Dual-role regulator of a novel miR-3040 in photoperiod-mediated wing dimorphism and wing development in green peach aphid
Abstract
Wing dimorphism is regarded as an important phenotypic plasticity involved in the migration and reproduction of aphids. However, the signal transduction and regulatory mechanism of wing dimorphism in aphids are still unclear. Herein, the optimal environmental conditions were first explored for inducing winged offspring of green peach aphid, and the short photoperiod was the most important environmental cue to regulate wing dimorphism. Compared to 16 L:8 D photoperiod, the proportion of winged offspring increased to 90% under 8 L:16 D photoperiod. Subsequently, 5 differentially expressed microRNAs (miRNAs) in aphids treated with long and short photoperiods were identified using small RNA sequencing, and a novel miR-3040 was identified as a vital miRNA involved in photoperiod-mediated wing dimorphism. More specifically, the inhibition of miR-3040 expression could reduce the proportion of winged offspring induced by short photoperiod, whereas its activation increased the proportion of winged offspring under long photoperiod. Meanwhile, the expression level of miR-3040 in winged aphids was about 2.5 times that of wingless aphids, and the activation or inhibition of miR-3040 expression could cause wing deformity, revealing the dual-role regulator of miR-3040 in wing dimorphism and wing development. In summary, the current study identified the key environmental cue for wing dimorphism in green peach aphid, and the first to demonstrate the dual-role regulator of miR-3040 in photoperiod-mediated wing dimorphism and wing development.
Introduction
Phenotypic plasticity is the ability of a genotype to produce different phenotypes in response to various environmental cues (Bradshaw, 1965). It aids insects to adapt to diverse external environments, enhancing their adaptive survival capabilities (Maynard et al., 2019). There are many diverse polymorphic phenomena in insect pests, such as the typical transition of locusts from solitary phase to gregarious phase, long- or short-winged morphs of planthoppers, and wing and reproduction dimorphisms of aphids (Wang & Kang, 2014; Zhang et al., 2019; Yan et al., 2020a). Wing dimorphism is a particularly intriguing phenomenon in aphids, which is directly related with their migration and reproduction. Wingless aphids usually have larger body size, and can reproduce more offspring, whereas winged aphids typically produce fewer offspring, and have the ability to migrate over long distances to search for suitable habitats (Wratten, 1977; Braendle et al., 2006; Hayes et al., 2019).
The wing dimorphism of aphids is dependent on multiple environmental cues such as photoperiod, population density, temperature, and host plant quality (Müller et al., 2001; Braendle et al., 2006; Ogawa & Miura, 2014). Short photoperiod usually leads to the production of winged aphids in the subsequent generations (Blackman, 1971; Dixon, 1971; Blackman, 1975; Miura et al., 2003; Liu et al., 2014). High aphid population density or low nutrient supply can also induce the production of winged offspring via parthenogenesis (Lees, 1967; Müller et al., 2001; Ishikawa et al., 2012). Meanwhile, the photoperiod variation can also influence the transition from parthenogenetic reproduction to sexual reproduction, resulting in the production of both female and male aphids (Liu et al., 2014; Ji et al., 2021; Ji et al., 2023). In order to adapt to environmental changes, wing dimorphism and wing development are indispensable aspects involved in the entire life cycle of aphids. Therefore, how aphids respond and adapt to external environmental cues is a crucial and fascinating issue, and systematic research on this topic is beneficial for understanding the mechanism underling phenotypic plasticity.
Micro RNAs (miRNAs) are single-stranded, non-coding small RNAs of approximately 22 nucleotides, and their primary function is to participate in post-transcriptional regulation of gene expression by binding to the 3′-untranslated region of target messenger RNA (mRNA) in a reverse complementary manner, thereby leading to the degradation of mRNA (Bartel & Chen, 2004; Asgari, 2013; Lucas & Raikhel, 2013; Moran et al., 2017). The miRNAs have been demonstrated to participate in regulating gene expression in various biological processes, including wing development, metamorphosis, circadian rhythm, sex determination, and so forth (Peng et al., 2016a; Ylla et al., 2017; You et al., 2018; Li et al., 2022b). The insects usually respond to external environmental cues via regulating the expression of miRNAs, thus the miRNAs may contribute to insect adaptation to constantly changing environmental conditions (Asgari, 2013). For example, miR-34 controls the switch between long- and short-winged morphs of planthoppers by modulating the juvenile hormone and insulin signaling pathways (Ye et al., 2019). In aphids, several miRNAs have been demonstrated to be involved in wing dimorphism. For instance, miR-9b mediates density-dependent wing dimorphism via regulating ABCG4, while miR-8 can activate the Wnt signaling pathway, leading to an increase in the proportion of winged offspring (Shang et al., 2020; Zhou et al., 2023). Similarly, some miRNAs have also been demonstrated to be involved in wing development in aphids, such as miR-147b, miR-2, miR-306, and so forth (Fan et al., 2020; Li et al., 2022b).
Green peach aphid (Myzus persicae) is one of the major insect pests damaging numerous crops worldwide (Dedryver et al., 2010; Margaritopoulos et al., 2021). Aphids cannot only cause direct damage by feeding on plant phloem but also transmit various plant viruses such as barley yellow dwarf and potato leafroll, leading to severe economic losses (Nilsa et al., 2011; Simon & Peccoud, 2018). The complicated and versatile phenotypic plasticity of aphids provides them strong adaptation ability to changing external environments (Lambers, 1966; Müller et al., 2001). The current study aimed to identify the crucial miRNA for wing dimorphism in green peach aphid. However, the efficient delivery of a miRNA mimic and inhibitor should be achieved in aphids before function analysis. With the successful construction of nanocarrier-mediated double-stranded RNA (dsRNA) delivery systems in various aphid species, the nanocarrier is expected to be able to deliver the miRNA mimic and inhibitor to penetrate the aphid cuticle for efficient delivery (Zheng et al., 2019; Yan et al., 2020b; Yang et al., 2022; Li et al., 2022a; Ma et al., 2022a; Zhou et al., 2023). In the current study, the optimal environmental conditions were first explored for inducing winged aphids. Subsequently, the differentially expressed miRNAs in aphids treated with long and short photoperiods were screened using small RNA sequencing. A novel miR-3040 was identified as a key miRNA involved in wing dimorphism and wing development via employing a miRNA mimic and inhibitor. Additionally, the activation or inhibition of let-7 expression resulted in a pronounced lethal effect on aphids.
Materials and methods
Insect rearing
Green peach aphids were reared on radish seedlings in an environmental chamber under a photoperiod of 16 h:8 h (light:dark) at 18 °C. All aphids were the descendants of a single parthenogenetic aphid, and were individually reared and cultured for at least 5 generations. Subsequent experiments were conducted using parthenogenetic female offspring.
Induction condition for winged aphids
The induction conditions such as population density, photoperiod, and so forth, for winged aphids were first explored. (1) Population density: 1, 10, 20, and 50 1st instar nymphs were inoculated using a paintbrush into plastic culture dishes with a diameter of 60 mm under a photoperiod of 16 h:8 h (light:dark) at 18 °C. The plastic culture dishes were filled with 1% agar, and radish leaves were placed on the agar. The numbers of winged and wingless offspring were recorded for the subsequent 10 d, and the proportion of winged offspring was then calculated. Each treatment was repeated 3 times. (2) Photoperiod condition: individual 1rst instar nymphs were reared in growth chambers set at 18 °C under various photoperiods (16 h:8 h, 12 h:12 h, and 8 h:16 h, light:dark). The numbers of winged and wingless offspring were recorded for the subsequent 10 d. Each treatment was repeated 15 times. (3) Developmental stage: individual aphid at different developmental stages was reared under a photoperiod of 8 h:16 h (light:dark) at 18 °C. The numbers of winged and wingless offspring were then recorded for the subsequent 10 d. Each treatment was repeated 15 times. (4) Temperature condition: individual 1st instar nymphs were reared under a photoperiod of 8 h:16 h (light:dark) at different temperatures (18 and 20 °C). The numbers of winged and wingless offspring were recorded for the subsequent 10 d. Each treatment was repeated 15 times.
Sample preparation and RNA extraction
Based on above results, the short photoperiod could induce the production of winged aphids. Thus, the 4th instar nymphs reared under the photoperiods of 16 h:8 h and 8 h:16 h (light:dark) were collected for miRNA transcriptomic profiling. Total RNA was extracted from the heads of treated aphids using TRIzol reagent (Invitrogen) following the manufacturer's instructions. We fixed the aphid under the stereo microscope, carefully twisted the head off, and placed it into TRIzol reagent. Each treatment consisted of 500 aphids with 3 biological replicates. The concentration and purity of RNA were examined using a NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific). The RNA integrity was assessed using an RNA Nano 6000 assay 46 kit of the Agilent Bioanalyzer 2100 system (Agilent Technologies).
Small RNA sequencing and quantitative real-time polymerase chain reaction
A total of 3 µg RNA per sample was used for sequencing, and the library preparation was conducted on the Illumina HiSeq 2500 platform, generating paired-end reads. Bowtie was used to align the filtered reads to the reference sequence and to analyze their distribution on the reference genome (Langmead & Salzberg, 2012). After the alignment to the reference sequence, the clean reads were further compared with the GtRNAdb database (Chan & Lowe, 2009), Rfam database (Gardner et al., 2009), Repbase database (Kohany et al., 2006), and National Center for Biotechnology Information database (Pruitt et al., 2009) using Blast software to filter out non-coding RNAs (ncRNAs) such as ribosomal RNA (rRNA), transfer RNA (tRNA), small nuclear RNA (snRNA), small nucleolar RNA (snoRNA), and repetitive sequences. Then, the final obtained reads were aligned with the specified range sequences in miRBase to obtain detailed information on the small RNAs matched in each sample, including the secondary structure of matched known miRNAs, the sequences, lengths, and occurrences of miRNAs in each sample. Additionally, miRDeep2 miRNA prediction software was used to analyze new miRNAs (Friedlander et al., 2011). Known and new miRNAs detected in the samples were subjected to family analysis to explore the presence of their respective miRNA families in other species. The expression levels of known and new miRNAs were statistically analyzed, and their expression levels were normalized using transcripts per million (TPM) (Zhou et al., 2010). Finally, RNAhybrid and miRanda software were employed to predict miRNA target genes (John et al., 2004; Rehmsmeier et al., 2004).
Small RNA sequencing revealed that 5 miRNA candidates might participate in the wing dimorphism and wing development in green peach aphid. The expression levels of 5 miRNA candidates were verified using quantitative real-time polymerase chain reaction (qRT-PCR). The fore-mentioned RNA samples were transcribed into complementary DNA (cDNA) using miRcute Enhanced miRNA cDNA First Strand Synthesis Kit (TIANGEN). The qRT-PCR was performed on a Step One PlusTM Real-Time PCR system (Applied Biosystems) using the Power SYBR® Green Master Mix (Applied Biosystems). The primers are listed in Table S1. The qRT-PCR conditions were set as follows: 95 °C for 15 min, and 40 cycles of 94 °C for 20 s, 60 °C for 20 s, and 72 °C for 20 s. The specificity of the qRT-PCR signal was further confirmed using a melting curve. The U6 snRNA was chosen as the internal standard reference, and the amount of each miRNA expression was normalized to the abundance of reference gene using the 2−ΔΔCt method (Livak & Schmittgen, 2001).
In vivo characterization of miRNA candidates on wing dimorphism
The mimics and inhibitors of 5 miRNA candidates and corresponding negative controls were designed and synthesized by GenePharma (Shanghai GenePharma Co., Ltd.) (Table S1). To improve the delivery efficiency and bioactivity of miRNA mimics and inhibitors, they were mixed with the star polycation (SPc) nanocarrier at the mass ratio of 1:1 in diethylpyrocarbonate water solution. The mixture was incubated for at least 5 min at room temperature, and the SPc could spontaneously assemble with miRNA mimics or inhibitors into the nucleic acid/SPc complex (Li et al., 2019; Yang et al., 2022; Ma et al., 2022b). A final concentration of 0.1% surfactant was added to reduce the surface tension of droplets. The miRNA mimics or inhibitors were directly applied on the notum of 4th instar nymphs, which were individually reared under the photoperiods of 16 h:8 h and 8 h:16 h (light:dark) at 18 °C. Each aphid was treated with 100 ng miRNA mimics or inhibitors.
The expression levels of 5 miRNA candidates were examined after the application of corresponding miRNA mimics or inhibitors using qRT-PCR similarly as above. Each treatment included 20 aphids, and was repeated 3 times. Meanwhile, the mortality was recorded at 5 d after the topical application. Each treatment consisted of 30 aphids, which was repeated 3 times. Finally, the numbers of winged and wingless offspring were recorded for the subsequent 10 d, and the proportion of winged offspring was then calculated. Each treatment was repeated 15 times.
In vivo characterization of miR-3040 on wing development
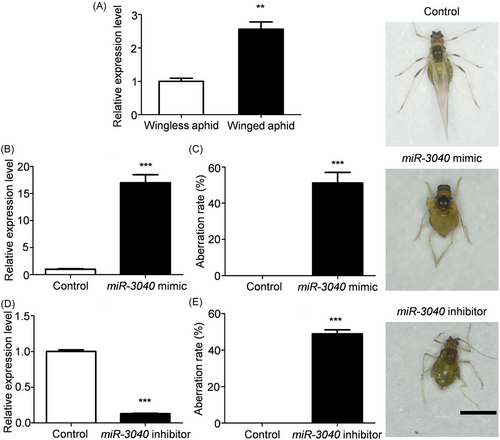
The effects of miR-3040 on wing development of the 3rd instar aphids were extensively explored. The expression levels of miR-3040 were first examined in wingless and winged aphids using qRT-PCR similarly as above. Each sample consisted of 20 aphids with 3 biological replicates. Meanwhile, the formulation containing 100 ng of miR-3040 mimics, inhibitors, or negative control was applied on the notum of the 3rd instar winged aphids, respectively. The expression level of miR-3040 was examined using qRT-PCR at 24 h after the topical application. Each treatment included 20 aphids, and was repeated 3 times. Furthermore, the aberration rate of adult aphids was finally recorded. Each treatment included 30 aphids, which was repeated 3 times.
Statistical analysis
Statistical analysis was conducted using DPS software (http://www.chinadps.net/index.html). Independent t-test (*P < 0.05; **P < 0.01; ***P < 0.001; ns, no significance) was used for pairwise comparison. The multiple groups were analyzed using Tukey's Honestly Significant Difference test. P < 0.05 was considered statistically significant. The descriptive statistics are shown as the mean values and standard errors of the means.
Results
Photoperiod is a vital environmental factor in wing dimorphism
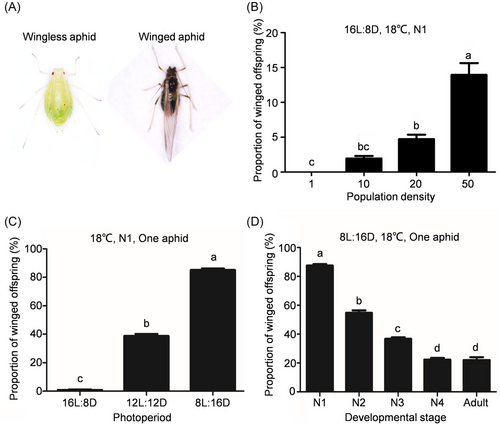
To determine the key factor regulating wing dimorphism, the impacts of various environmental factors such as photoperiod, population density, developmental stage, and temperature on wing dimorphism were evaluated. The morphological differences between winged and wingless aphids are shown in Fig. 1A. Under long photoperiod, the proportion of winged offspring was slightly influenced by population density with the highest proportion of merely 14% (Fig. 1B). However, the proportion of winged offspring remarkably increased under short photoperiod (Fig. 1C). Compared to 16 L:8 D photoperiod, the proportion of winged offspring increased to 32.6% under 12 L:12 D photoperiod, which further reached up to 90% under 8 L:16 D photoperiod. Furthermore, the 1st instar nymphs reared under short photoperiod produced the highest proportion of winged offspring (Fig. 1D), and slight temperature variation did not significantly alter the proportion of winged offspring (Fig. S1). Thus, the 1st instar nymphs reared under short photoperiod produced a high proportion of winged offspring.
Differentially expressed miRNAs in aphids treated with short and long photoperiods
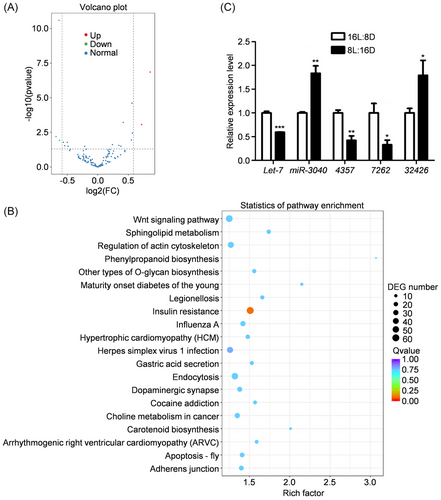
To explore the molecular mechanism underlying the transduction of photoperiodic signals, the heads of aphids as a critical site responding to photoperiod were harvested for small RNA sequencing. The sequencing samples exhibited high quality and good biological reproducibility (Table S2 and Fig. S2). According to the sequencing data, there were only 5 differentially expressed miRNAs in green peach aphid, with 2 upregulated miRNAs and 3 downregulated miRNAs in the treatment of short photoperiod (Fig. 2A). Using the RNAhybrid and miRanda software, 5 591 putative target genes within the untranslated regions (UTRs) of mRNAs were predicted, which might serve as seed regions for conservatively differentially expressed miRNAs. Among them, miR-3040 was predicted to target 4 845 genes, while let-7 targeted 660 genes (Fig. S3). Subsequently, all predicted target genes were subjected to Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis, resulting in a total of 304 enriched signaling pathways (Fig. 2B). The most enriched pathways included the Wnt signaling pathway, insulin resistance, endocytosis, regulation of actin cytoskeleton, and so forth. The expression levels of 5 differentially expressed miRNAs were verified by qRT-PCR, and the results were in accordance with the sequencing results (Fig. 2C).
Crucial function of miR-3040 in wing dimorphism
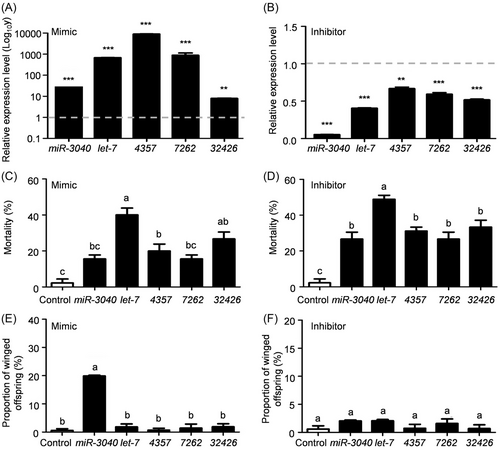
To explore the biological function of 5 candidate miRNAs in wing dimorphism, the miRNA mimics or inhibitors were synthesized to successfully up- or downregulate the expression of corresponding miRNA under long photoperiod (Fig. 3A, B). It was found that let-7 mimic resulted in the highest aphid mortality of 40%, and the mortalities induced by miR-3040 and 7262 mimics were the lowest with 15.6% (Fig. 3C). Encouragingly, miR-3040 mimic could significantly increase the proportion of winged offspring to 19.8% under long photoperiod (Fig. 3E). Similarly, the inhibitors of all 5 candidate miRNAs could lead to aphid death, and let-7 inhibitor induced the highest mortality of 48% (Fig. 3D), whereas their application could not change the proportion of winged offspring (Fig. 3F).
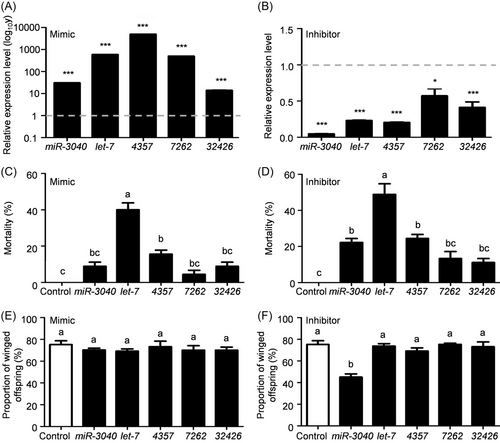
Successful regulation in the expression of 5 candidate miRNAs was achieved in green peach aphid under short photoperiod (Fig. 4A, B). Interestingly, both up- and downregulation of let-7 led to aphid death, and its mimic and inhibitor resulted in 40% and 48.9% mortalities, respectively (Fig. 4C, D). The application of 5 candidate miRNA mimics could not decrease the proportion of winged offspring, whereas the proportion of winged offspring decreased by 40% after the application of miR-3040 inhibitor (Fig. 4E, F). These above results suggested that the miR-3040 expression level was directly related with wing dimorphism, and its high expression level was crucial for the transition from wingless aphids to winged aphids.
Crucial function of miR-3040 in wing development
To further validate the function of miR-3040, its expression levels were first examined in winged and wingless aphids, respectively. As expected, the expression level of miR-3040 in winged aphids was 2.5 times higher than that in wingless aphids (Fig. 5A). Further experiments revealed that the activation of miR-3040 expression via mimic resulted in a wing deformity rate of 51.1% in winged aphids (Fig. 5B, C). Similarly, the downregulation of miR-3040 also led to wing deformities in green peach aphid, with the deformity rate of 48.9% (Fig. 5D, E). Based on the current results, both up- and downregulation of miR-3040 expression hindered the wing development in aphids, and the wings exhibited wrinkling, curling, folding, and so forth. Both the forewings and hindwings of green peach aphid could not spread normally with smaller size.
Potential miR-3040-invovled signaling pathways
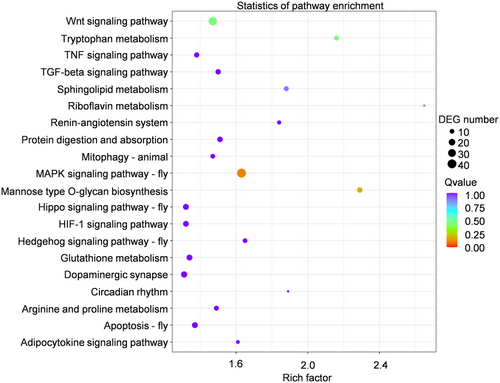
There were more than 4 000 potential target genes of miR-3040, and it was impossible to investigate the function of all suspected genes. Thus, the KEGG analysis was conducted to analyze all potential miR-3040-invovled signaling pathways. The potential target genes were enriched in multiple signaling pathways related to wing development, including the Wnt signaling pathway, mitogen-activated protein kinase (MAPK) signaling pathway, Hippo signaling pathway, Hedgehog signaling pathway, apoptosis, and so forth. (Fig. 6). Our results supported that the miR-3040 played an important role in wing dimorphism and wing development of green peach aphid.
Discussion
As a vital environmental factor in insect growth and development, photoperiod plays a crucial role in wing dimorphism. For instance, several studies have documented that wing dimorphism is influenced by photoperiod and temperature in planthopper Nilaparvata lugens (Kismoto, 1965; Zhang et al., 2019). In crickets, the percentage of macropterous individuals greatly increases in both males (31.6%) and females (40%) when exposed to long photoperiod (Tanaka et al., 1976; Zeng et al., 2010; Wu et al., 2014). Photoperiod can also influence the transition between winged and wingless aphids, and the proportion of winged offspring is around 70% under short photoperiod (Müller et al., 2001; Braendle et al., 2006; Liu et al., 2014). In the current study, the proportion of winged aphids was dependent on the photoperiod, and the short photoperiod could induce the formation of winged offspring. In addition, the population density is another crucial factor determining wing morph differentiation in aphids (Lees, 1967; Sutherland, 1969; Simpson et al., 2001; Braendle et al., 2006). In Acyrthosiphum pisim, high population density can induce approximately 80% winged offspring, while co-application of density and starvation treatment can increase the proportion of winged offspring to as high as 94% (Brisson et al., 2010; Vellichirammal et al., 2016; Zhou et al., 2023). Our results showed that the high density treatment did not induce a sufficiently high proportion of winged offspring, suggesting that the sensitivity of green peach aphid to population density was not as strong as that of pea aphids. Furthermore, we found that early instar aphids exposed to short photoperiod could produce a high proportion of winged offspring. Therefore, we identified the optimal induction conditions for winged green peach aphid.
The small RNA sequencing was performed to identify 5 differentially expressed miRNAs in aphids treated with short and long photoperiods. Among them, the novel miR-3040 was identified as a key regulator that controlled photoperiod-induced wing dimorphism in green peach aphid. Specifically, the inhibition of miR-3040 expression could reduce the proportion of winged offspring induced by short photoperiod, whereas its activation increased the proportion of winged offspring under long photoperiod. These forward and reverse experiments confirmed the involvement of miR-3040 in photoperiod-mediated wing morph differentiation in green peach aphid. miR-3040 has been mentioned in only a few studies, and its function remains unclear (Peng et al., 2016b). Although many miRNAs have been confirmed to participate in insect wing dimorphism, miR-3040 was demonstrated for the first time to be involved in aphid wing dimorphism (Rubio & Belles, 2013; Ye et al., 2019; Shang et al., 2020; Zhou et al., 2023). In addition, the inhibition or activation of all 5 miRNAs led to varying degrees of mortality in green peach aphid with the highest mortality with let-7 mimic or inhibitor, which might be related to its important function in various signaling pathways. The previous studies have demonstrated that let-7 can target the juvenile hormone or ecdysone signaling pathway, thereby inhibiting insect growth and development (Song et al., 2018; Peng et al., 2019; Inui et al., 2022). Depletion of let-7 also leads to heterochronic defects and failure of larval-to-adult transition (Bushati & Cohen, 2007). Our results revealed that let-7 could serve as a target site for green management of aphids.
Multiple miRNAs are involved in various processes of insect life and development, and they have also been confirmed to participate in the wing development of aphids (Bartel, 2004; Carthew et al., 2017). In brown citrus aphid Aphis citricidus and pea aphid A. pisum, miR-9b can mediate the wing development by regulating ABCG4, and the activation of miR-9b expression results in wing deformities with 41% for citrus aphids and 50% for pea aphids, respectively (Shang et al., 2020). miR-147b participates in wing development via vestigial in bird cherry oat aphid, Rhopalosiphum padi (Fan et al., 2020). In addition, several miRNAs have been found to induce varying degrees of wing deformities in English grain aphid Sitobion avenae, and miR-2 can induce 11% wing deformities (Li et al., 2022b). Interestingly, our study demonstrated that the miR-3040 was highly expressed in winged aphids compared to wingless aphids, and the activation or inhibition of miR-3040 expression in winged aphids led to the formation of malformed wings, revealing that the proper expression level of miR-3040 was critical for the normal development of aphid wings. Thus, miR-3040 acted with a dual role in regulating both photoperiod-mediated wing dimorphism and wing development.
The potential target signaling pathways of miR-3040 were interesting, which were further analyzed using KEGG analysis. However, there were too many potential target genes of miR-3040, which were primarily enriched in Wnt, Hedgehog, Hippo, MAPK, transforming growth factor-beta signaling pathways, and so forth. The Wnt signaling pathway is conserved in insect wing growth and wing dimorphism, which is required for proper polarity within wing imaginal discs (Wodarz & Nusse, 1998; Murat et al., 2010). A recent publication has demonstrated that the activation of the Wnt signaling pathway can promote wing primordium cells to overcome apoptosis, thereby developing into adult wings in aphids (Zhou et al., 2023). The downregulation of target gene vestigial in the Wnt signaling pathway leads to wing malformation in aphids (Fan et al., 2020; Zhang et al., 2022). The hedgehog protein is a typical diffusible intercellular morphogen that affects gene expression of Drosophila in a concentration-dependent manner, regulating in wing development via influencing downstream targets (Torroja et al., 2005; Nahmad & Stathopoulos, 2009; Brisson et al., 2010). The Hippo signaling pathway controls tissue growth by controlling cell growth, proliferation and apoptosis, which regulates the wing development and growth of Drosophila via the c-Jun N-terminal kinase signaling pathway (Ma et al., 2015). The current results revealed that the miR-3040 could regulate various signaling pathways related with wing dimorphism and wing development. The acting mechanism of miR-3040 was complicated, and its pivotal target genes should be further identified.
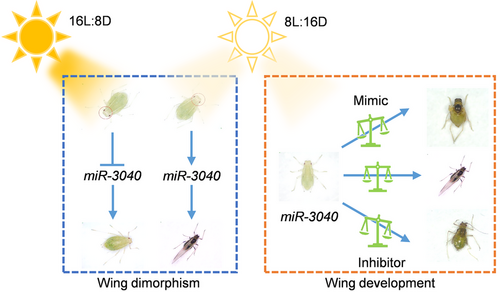
In conclusion, the current study identified a novel miR-3040 with dual roles that could not only regulate photoperiod-mediated wing dimorphism, but also modulate wing development in green peach aphid (Fig. 7). The short photoperiod could induce the formation of winged offspring via activating miR-3040 expression. Meanwhile, the expression level of miR-3040 was critical for wing development, and both up- or downregulation of miR-3040 expression led to wing deformity. In addition, the current study also identified let-7 as a potential target for sustainable management of green peach aphid. However, the specific signaling pathway and target genes regulated by miR-3040 require further exploration.
Acknowledgments
The authors would like to acknowledge the National Natural Science Foundation of China (32030012) and National Key Research and Development Program of the Ministry of Science and Technology (2022YFD1401800).
Disclosure
The authors have declared that no competing interests exist.




