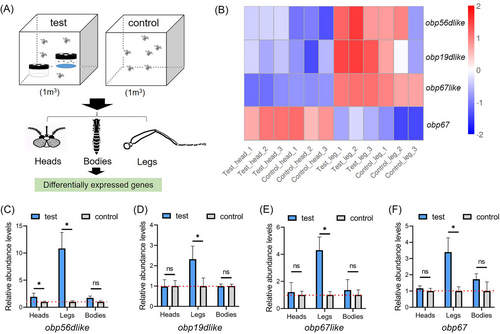
The RT-qPCR analyses of olfactory gene transcripts showed significantly greater accumulations of the products of obp56d-like in the heads and legs than were observed in the corresponding controls (Fig. 2C, P < 0.05). Transcript accumulations of obp19d-like, obp67-like and obp67 were higher in the legs than the respective controls (Fig. 2D–F, P < 0.05). These data are consistent with the transcriptome sequencing, with the only difference being that obp56d-like is more weakly expressed in the legs (Fig. 2C, P < 0.05).
Gravid Ae. albopictus females usually deposit eggs at 72 h PBM (Xiao et al., 2024). Temporal expression profile analyses of the differentially-expressed olfaction-related genes performed with whole bodies showed that the accumulation of obp67 and obp67-like transcripts starts increasing at 3 d PBM, and continues to rise for the next 2 d (Fig. S3). The accumulation of obp56d-like gene transcript started to increase at 3 d PBM, reaching a maximum at 5 d PBM (Fig. S3). The accumulation of obp19d-like transcript modulated, and first fell during 2–4 d PBM and then rose later at 5 d PBM (Fig. S3). Tissue-specific transcript accumulation profiles revealed that the strong accumulation of obp67-like was principally associated with expression in the legs, whereas the accumulation of obp67, obp56d-like and obp19d-like resulted from expression in both the head and the legs (Fig. S3). The results support the conclusion that changes in the transcript accumulation of these 4 genes may be associated with oviposition site-searching behavior.
RNAi-mediated knockdown of obp56d-like, obp67, obp19d-like and obp67-like transcripts affects oviposition site-searching behavior
RNAi-mediated transcript knockdown was designed to be effective for >3 d. Gravid females were recovered at 3 d PBM and used for the intrathoracic dsRNA injections (Fig. 3A). The protocol used was able to knock down obp19d-like, obp56d-like and obp67 transcript levels within 1–3 d after injection of the corresponding dsRNAs, with the relative abundance of obp67-like transcripts decreasing by >60% (Fig. 3B–E). The results show that the knockdown efficiency is good, allowing subsequent behavioral experiments to be performed.
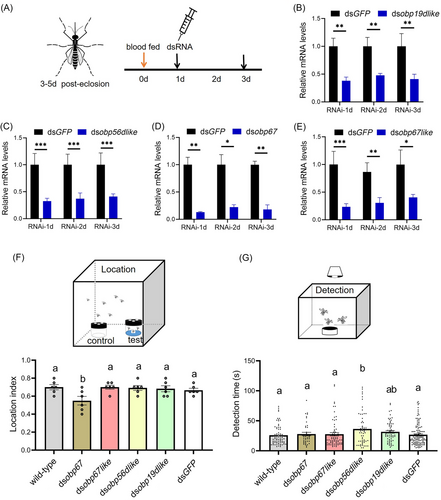
RNAi experiments performed with dsGFP-injected control mosquitoes showed a location index of 0.70 ± 0.05, comparable with that of the wild types (0.70 ± 0.03) (Fig. 3F). The 4 experimental groups had location indices of 0.55 ± 0.05 (dsobp67), 0.70 ± 0.02 (dsobp67-like), 0.69 ± 0.03 (dsobp56d-like) and 0.68 ± 0.03 (dsobp19d-like). The only significant difference between the dsGFP-injected group and the wild-type control group was observed for dsobp67 (P < 0.05). This result supports the conclusion that the obp67 molecule may have a role in oviposition site-searching and locating behavior.
RNAi knockdown impacts gravid females during the detection phase, with detection times of 25.88 ± 1.97 s (wild type), 27.2 ± 1.44 s (dsobp67), 27.67 ± 1.52 s (dsobp67-like), 37.02 ± 3.52 s (dsobp56d-like), 31.62 ± 8.28 (dsobp19d-like) and 26.84 ± 1.97 (dsGFP) (Fig. 3G). Only the detection time of females with the knockdown of obp56d-like significantly differed from that of the wild type and the dsGFP control groups (P < 0.05), supporting the conclusion that the obp56d-like gene products may play a role in detecting water at close range.
Cas9/sgRNA-mediated ablation of obp56d-like affects oviposition site-searching behavior
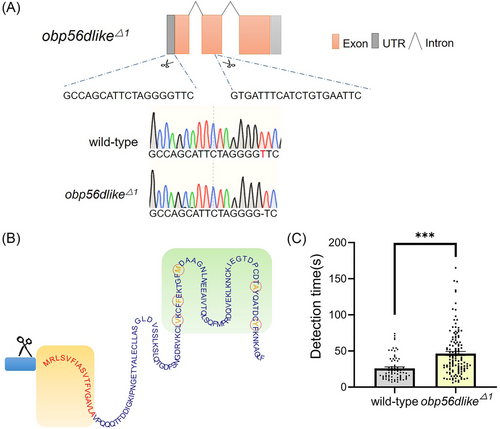
The RNAi experimental results targeting the obp56d-like and obp67 gene transcripts support a role for their involvement in the sensory mechanisms associated with locating oviposition sites. Gene editing techniques were used to create a strain with a nonfunctional obp56d-like allele. The obp56d-like gene (LOC115257664) is 691 base pairs (bp) in length and encodes a protein of 137 amino acids with a predicted secretory signal peptide region (https://services.healthtech.dtu.dk/services/SignalP-6.0/). As OBPs are generally small, we employed a dual sgRNA approach to disrupt the signal peptide and the first exon of the corresponding gene using the targeting sequences listed in Table S1. Detailed data on the knockout strains are shown in Table S3. The results indicate that only the sgRNA targeting the untranslated region (UTR) created a nonfunctional allele, designated obp56d-likeΔ1, with a 1-bp deletion in the UTR (Fig. 4A). Homozygous gravid mutant females had a detection time of 45.68 ± 2.79 s, which is significantly longer compared with that of wild-type controls (25.88 ± 1.98 s) (Fig. 4B, P < 0.05). This finding is consistent with the observed behavioral alterations from the obp56d-like RNAi experiments. Although the deletion in the UTR does not alter mRNA expression levels, it may modulate the translation of the subsequent protein, potentially leading to diminished levels of obp56d-like protein and thereby impacting its functionality (Li et al., 2022). No homozygous obp67 mutant line was recovered, despite significant effort, supporting the hypothesis that the recovered mutant alleles could confer a recessive lethal phenotype. Detailed data on the knockout strains are shown in Table S2.
The number of eggs laid by the wild type was 75.75 ± 4.49, whereas the number of eggs laid by the obp56dlike△1 mutant was 54.75 ± 3.48. A significant difference was observed between the 2 groups (Fig. S5, P < 0.01). The observed reduction in spawning number may be attributed to the involvement of obp56d-like in water recognition, a phenomenon that requires further investigation.
Discussion
Oviposition site selection in mosquitoes is an important component of their reproductive behavior. Both olfactory and visual cues influence female choice for an appropriate site (Hoel et al., 2011; Reiskind & Zarrabi, 2012;Tsunoda et al., 2020; Khan et al., 2023). Aedes albopictus gravid females prefer small bodies of stagnant (nutrient-rich) water in dark containers (Parker et al., 2020). Using a technique that blocks the olfactory receptors in the antennae and the maxillary palps, we first show that olfaction is necessary for oviposition site identification and utilization, and that the products of the OBP genes obp67 and obp56d-like have a role in this complex behavior.
Mosquito oviposition site selection is a complex and integrated perceptual process, with multiple steps. We delineated aspects of the behavior into phases that include locating the container and detecting the water. Visual cues such as container color and size are likely to be involved first, with olfaction playing a role in the final selection of a preferred site (Hoel et al., 2011; Reiskind & Zarrabi, 2012;Tsunoda et al., 2020).
Mosquito olfactory systems are able to distinguish numerous volatile compounds in nature, and previous research shows that they use olfaction to recognize moisture and specific odors emitted by water and microorganisms in a potential oviposition site (Bentley & Day, 1989; Girard et al., 2021; Mosquera et al., 2023a). The adult antennae and maxillary palps have a large number of different types of odorant receptors and are the organs likely to be involved in oviposition site selection (Mitra et al., 2021). The application of glue to these organs was expected to prevent the binding of odors to the receptors and provide a basis for assigning roles to them. The experiments with treated antennae support the conclusion that they have a role both in container location and water detection, whereas the maxillary palps have a more significant role in the water-detection phase.
Odorant binding proteins (OBPs) were discovered almost 3 decades ago, but there is still much to be revealed about their role(s) in insect olfaction. Moisture detection (hygrosensation) is a fundamental sensory modality in insects (Knecht et al., 2017). Electrophysiological techniques provided novel insights into humidity sensing in both D. melanogaster and mosquitoes (Chown et al., 2011). Stimulus responses to sensory-sensitive recordings (SSRs) detect humidity and related heat dynamics (Greppi et al., 2020). Previous work with D. melanogaster reported that the products of the obp59a gene localized in the antennae detect humidity, and that mutant individuals lacking the respective protein have defects in humidity perception (Sun et al., 2018).
Furthermore, receptors such as IR25a, and sensory pathways involving IR93a, IR68a and IR40a, play a crucial role in D. melanogaster for detecting temperature and humidity (“hygrometric behavior perception”) (Enjin et al., 2016). Similarly, IR93a has been implicated as important to the search for moist oviposition sites in the mosquitoes Anopheles gambiae and Ae. aegypti (Laursen et al., 2023). In addition, other gene products in mosquitoes that respond to osmotic pressure, such as the mechanosensory channels represented by the ppk301 ion channel, an isoform similar to the ppk28 and ppk301 genes in D. melanogaster, are associated with freshwater oviposition behavior in Ae. aegypti (Matthews et al., 2019). We show here that OBP67 and OBP56d-like gene products guide oviposition site selection in Ae. albopictus. Furthermore, oviposition site selection in this mosquito involves multiorgan-based sensory detection mediated by a combination of factors. This complexity is evident in the data that show that a single mutation in the OBP56d-like gene and OBP67 RNAi-mediated transcript knockdown did not completely abolish the ability to search for oviposition sites. Further study of these genes and others could provide the basis for the development of novel mosquito control programs targeting this important reproductive behavioral phenotype.
Conclusion
This study reveals aspects of the olfactory role played in Ae. albopictus oviposition site selection behavior.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (31830087 and 82261128003/2022YFML1001), the Key R&D Program of Guangdong Province (2022B1111030002) and the National Institutes of Health, USA (AI136850) to X.-G.C. A.A.J. is a Donald Bren Professor at UCI.
Disclosure
The authors declare that they have no conflicts of interest associated with this work.




