Characterization of two Bursicon genes and their association with wing development in the brown citrus aphid, Aphis citricidus
Abstract
The tanning hormone, Bursicon, is a neuropeptide secreted by the insect nervous system that functions as a heterodimer composed of Burs-α and Burs-β subunits. It plays a critical role in the processes of cuticle tanning and wing expansion in insects. In this study, we successfully identified the AcBurs-α and AcBurs-β genes in Aphis citricidus. The open reading frames of AcBurs-α and AcBurs-β were 480 and 417 bp in length, respectively. Both AcBurs-α and AcBurs-β exhibited 11 conserved cysteine residues. AcBurs-α and AcBurs-β were expressed during all developmental stages of A. citricidus and showed high expression levels in the winged aphids. To investigate the potential role of AcBurs-α and AcBurs-β in wing development, we employed RNA interference (RNAi) techniques. With the efficient silencing of AcBurs-α (44.90%) and AcBurs-β (52.31%), malformed wings were induced in aphids. The proportions of malformed wings were 22.50%, 25.84%, and 38.34% in dsAcBurs-α-, dsAcBur-β-, and dsAcBurs-α + dsAcBur-β-treated groups, respectively. Moreover, feeding protein kinase A inhibitors (H-89) also increased the proportion of malformed wings to 30.00%. Feeding both double-stranded RNA and inhibitors (H-89) significantly downregulated the wing development-related genes nubbin, vestigial, notch and spalt major. Silence of vestigial through RNAi also led to malformed wings. Meanwhile, the exogenous application of 3 hormones that influence wing development did not affect the expression level of AcBursicon genes. These findings indicate that AcBursicon genes plays a crucial role in wing development in A. citricidus; therefore, it represents a potential molecular target for the control of this pest through RNAi-based approaches.
Introduction
Tanning hormone, known as Bursicon, was first discovered in 1962 as a peptide neurohormone in Calliphora vicina; it consists of 2 subunits (Burs-α and Burs-β) and is highly conserved among multiple species (Fraenkel & Hsiao, 1962; Honegger et al., 2008). Both Burs-α and Burs-β subunits share a common feature of containing 11 conserved cysteine residues that play a crucial role in establishing disulfide bonds, which enable the subunits to form a ring-like structure (Dong & Song, 2013). In recent years, Bursicon was identified in many insects from Blattaria (Kostron et al., 1995), Diptera (Bowser & Tobe, 2006; Honegger et al., 2011), Coleoptera (Arakane et al., 2008), Lepidoptera (Huang et al., 2007; Luo et al., 2023), and Hemiptera (Yu et al., 2016; Li et al., 2021).
Functional research on the tanning hormone gene has mainly focused on the development of insect epidermis and its melanosis and hardening, wing maturation and extension, preventive immunity, muscle contraction, and migration of egg marginal cells (An et al., 2012; Dong & Song, 2013; Zhang et al., 2017; Kong et al., 2021; Zhang et al., 2022a). For example, in Mythimna separata, the MsBursicon gene expression level was found to be significantly influenced by larval density and regulated the expression of antimicrobial peptide genes, which enhanced the prophylactic immunity response in crowded larval populations (Kong et al., 2021). Bursicon was also found to contribute to the cuticle tanning in Drosophila melanogaster; it stimulates phosphorylation of tyrosine hydroxylase (TH) via the protein kinase A (PKA) pathway, which activates TH to convert L-tyrosine into L-DOPA, a precursor of dopamine, in the epidermis (Davis et al., 2007; Moussian, 2010; White & Ewer, 2014). In another study, Bursicon influenced LGR2 (leucine-rich repeat-containing G protein-coupled receptor 2) and regulated the cyclic adenosine monophosphate/PKA (cAMP/PKA) signaling pathway, which is crucial for transmitting the hormonal signal responsible for inducing cell death in the wing epidermis and consequently impacts wing expansion in D. melanogaster (Luo et al., 2005). Similarly, the silencing of Bursicon is mediated by RNA interference (RNAi)-induced defects in the wing expansion of Bombyx mori and Manduca sexta (Huang et al., 2007; Dai et al., 2008). In Tribolium castaneum, a coleopteran, embryonic injections of Bursicon double-stranded RNA (dsRNA) also caused incomplete wing expansion and resulted in wrinkles; however, unlike in D. melanogaster, this did not affect cuticle tanning (Arakane et al., 2008; Bai & Palli, 2010).
Aphis citricidus belongs to Aphidae and is an important citrus pest and the primary vector for Citrus tristeza virus (Komazaki, 1994; Harper et al., 2016). The life history of A. citricidus is mostly parthenogenetic with no sexual reproduction stage, which means it has high reproductive ability all year round (Halbert & Brown, 1996; Michaud, 2001). In addition, the number of winged aphids significantly increases under high population density, which enables them to spread widely in citrus-producing areas (Shang et al., 2020). Therefore, the investigation of aphid wing development has significant scientific and practical implications for controlling aphid migration and spread.
To clarify the relationship between Bursicon and genes related to aphid wing development, and their roles in this development, we analyzed the function of Bursicon in A. citricidus by RNAi. The dsRNAs dsAcBurs-α and dsAcBurs-β were synthesized in vitro, and the expression level of AcBursicon genes was reduced via artificial feed. In this study, we identified AcBursicon genes and validated its relative expression levels in different developmental stages of A. citricidus through real-time quantitative polymerase chain reaction (RT-qPCR). We also explored the function of AcBursicon genes using RNAi technology, the effects of 3 hormones (20-hydroxyecdysone [20E], juvenile hormone III [JHIII,] insulin) on AcBursicon genes, and the impact of AcBursicon genes on classical wing development-related genes. Overall, our study provides insight into the relationship between Bursicon and genes involved in wing development in aphids and provides valuable information that can support future research in this field and potentially aid in the development of effective strategies for aphid control.
Materials and methods
Experimental insects, rearing, and sample preparation
A. citricidus used in this experiment were collected in the field at Southwest University, Chongqing, separately reared on Citrus sinensis and placed in a chamber (light : dark = 16 h : 8 h, 24 °C, and 70% relative humidity). All progeny were exclusively generated through parthenogenesis from the stock colony.
Samples from various developmental stages of A. citricidus were meticulously collected to investigate the relative expression levels of AcBurs-α and AcBurs-β. The samples included 1st instar nymphs (N1), 2nd instar nymphs (N2), 3rd instar nymphs (N3), 4th instar winged nymphs (N4-WD), 4th instar wingless nymphs (N4-WL), winged adults (AD-WD), and wingless adults (AD-WL). All adults were 1-d-old adult aphids. Each treatment had 4 biological replicates consisting of 20 aphids per replicate. After collection, all samples were immediately immersed in TRIzol reagent and stored at −80 °C to preserve the RNA for subsequent extraction.
Sequence alignments and phylogenetic analysis
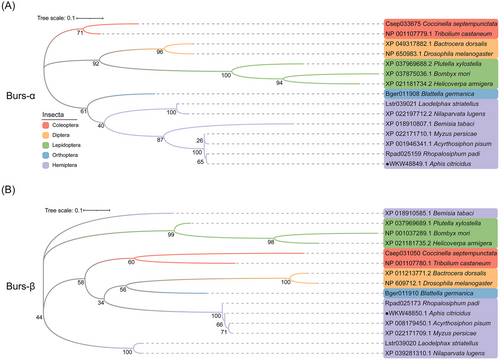
AcBurs-α and AcBurs-β were identified according to the A. citricidus genome database. The nucleotide and amino acid sequences of AcBurs-α and AcBurs-β have been uploaded to the National Center for Biotechnology Information (NCBI) database (https://www.ncbi.nlm.nih.gov/). Amino acid sequences of other representative species were obtained from the NCBI database. The molecular weight and theoretical isoelectric point (pI) of AcBursicons were predicted by the ExPASy server (https://www.expasy.org/). Amino acid sequence alignment was performed by Jalview software (https://www.jalview.org/download/). Six Bursicon amino acid sequences from other Aphididae species were chosen for multiple sequence alignment with AcBursicons. The phylogenetic tree was constructed using the maximum likelihood method in MEGA11 (https://megasoftware.net/) with 1 000 bootstrap replicates.
RNA extraction and RT-qPCR
Total RNA was extracted using the TRIzol Kit (Invitrogen, Carlsbad, CA, USA), followed by the removal of genomic DNA using DNase I (Promega, Madison, WI, USA) reagent. Subsequently, complementary DNA (cDNA) synthesis was carried out using the PrimeScript RT Reagent Kit (TAKARA, Dalian, Liaoning, China). RT-qPCR analysis was performed on a BIO-RAD CFX Connect Real-Time System (Bio-Rad, Hercules, CA, USA). β-actin and EF1α (elongation factor 1-alpha) (Shang et al., 2015) were used as reference genes to calculate the relative expression levels of genes with qBase+ (Hellemans et al., 2007). Quantitative primers were designed using Primer Blast, a tool available from the NCBI (https://www.ncbi.nlm.nih.gov/tools/primer-blast/). A comprehensive list of all primers used in this study is provided in Table S1.
RNAi
The dsRNA (dsGFP, dsAcBurs-α, dsAcBurs-β, dsvestigial) were synthesized using the Transcript Aid T7 High Yield Transcription Kit (Thermo Fisher Scientific, Waltham, MA, USA) and subsequently purified via phenolic chloroform extraction. Aphids that just molted from the 3rd instar to the 4th instar were tested for the treatment. Specifically, adult aphids were first selected and placed on citrus plants. Immediately, we started to collect newborn nymphs for 6 h. After 5–6 d, all aphids developed into 4th instar nymphs, and the morphs belonging to winged/wingless morphs were distinguished by wing buds. The dsRNAs were added into the artificial diet and fed to aphids (N4-WD) for RNAi. The silencing efficiency of genes was detected using RT-qPCR after 24 h of feeding. The wing morphology and mortality of adults were observed and recorded when all aphids emerged. Each treatment included 4 biological replicates with 30 aphids per replicate. Samples with stable silencing efficiency were selected for transcriptome sequencing.
Transcriptome sequencing
Purity, concentration, and integrity of RNA samples were assessed with a spectrophotometer (Nanodrop 2000, Thermo Fisher, Waltham, MA, USA). Qualified RNA was processed for cDNA library construction and then sequenced on the Illumina sequencing platform. Transcriptome assembly and RNA-seq analyses used the same strategy as a previous study (Shang et al., 2020). Genes with a false discovery rate <0.01 and the absolute value of |log2Fold change| > 1 found by DESeq algorithm were assigned as differentially expressed.
Hormone and PKA inhibitor (H-89) treatment
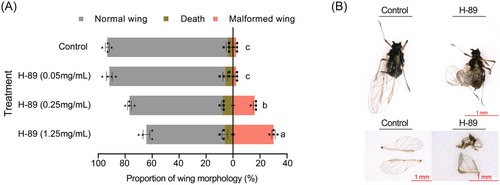
The effect of hormones on AcBurs-α and AcBurs-β expression was investigated by feeding aphids 20E (250 μg/mL) (Vellichirammal et al., 2017), JHIII (1 μg/mL) (Ishikawa et al., 2013), and insulin (5 mg/mL) (Shang et al., 2020). The role of the cAMP/PKA pathway in AcBurs-α and AcBurs-β regulation of wing development was studied by treating aphids (N4-WD) with PKA inhibitors, and aphids were fed H-89 through artificial feed. Samples were collected 24 h after feeding for total RNA extraction. Each treatment included 4 biological replicates with 30 aphids per replicate. The wing morphology (normal or malformed) of adults was observed and recorded. Photographs were taken using a digital microscope (VHX-5000) (Keyence, Osaka, Japan).
Statistical analysis
All data are represented by the mean ± standard error of 4 independent biological replicates. Statistical analyses were performed using SPSS v.25.0 for Windows (IBM, Armonk, NY, USA). One-way analysis of variance (ANOVA) (P < 0.05) was used for multiple comparisons. Student's t-test (*P < 0.05; **P < 0.01; Bursicons P < 0.001; ns, no significance) was used for pairwise comparison, and multiple comparisons analyzed by one-way ANOVA followed by Tukey's test for post hoc comparisons (P < 0.05).
Results
Identification and characterization of AcBursicon genes
The open reading frames (ORFs) of AcBurs-α and AcBurs-β were 480 and 417 bp in length, respectively (Fig. S1). These ORFs encode polypeptides consisting of 159 and 138 amino acid residues, with predicted molecular weights of 17.46 and 15.55 kDa and pI of 7.96 and 4.84, respectively.
In the multiple sequence alignment, the 11 cysteine residues of AcBurs-α and AcBurs-β were highly conserved in the homologs obtained from multiple aphids, and the sequence homologies were 98.13% and 99.07%, respectively (Fig. 1A, B). Furthermore, AcBurs-α and AcBurs-β phylogenetically clustered with homologs from multiple aphids (Fig. 2A, B).

Developmental expression profiles of AcBursicon genes
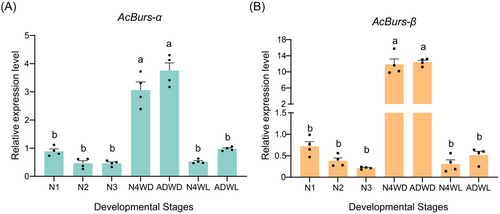
RT-qPCR was used to determine the messenger RNA (mRNA) expression levels of AcBurs-α and AcBurs-β. AcBurs-α and AcBurs-β were expressed at all developmental stages, and the expression level was relatively low and stable at the 1st–3rd instar nymphs. The expression level was the lowest in the 3rd instar aphid, and highest in the 4th instar winged nymphs and winged adults. The expression levels of AcBurs-α and AcBurs-β in the 4th instar winged nymphs were 6.62 and 55.39 fold higher than in the 3rd instar nymphs, respectively. During the adult stage, AcBurs-α and AcBurs-β expression levels in adult winged aphids were 3.84 and 24.23 fold higher than those in adult wingless aphids, respectively (Fig. 3A, B).
Functional analysis of AcBursicon genes based on RNAi and PKA inhibitor approaches
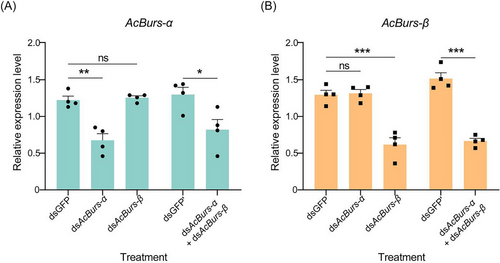
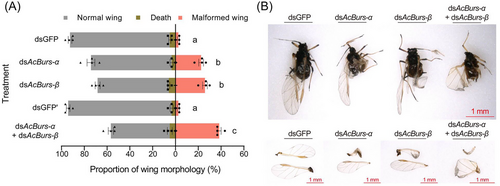
RNAi technology was used to explore the biological functions of AcBursicon genes. The 4th instar winged nymphs were fed an artificial diet mixed with dsRNA. The silencing efficiencies of AcBurs-α were 44.81% and 36.83% after feeding dsAcBurs-α or dsAcBurs-α + dsAcBurs-β for 24 h, respectively (Fig. 4A). Moreover, the silencing efficiency of AcBurs-β reached 52.32% and 55.37% after feeding dsAcBurs-β or dsAcBurs-α + dsAcBurs-β for 24 h, respectively (Fig. 4B). In addition, silencing AcBurs-α or AcBurs-β alone did not affect the expression level of the other genes (Fig. 4A, B). The proportions of malformed wings were 22.50%, 25.84%, and 38.34% in dsAcBurs-α-, dsAcBurs-β-, and dsAcBurs-α + dsAcBurs-β-treated groups, respectively (Fig. 5A, B). The mortality of aphids under different treatments showed no significant difference.
Artificial feed mixed with PKA inhibitor was fed to 4th instar winged nymphs. Similar to RNAi, feeding PKA inhibitors (H-89) also caused malformed wings in A. citricidus, and the proportion of malformed wings increased with increasing H-89 concentration. When 0.05 mg/mL H-89 was added to the feed, there was no difference in the proportion of malformed wings compared with the control group. When the H-89 concentrations were 0.25 and 1.25 mg/mL, the proportion of malformed wings significantly increased to 16.00% and 30.00%, respectively (Fig. 6A, B).
Transcriptome analysis after silencing AcBursions
We conducted transcriptome analysis on the samples after silencing AcBursicon genes. Of the 761 differentially expressed genes (DEGs) in double-stranded Green Fluorescent Protein (dsGFP) versus dsAcBursicon genes (dsAcBurs-α + dsAcBurs-β), the treatment group (that was fed dsAcBurs-α + dsAcBurs-β for 24 h) had 199 genes that were up-regulated and 562 genes that were downregulated compared with the control (that were fed dsGFP for 24 h) (Fig. 7A).
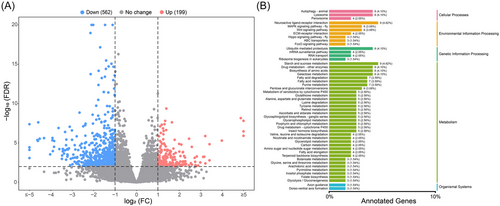
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis results showed that the DEGs were mainly concentrated on metabolism, followed by environmental information processing and cellular processes. In metabolism, DEGs were mainly related to the synthesis and metabolism of glycolipids and the biosynthesis of amino acids; in environmental information processing, DEGs were enriched in 7 pathways, among which the Wingless/integrated (Wnt) signaling pathway, Hippo signaling pathway, and Forkhead Box O (FoxO) signaling pathway were highly correlated with wing development and widely reported among various insects (Fig. 7B). In cellular processes, the enriched pathways included autophagy and lysosomal pathways, but identification revealed no significant changes in the marker genes for autophagy and apoptosis (Figs. S2 and S3).
Wing development-related gene expression in response to RNAi and PKA inhibitor
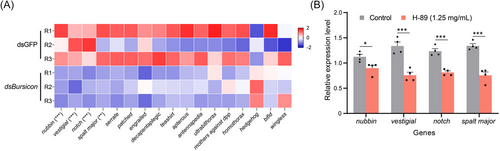
To study the regulation of AcBursicon genes in wing development, the expression levels of wing development-related genes of the transcriptome were identified. Among 17 genes, the expression levels of nubbin, vestigial, notch, and spalt major were significantly downregulated after dsRNA feeding, and most of the other genes showed a trend toward downregulation (Fig. 8A). After feeding PKA inhibitors, the expression levels of nubbin, and vestigial, notch and spalt major were also significantly downregulated (Fig. 8B).
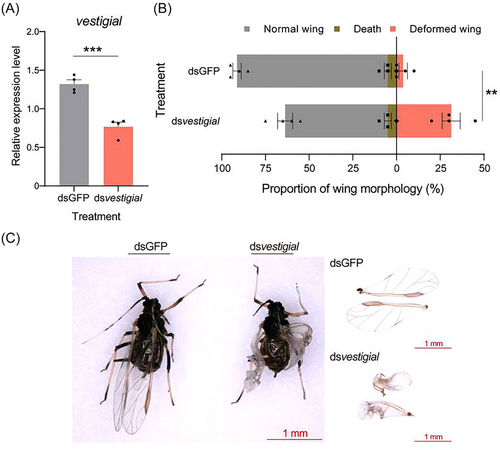
To investigate the effects of wing development-related genes on the wing development of A. citricidus, we used RNAi technology to silence vestigial, the most significantly downregulated wing development-related gene. The silencing efficiency of the vestigial was 41.86% after feeding dsvestigial for 24 h (Fig. 9A). The proportion of the malformed wings was 31.25% in dsvestigial treatment (Fig. 9B, C).
Effect of hormones on AcBursicon genes
The 4th instar winged nymphs were fed an artificial diet mixed with 20E, JHIII, and insulin. The RT-qPCR results showed that feeding these 3 hormones did not significantly affect the expression levels of AcBurs-α and AcBurs-β (Fig. S4A and S4B).
Discussion
Bursicon is a homodimer or heterodimer neuropeptide encoded by Burs-α or Burs-β, which plays an important role in insects’ skin tanning, wing development, and immunity (Vitt et al., 2001; Honegger et al., 2008; Dong & Song, 2013). In our study, we discovered the presence of AcBursicon genes in A. citricidus. Notably, the 11 cysteine residues of AcBurs-α and AcBurs-β exhibited a high level of conservation in the homologs obtained from 6 other aphid species (Fig. 1). These cysteine residues form 5 intramolecular disulfide bonds and an intermolecular dimer (Dong & Song, 2013).
AcBurs-α and AcBurs-β were highly expressed in 4th instar winged nymphs and adult winged aphids and were highly correlated with A. citricidus wing development. The higher expression levels of Bursicon genes at the pupal stage compared with the larvae and adults in Anopheles gambiae indicate that Bursicon may play a role in pupal development and metamorphosis (Honegger et al., 2011). This finding is consistent with similar relative expression levels observed in other insects such as Bombyx mori (silkworm), Lymantria dispar (gypsy moth), and M. separata (oriental armyworm) (Huang et al., 2007; Kong et al., 2021; Zhang et al., 2022a). Bursicon (also called the tanning hormone) is a hormone that is involved in the regulation of cuticle hardening and wing expansion during metamorphosis, and its higher expression at the pre-eclosion stage (last pupal stage or last nymph stage) indicates its importance in wing development.
Bursicon has been reported to regulate insect wing development by activating cAMP/PKA signals to induce apoptosis in wing dermal cells (Kimura et al., 2004; Honegger et al., 2011). Our research showed that feeding PKA inhibitors or silencing the AcBursicon genes through RNAi can lead to wing malformation in the 4th instar winged nymphs after emergence. Bursicon is widely believed to be the hormone responsible for cuticle tanning in many insects (Dong & Song, 2013). Wing expansion was regulated by specific Bursicon- and crustacean cardioactive peptide (CCAP)-expressing brain cells at the time of the adult molt (Luan et al., 2006; Luan et al., 2012; White & Ewer, 2014). Bursicon participates in cuticle tanning and wing expansion after molting. In this study, although no significant difference in mortality was observed between the RNAi-treated and control groups, the effects on the internal physiological structures are still unclear. Bursicon subunits result in wrinkled elytra due to incomplete wing expansion and are involved in post-ecdysis behavior modulation for wing expansion in T. castaneum (Arakane et al., 2008). In other studies, Bursicon affects cuticle tanning and causes behavioral deficits responsible for wing expansion and wing deformity (Luo et al., 2005; Luan et al., 2006; Huang et al., 2007; Dai et al., 2008). Thus, behavioral deficits, disrupted processes of tanning or hardening of the wings, or insufficient wing hardening may be the main reasons for the observed wing defects in A. citricidus. In future work, we will pay more attention to the effects of silencing AcBursicon genes on the tanning and hardening of the epidermis as well as on the wing structures (such as venation, wing size, or shape).
The regulation of wing development by tanning hormones may also be related to classical wing development-related genes. In Bactrocera dorsalis, Bursicon can affect the expression of hedgehog, a classic wing development-related gene, and thus regulate wing expansion (Zhou et al., 2024). In our research, after silencing AcBursicon genes, 4 classic wing development-related genes, including nubbin, vestigial, notch, and spalt major, were significantly downregulated. Silencing the expression of vestigial in the 4th instar winged nymphs through RNAi also led to wing malformation after emergence, the same phenotype as after silencing Bursicon genes. In Drosophila, the Notch signaling pathway is involved in wing-cell proliferation and wing morphogenesis because it regulates several genes at the dorsal–ventral boundary, including vestigial, which regulates proper wing margin formation (Baonza & Garcia-Bellido, 2000; Zecca & Struhl, 2007). In T. castaneum, RNAi of vestigial and nubbin led to a reduction of elytra and hindwings (Tomoyasu et al., 2009; Clark Hachtel et al., 2013). Similarly, silencing the expression of vestigial also led to wing malformation in Myzus persicae (Zhang et al., 2022c). The function of spalt major was regulated by the early wing disc cell, which develops into the dorsal body wall cell and delimits the wing primordium of D. melanogaster (Grieder et al., 2009). This indicates that AcBursicon genes regulates the expression of wing development-related genes, which is a potential mechanism for regulating the wing development of A. citricidus.
The diverse structures of insect wings confer multiple advantages; they make insects more proficient in a variety of activities, such as foraging, courtship, evasion, spreading, and migration, surpassing other terrestrial animals in these aspects. Wing-based diffusion, in particular, is an ecologically significant strategy employed by pests to adapt and respond to environmental changes (Zhang et al., 2019). In the formation and development of insect wings, the final developmental form of insect wings is mainly determined by the Dpp, Hippo, and Wnt signaling pathways through mutual interaction and coordination (Simoes Da Silva et al., 2019; Guo et al., 2020; Yin et al., 2020; Yuan et al., 2022). In recent years, insulin/insulin-like growth factor signaling has also been reported to be involved in wing development regulation in Nilaparvata lugens and Acyrthosiphon pisum (Xu et al., 2015; Yuan et al., 2022; Zhang et al., 2022b).
In our study, the KEGG pathway enrichment results showed that multiple wing development-related pathways such as the Wnt, Hippo, and FoxO signaling pathways were enriched. Moreover, in cellular processes, pathway enrichment results also showed a high correlation with cellular autophagy. REPTOR2, which regulates the expression of autophagy-related genes (ATGs), can transform winged pea aphids into wingless forms by activating wing disc autophagy to inhibit wing development (Yuan et al., 2023). Similarly, the Wnt signaling pathway also suppresses apoptosis during early wing development (Zhou et al., 2023). However, in our study, most autophagy-related genes and apoptosis genes did not undergo significant changes (Figs. S2 and S3). We speculate that despite enrichment for autophagy and lysosomal pathways by KEGG analysis, the enriched genes (Table S2) did not play a dominant role in autophagy. Therefore, there was no significant change in the expression level of ATGs. This also indicates that the effect of tanning hormones on wing development may not be related to the mRNA levels of autophagy-related or apoptosis genes but may be regulated through protein levels.
Three hormones (20E, JHIII, and insulin) have been widely reported to be involved in aphid wing development (Ishikawa et al., 2013; Vellichirammal et al., 2017; Shang et al., 2020; Yuan et al., 2022). After feeding A. citricidus 3 hormones, there was no significant change in AcBursicon gene expression levels, which indicated that AcBursicon genes regulation of wing development in A. citricidus was independent of these hormones.
In summary, we identified AcBurs-α and AcBurs-β, and mixed in vitro-synthesized dsRNAs (dsAcBurs-α; dsAcBurs-β; dsAcBurs-α + dsAcBurs-β) with artificial aphid feed that was fed to 4th instar winged nymphs to interfere with AcBursicon gene expression. The results showed that, regardless of whether dsAcBurs-α/dsAcBurs-β were fed alone or in combination with dsAcBurs-α + dsAcBurs-β, the AcBursicon gene transcription level was significantly downregulated, and interference with AcBursicon genes resulted in curled and malformed wings, which could not be normally flattened, and restricted development. At the same time, decreased expression levels of Bursicon can also lead to downregulated expression levels of classical wing development genes (nubbin, vestigial, notch, and spalt major). We also confirmed that reducing the expression level of vestigial through RNAi can also lead to wing malformation. This indicates that AcBursicon genes play an indispensable role in the wing expansion of A. citricidus and could be a valuable target for controlling A. citricidus.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (32272526, 32302338, and 32020103010), Natural Science Foundation of Chongqing, China (CSTB2022NSCQ-MSX0750), and China Agriculture Research System of the Ministry of Finance and the Ministry of Agriculture and Rural Affairs.
Disclosure
The authors declare they have no conflicts of interest.
Open Research
Data availability statement
RNA-seq raw sequences have been submitted to the Sequence Read Archive at NCBI under the Accession number PRJNA1017533. Further inquiries can be directed to the corresponding author.




