Lung ultrasound in a respiratory pandemic
How to use lung ultrasound to assess the severity of COVID-19, exclude alternative causes and ensure endotracheal intubation at the bedside in real time in the patient presenting to ED with respiratory symptoms during a surge in COVID-19 presentations.
Abstract
Australian hospitals have prepared for a major surge in patients due to the infectious respiratory pandemic COVID-19. In other nations, patient presentations have overwhelmed resources. Ultrasound has been shown to be an effective tool to exclude significant life-threats in resource poor settings. In this article, we will describe three lung ultrasound algorithms for the emergency diagnosis of patients presenting with respiratory symptoms during a COVID-19 pandemic: (i) LUSC19: lung ultrasound to assess the severity of COVID-19; (ii) LUSAC: lung ultrasound to exclude alternative causes of respiratory distress; and (iii) LUSI: lung ultrasound following intubation. We anticipate that emergency physicians will use these algorithms during the upcoming respiratory pandemic to rapidly determine the severity of COVID-19 infection, to seek and treat significant alternative diagnoses and ensure endotracheal intubation.
Lung ultrasound (US) is at the forefront of patient assessment during the COVID-19 pandemic, superseding traditional means such as auscultation, chest X-ray (CXR) and computed tomography (CT). Descriptive studies emerging from Italy describe the effective use of lung US in an overwhelmed system.1
Lung US is as sensitive as chest CT in experienced hands2 and international guidelines have been developed for its use in critical care.3 In a respiratory pandemic, lung US enables assessment of the severity of interstitial lung disease caused by COVID-19, the presence of alternative causes for respiratory distress and the confirmation of endotracheal intubation.
On CT, COVID-19 is a bilateral, peripherally located interstitial lung disease, beginning at the posterior lower lobes and progressing superiorly.4 Patients with severe disease may have consolidation, increased interstitial oedema, extensive lung involvement and small effusions.4, 5 Lung US signs closely match CT findings.6, 7
In a respiratory pandemic, some patients will present with an alternative cause for respiratory distress which may be missed. Lung US can accurately diagnose common differential diagnoses such as acute pulmonary oedema (APO), pneumothorax and pleural effusions.8
Finally, following intubation, lung US enables confirmation of endotracheal intubation at the bedside prior to CXR availability, with a higher accuracy than auscultation.9
LUSC19: lung US to assess the severity of COVID-19
To determine the severity of COVID-19 in patients with typical bilateral changes (Table 1).
| Score | Pleura | B lines | Consolidation |
|---|---|---|---|
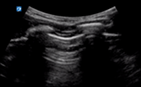
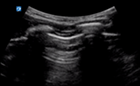
| 0 | 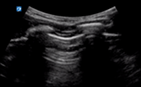
|
|
|
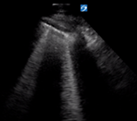
| Thin regular pleural line | A lines and/or <3 B lines | No consolidation | |
| 1 | 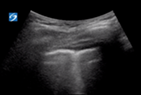
|
|
|
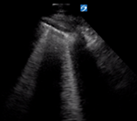
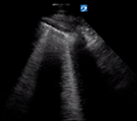
| Irregular but thin pleural line | >3 separated B lines | No consolidation | |
| 2 | 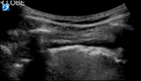
|
|
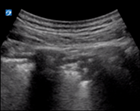
|
| Thick pleural line | >3 separated B lines | Subpleural <1 cm | |
| 3 | 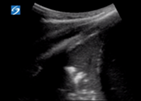
|

|
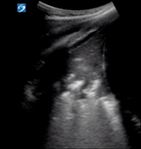
|
| Pleural line may be irregular, thick or not seen due to hepatisation | Confluent B lines | Subpleural >1 cm or hepatisation with shred sign | |
| 4 | 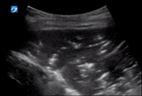
|
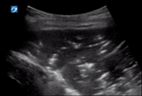
|

|
| Pleural line not seen due to hepatisation | No B lines due to hepatisation | Lobar consolidation with air bronchograms |
Where to scan
By dividing the chest into six segments bilaterally, all lobes of the lung may be examined with US. Begin anteriorly, proceed to the lateral and then posterior chest. The nomenclature (R/L 1–6) is then consistent even if the posterior chest is not examined (Figs 1-3).
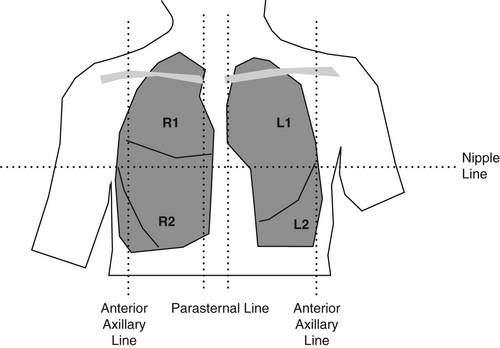
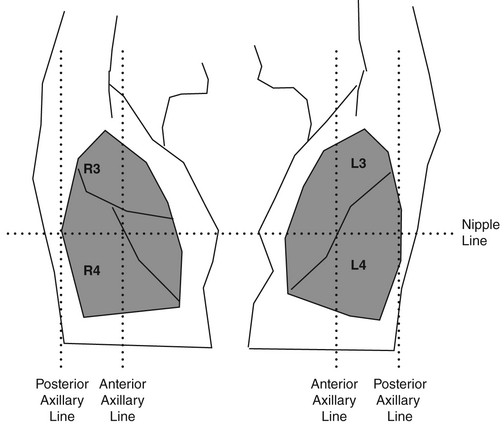
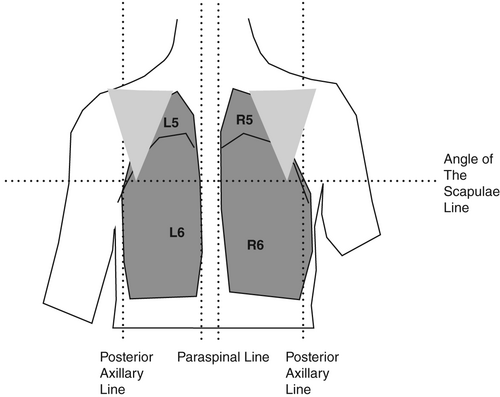
Machine settings
Transducer: curvilinear, lung preset (compound and harmonic imaging off, dynamic range decreased).
Depth: initially 5–6 cm (pleura in the centre of the screen) then increase depth to 15–20 cm to assess the parenchyma and pleural effusions.
Gain: adjust gain so that the pleura is bright white and the rib shadows are black.
How to scan
Place the transducer vertically with the marker orientated cranially. Image two ribs with corresponding shadows (Fig. 4). Angle the transducer medially and laterally until the US beam is perpendicular to the pleura (i.e. the pleura becomes bright white and as thin as possible). Assess the pleura for changes. Where artefacts other than A lines are seen, increase the depth to assess these better. Do this for each lung zone. In R4 and L4, increase the depth (15–20 cm) to assess for the spine sign (Fig. 5).

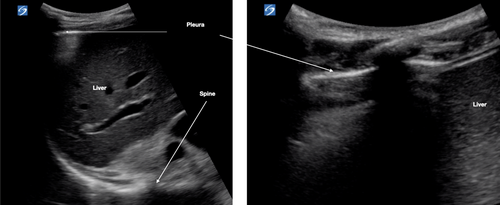
Lung US features in COVID-19
US of non-diseased lung shows a thin pleura (<3 mm) with horizontal reverberation artefacts deep to the pleura (A lines) (Fig. 4).
- Thickened, irregular pleura
- B lines
- Consolidations.
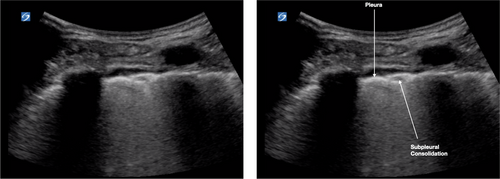
Pleura
Interstitial lung disease causes the pleural line to become shredded, irregular and thick with hypoechoic pock-marked areas (Fig. 6). These hypoechoic areas are subpleural consolidations.
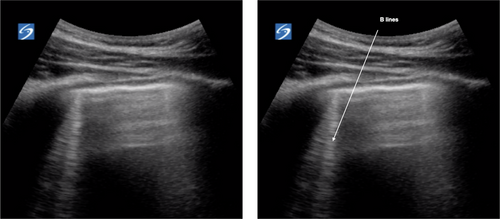
B lines
B lines are vertical laser-like lines extending from the pleura to the inferior edge of the screen (Fig. 7). They move synchronously with respiration. More than three B lines in one lung segment is abnormal. In interstitial disease the B lines are often localised to an area of diseased pleura. As COVID-19 severity increases B lines increase in number. There may be areas of normal lung with A lines interspersed between areas with B lines.
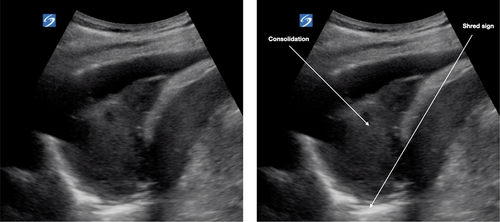
Consolidations
Subpleural consolidations (Fig. 6) are hypoechoic areas <1 cm in depth within a thickened pleural line. These progress to hypoechoic triangular areas of hepatisation (>1 cm) with a deeper shred sign (Fig. 8). Lobar consolidations are large areas of consolidation with air bronchograms, without a visible shred sign.
Pleural effusions
Pleural effusions are uncommon and if present are small in patients with COVID-19 pneumonitis. On lung US, these would be seen as the quad sign (Fig. 9) or small effusions near the diaphragm.
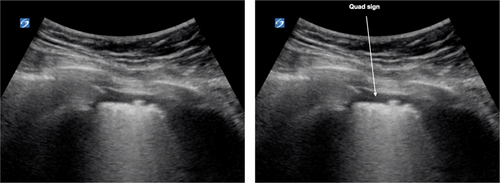
In summary, the lung US features of COVID-19 are bilateral, begin in the lower zones and progress superiorly. The pleural line is irregular and becomes thick and develops subpleural consolidations with increased severity. Subpleural consolidations progress to lobar consolidation in severe disease. More than three separated B lines are seen initially and increase in number with more severe disease. Pleural effusions, if present, are small.
Scoring
Extrapolated from CT, we have developed a severity scoring system for COVID-19 in patients with bilateral lung US signs (patients with unilateral signs should be excluded) (Table 1):
0: Thin pleura and A lines only or <3 B lines.
1: Irregular pleura and >3 separated B lines.
2: Thickened pleura and/or hypoechoic subpleural consolidation <1 cm.
3: Consolidation with shred sign and/or confluent B lines.
4: Lobar consolidation without shred sign.
A final severity score is the sum of the individual scores of all 12 segments. The severity score could be used to monitor progress and guide inpatient treatment. This score is currently unvalidated, and research is required to evaluate its utility in tailoring management and predicting significant clinical outcomes.
Pitfalls
The changes of COVID-19 are not specific. Bilateral lower lobe changes may also be seen in other viral pneumonitis, interstitial lung diseases such as pulmonary fibrosis and adult respiratory distress syndrome.
LUSAC: lung US for alternative causes of respiratory distress
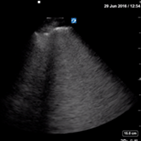
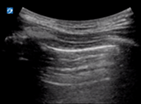
Alternative causes of respiratory distress (Table 2) easily diagnosed with lung US are APO, pneumothorax and large pleural effusions.
| Acute pulmonary oedema | L1/2 and R1/2 | L4/6 and R4/6 | |
|

|
||
| Thin pleural line, confluent B lines | Bilateral anechoic effusions | ||
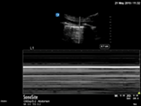
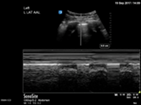
| Pneumothorax | L1/2 or R1/2 | M mode | Lung point |
|
|
|
|
| Thin regular static pleural line, A lines only | Barcode sign | Seashore sign interspersed with barcode sign | |
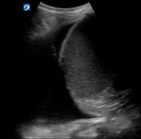
| Pleural effusion | L4/6 and R4/6 | ||
|
|||
| Spine extends above the diaphragm | |||
Acute pulmonary oedema
Insonate R1/2 and L1/2. Confluent homogenous B lines with a thin pleura are more likely to be due to APO.
Pneumothorax
In the supine patient, assess for pleural sliding at L1/2 and R1/2. Pneumothorax is likely if there are A lines, thin pleura and an absence of pleural sliding. Translate the transducer laterally to find the lung point at which pleural sliding is seen with inspiration. M mode over the area of absent pleural sliding will show a barcode sign. At the lung point, the M mode will show interspersed barcode and seashore sign.
Pleural effusion
With the patient supine, insonate the inferior pleural space at L4 and R4. We suggest placing the transducer as close to the bed as possible to visualise pleural fluid which will be located posteriorly.
In the normal chest, aerated lung prevents the US beam penetrating down to the spine and thus the spine is not visualised above the diaphragm. In the presence of pleural fluid, the spine is seen to extend above the diaphragm. As the volume of pleural fluid increases, more thoracic vertebrae will be visualised. Large pleural effusions are uncommon in COVID-19.
LUSI: lung US following intubation
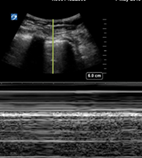
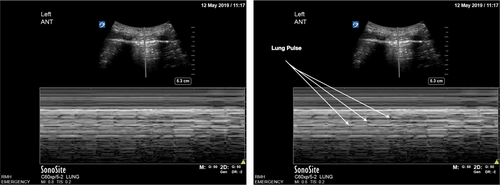
Following intubation, we suggest insonation at L1/2 and R1/2 for pleural sliding (Table 3). The presence of pleural sliding bilaterally indicates endotracheal intubation. Absent pleural sliding unilaterally may be due to endobronchial intubation or pneumothorax. If there is absent pleural sliding, M mode at the static pleura will show a barcode sign in pneumothorax and a lung pulse in non-ventilated lung (Fig. 10). Absent pleural sliding bilaterally may be due to oesophageal intubation or bilateral pneumothoraces (less likely).
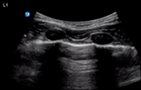
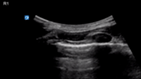
| Endotracheal intubation | L1/2 | R1/2 | M mode |
|
|
|
|
| Pleural sliding | Pleural sliding | Seashore sign | |
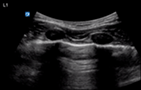
| Endobronchial intubation | L1/R1 | M mode | |
|
|
||
| Static pleural line in non-ventilated lung | Lung pulse in non-ventilated lung | ||
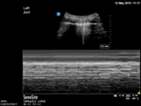
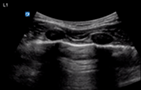
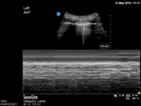
| Oesophageal intubation | L1 and R1 | M mode L1 and R1 | |
|
|
||
Static pleural line bilaterally |
Lung pulse bilaterally |
||
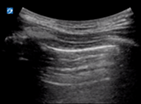
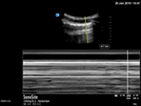
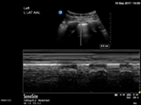
| Pneumothorax | L1/R1 | M mode | Lung point |
|
|
|
|
| Static pleural line | Barcode sign | Barcode and seashore sign |
Discussion and conclusion
These algorithms may be used in Australian EDs during the impending COVID-19 pandemic allowing the rapid, bedside assessment of patients presenting with respiratory symptoms. Use of US enables the assessment of COVID-19 severity and may decrease misdiagnosis, potentially improving patient outcomes. US machine cleaning should comply with local and international guidelines to decrease the risk of cross infection.
Author contributions
AL wrote the majority of the text. However, JA, BL and AM provided insightful editing and clarification.
Competing interests
None declared.




