Is it really possible to kill with insulin without leaving traces? From lifesaver to killer, the issues surrounding the analytical characterization of postmortem insulin illustrated by an exemplary case
Abstract
Evidence of an insulin overdose is very complicated in the medico-legal field. The analysis and subsequent interpretation of results is complex, especially when treating postmortem blood samples. The instability of insulin, the special pre-analytical conditions and the absence of specific analytical methods has led most laboratories not to analyze insulin in their routine with a consequent underestimation of cases. This paper aims to assess the difficulties associated with the analytical characterization of insulin by describing a case that typically represents most of the inconveniences encountered following a suspected insulin overdose. The case concerns a man found dead at home by his brother. After an external examination, which did not reveal a specific cause of death, toxicological analysis was requested which did not reveal any substance of toxicological interest. Only 9 months later, it was reported to the toxicologist that the subject was diabetic, on insulin lispro treatment and that three empty syringes were found next to his body. Following analysis by LC-high-resolution mass spectrometry, the presence of insulin lispro at a concentration of 1.1 ng/mL, a therapeutic concentration, was evidenced. Despite the low concentration found, overdose cannot be excluded and this paper will describe the criteria evaluated to reach this conclusion. This case highlights that the interpretation of a postmortem insulin concentration is very complex and requires the evaluation of various elements including the circumstances of death, the subject's medical history, the interval between death and sampling and the sample storage.
Highlights
- Interpretation of a postmortem insulin concentration is difficult.
- Anamnesis and context of death are critical in insulin overdose investigation.
- Even if insulins are measured at therapeutic concentration, overdose cannot be excluded.
- The criteria to be evaluated in a insulin overdose will be described in this paper.
1 INTRODUCTION
Deaths from insulin overdose are still not free of difficulties in the investigations that follow, due to the challenges in the analytical characterization of insulin itself.
Although the discovery and synthesis of synthetic insulins has saved numerous lives over the years, their misuse has often been described. Several nontherapeutic uses of these substances have been reported, from doping to suicidal or criminal purposes [1-3]. Its misuses therefore pose serious health risks. Indeed, insulin overdoses, whether accidental or intentional, are potentially fatal due to hypoglycemia-induced alteration of central nervous system metabolism, which can result in brain dysfunction, cerebral edema, and if not treated in time, coma and neurological death [4].
In the medico-legal field, documenting insulin intoxication is quite complex. The difficulty lies in the absence of morphological evidence, nonspecific pathological changes, and the lack of specific tests capable of discriminating between different types of insulin, remaining sensitive enough to measure low concentrations [3].
1.1 Insulin analogues structural similarity and unspecificity of immunological methods
Several formulations of insulin analogues have been synthesized. Synthetic insulin analogues can be divided into two categories: fast-acting insulins (lispro, aspart, and glulisine), and long-acting insulins (glargine, detemir and degludec). Depending on the desired pharmacokinetic profile, the initial peptide sequence is modified by the addition, substitution, or inversion of amino acids and the result leads to insulin analogues with very similar structures and molecular weights [5]. That is the first difficulty in evidencing an insulin overdose. Before the advent of modern technologies such as high-resolution mass spectrometry, the main difficulty in revealing insulin overdose was related to the inability to discriminate endogenous insulin from exogenous synthetic insulins. In fact, the most widely used analytical methods are immunoassay techniques, which have some drawbacks with regard to their specificity (unable to discriminate between insulin analogues) [6]. Most importantly, these methods are only suitable for serum and not for postmortem whole blood that has been hemolyzed and full of clots because of the degradation processes [7, 8]. Nowadays, current technologies such as high-resolution mass spectrometry make this discrimination possible without any difficulty by using multi-charged ions specific for each type of synthetic analogue of human insulin [9-13].
1.2 High molecular weight proteins
In addition to the issue of the high structural similarity between the various types of insulin, it must be considered that insulin is a protein with a high molecular weight (>2000 Da).
With classical ionization methods such as electrosprays, the m/z ratios produced are often between 500 and 2000. For high-mass molecules, multiply charged ions are formed, and therefore these are polyprotonated molecules. The formation of multiply charged ions is important as it reduces the mass-to-charge ratio of the ions, allowing the analysis of very heavy molecules such as proteins. The observed spectrum will, however, be more complex than unit-charged molecules [14]. Unfortunately, not all toxicology laboratories have the possibility to develop analytical methods for protein extraction and analysis. For this reason, the number of insulin overdoses will certainly be underestimated as most laboratories cannot perform this type of analysis in their routine.
1.3 Insulin postmortem instability
The biggest challenge with postmortem insulins analysis, however, it is the instability of insulins. Whole blood is usually used for postmortem toxicological analysis, which undergoes degradation, hemolysis and coagulation phenomena [15, 16]. This makes the amount of insulin remaining in the circulation almost undetectable. In addition to postmortem degradation, insulin has a propensity to undergo adsorption and aggregation. When insulin is in a low concentration solution, it is mainly in the form of monomers. Insulin monomers have hydrophobic groups oriented externally, which have a tendency to interact with the hydrophobic groups of the material used (polypropylene, glass, or borosilicate vials or tubes) to form bonds. Once adsorbed to the wall, these monomers interact with other monomers in solution to form aggregates [17].
If the amount of insulin present at the time of death was already low, it will become even lower after this phenomenon, becoming practically undetectable.
1.4 The time factor and sample preservation
The time factor plays a key role in the analytical characterization of insulin. The time between administration and death is often unknown and given that death from an insulin overdose will not occur immediately the concentration of insulin to be researched will be low as time passes. The longer this time is the more degradation phenomena will increase and the less it will be possible to establish a true concentration of insulin at the time of death [3].
Another key factor, where condition of storage comes into play, is the time between collection and analysis. In fact, degradation continues even in the test tube and if proper precautions are not taken, insulin will be impossible to find even with the most sensitive technologies simply because there will be no more [18]. It is recommended to centrifuge the sample immediately after collection to limit degradation [19]. Freezing the sample and collection in vials or test tubes made of materials that prevent insulin adsorption to the walls is also advised [19]. Unfortunately, however, these good practices in cases of suspected insulin overdose is difficult to apply in forensic medicine.
To answer the question raised in the title, yes, even today, if all the elements listed are not well evaluated, it is still possible to kill with insulin leaving traces, and in this paper, the authors will explain why by illustrating a case that fully represents the difficulties in revealing an insulin overdose.
2 THE CASE REPORT
A 63-year-old man was found dead at home lying on his bed by his brother. On request of the prosecutor, a body external examination was performed which revealed a marked asphyxiation syndrome not specific of a cause of death and the presence of a blood glucose sensor located on the left arm. Moving more in the direction of a toxic death, blood and urine samples were collected in order to perform toxicological tests. Conventional toxicological screening did not reveal any substance of toxicological interest at a toxic concentration. Nine months after the death, it is reported to the toxicologist that the subject was diabetic and that three syringes of Humalog® insulin (trade name of insulin lispro) and a letter expressing his suicidal intention were present next to the corpse. In response to this new information, an antidiabetic screening was performed. The blood sample was stored for 9 months in a dry tube at +4°C before insulin testing.
3 MATERIALS AND METHODS
3.1 Chemical and reagents
Human, aspart, lispro, glargine, detemir, and bovine insulin standards were supplied by Merck (Saint Quentin Fallavier, France). Methanol, acetonitrile for high-performance liquid chromatography isocratic grade and glacial acetic acid were obtained from V.W.R. Chemicals ProLabo (Fontenay-sous-Bois, France). Formic acid for liquid chromatography coupled with mass spectrometry (LC–MS) analysis was obtained from Carlo Erba (Chaussée du Vexin, France). Tween 20, Amicon Ultra Filters (3 kDa, 500 μL), and Eppendorf (low-binding protein) were purchased from Merck (Fontenay-sous-Bois, France). Mercodia Iso-Insulin enzyme-linked immunosorbent assay immunopurification plates were obtained from Mercodia (Paris, France). For all aqueous buffers and solutions, ultrapure water was used.
3.2 Insulin blood analysis
Two hundred fifty microliters of whole blood were subjected to protein precipitation with 250 μL of a mix of acetonitrile/methanol (1:1) in the presence of 20 ng/mL of bovine insulin used as an internal standard. Subsequently, ultra-filtration was performed using centrifugal concentrators and after two washes with 100 and 300 μL of phosphate-buffered saline (PBS), the retentate was deposited on the wells of the immunoassay plate for immunopurification. After 1 h of agitation in a stirring plate (800 rpm), the wells were washed three times with 300 μL of PBS and the insulin was eluted with 115 μL of a 2% formic acid solution of H2O/acetonitrile (80/20). Five microliters were injected into the LC-high-resolution mass spectrometry (HRMS) system. A 7-point calibration curve was prepared using the following concentrations of insulin lispro: 1, 5, 10, 50, 100, and 200 ng/mL.
3.3 Validation procedure
The method was validated according to SFTA (Societé Française de Toxicologie Analytique) guidelines [20] for linearity, precision, detection and quantitation limits and carry-over.
Calibrators and quality controls (QCs) for method validation were prepared in whole blood sample obtained from the French blood establishment (EFS). The blood was chosen after checking the absence of response to the retention times of the different insulins and the internal standard.
Three calibration curves, which included seven points (concentrations ranging from 1 to 200 ng/mL) and obtained over a 3-day period, were established for the study of linearity.
Repeatability and reproducibility were determined for two QC levels, 1 and 10 ng/mL. For repeatability, six replicates of each QC level were processed the same day. For reproducibility, each QC level was processed three times for four different days over a period of 2 weeks.
The limit of detection (LOD) was defined as the lowest concentration of the compound that can be detected with a signal-to-noise ratio greater than 3:1 for the target multi-charged ion. The limit of quantification (LOQ) was defined as the first point of the calibration curve.
Carry-over was also determined by analyzing for 5 days, three spiked blood samples with a concentration of 100 ng/mL followed by three blank blood samples (without standard). The percentage of carry-over was calculated as the ratio of the difference between the average of the values of the blanks passed just after the sequence of elevated concentrations and the average of the values of the blanks passed at the end and the difference between the averages of the elevated values and the average of the last blanks.
3.4 Instrument
Liquid chromatography separation was achieved using a Waters Acquity HSS C18 column (150 × 2.1 × 1.8 μm) with a controlled temperature maintained at 50°C. Five-microliter injection with a 0.25 mL/min flow of water with 0.1% of formic acid (Solvent A) and acetonitrile with 0.1% of formic acid (Solvent B) was used. The gradient elution was as follows: the initial 20% B was increased to 65% over 5.4 min, 65%–98% over 0.3 min and returned to initial conditions over 4.2 min. The total run time was 9.9 min, and lispro insulin was eluted at 2.7 min.
A Xevo G2-XS Q-TOF high-resolution mass spectrometer (Waters Corporation, Milford, MA, USA) was used, operating in positive ion mode and in sensitivity mode. Acquisition was performed in scan with a range of collision energies from 20 to 80 eV.
Desolvation gas flow was set at 1000 L/h at a temperature of 600°C, the cone gas to 50 L/h and the source temperature set to 120°C. The capillary voltage and the cone voltage were set to 3 and 15 V, respectively. In MS scanning, data were acquired from 100 to m/z 2000. Collision energy ranged from 20 to 80 eV. HRMS parameters were optimized by infusing 1 mg/mL solution of the analytes into the ion source and manually optimizing the parameters.
In addition to lispro, the presence of other analogues (aspart, glargine, detemir, and glulisine) and human insulin were tested, as described in a previous paper published by Arbouche et al. [13]. For lispro insulin, the 5-fold charged molecule was observed at m/z 1162.53665 (which deconvolutes to 5812.69504), while for human and bovine insulin the 5-fold charged ions were observed at m/z 1162.34989 and 1147.53238, respectively.
The high structural similarity of insulin lispro and human insulin results in almost identical chromatographic characteristics and spectra. Therefore, their ionic products, m/z 217.11830 and m/z 226.15516 found in the spectra obtained at high collision energy, are used to discriminate between the two types of insulin (Figure 1).
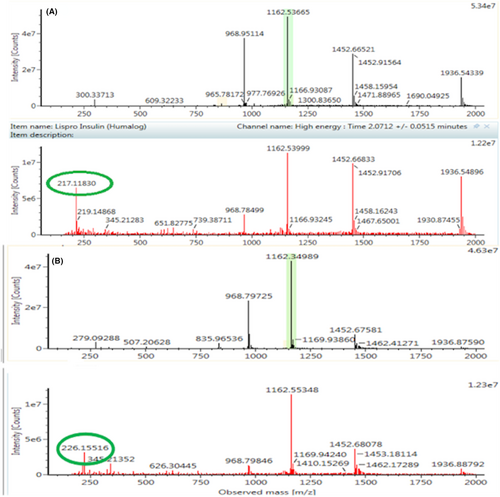
UNIFI software was used for data, chromatograms and spectra acquisition.
4 RESULTS AND DISCUSSION
For the method validation, the 7-point calibration curve showed a good linearity in the range 0–200 ng/mL with a correlation coefficient ranging from 0.9621 to 0.9999 during three tests. Repeatability was 15.4% and 10.2% at 1 and 10 ng/mL, respectively, and reproducibility was 18.1% and 9.5% at 1 and 10 ng/mL, respectively. The LOD and the LOQ are 0.5 and 1 ng/mL, respectively. Figure 2 shows the LOQ for insulin lispro in blood. The evaluation of carry-over showed an absence of carry-over providing values below 0.1%.
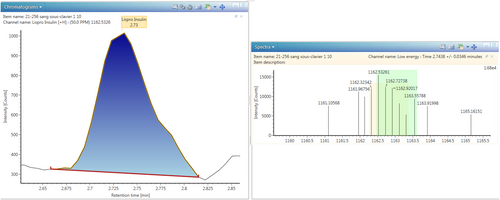
During the first toxicological screening no substance of toxicological interest was detected at a concentration that could have caused death. Only nordiazepam was revealed at a therapeutic concentration of 36 ng/mL. A toxic death therefore seemed to be excluded.
However, a very important information was not immediately communicated to the toxicologist: the subject's state of diabetes and the presence of three empty lispro insulin syringes in the night table. This information was not communicated to the toxicologist until 9 months after the blood sample collection and therefore 9 months after the death. The analysis of insulin and its synthetic analogues is generally not included in a toxicological screening. The reason is that this is an expensive method in terms of both material and time, which results in a quite costly analysis for the justice system. If the context of the death does not reveal any information that could suggest insulin intoxication (knowledge of a diabetic condition in the subject or the presence of empty syringes next to the corpse), this will not be searched. For this reason, the anamnestic-circumstantial criteria are of fundamental importance.
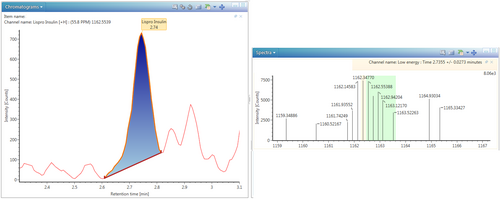
After acquiring this important information, an insulin screening was performed by LC-HRMS, which revealed the presence of insulin lispro at a concentration of 1.1 ng/mL (Figure 3).
Insulin lispro is a synthetic analogue of human insulin marketed under the name Humalog®. The difference with human insulin lies in the inversion of the amino acids lysine and proline at the C-terminal end of the β chain at position B28–B29. This substitution makes it less prone to aggregation in hexamers and leads to rapid monomer formation that will result in rapid absorption following subcutaneous injection and a more rapid onset of hypoglycemic action [21].
Regarding the concentration of insulin lispro found in this case, the toxicological significance of a postmortem exogenous insulin concentration is not easy to interpret. If the subject was not diabetic, the interpretation would have been easy as the mere presence of an exogenous insulin would create the suspicion of a suicide or murder attempt or an in-hospital mistake. The use of exogenous insulins in nondiabetic subjects can indeed have fatal consequences. However, the subject was diabetic and also on insulin lispro treatment. This detail makes the interpretation more complicated. At this point, interpretation of the found concentration is required. According to the European Medicines Agency (EMA) following the administration of a subcutaneous therapeutic concentration of 0.2 U/kg of insulin lispro the maximum blood concentration is approximately 1.66 ± 0.42 ng/mL (the dosage depends on the type of diabetes, physical activity and fasting state) [22]. Consequently, the concentration found in our case is within the therapeutic range. However, it is quite difficult to determine when an insulin concentration has to be considered toxic or therapeutic in the postmortem blood. In the literature, few papers report cases of insulin lispro overdose. Brvar et al. in 2005 [23] reported the case of a suicide attempt by a nondiabetic subject in which, 4 hours after the injection, insulin lispro was found at a concentration of 59.1 ng/mL, analyzed by enzyme immunoassay. Again in 2013, Hess et al. [24] reported a case of suicide by insulin lispro in which the latter was not found in the blood but only in the vitreous humor at 4 ng/mL. The explanation was attributed to an interval between injection and death of 7 h, which probably led to the total degradation of insulin in the blood. In 2019, Legg et al. [25] report a case of suicide using insulin lispro, in which once again insulin lispro was not found in the blood but only in the vitreous humor at a concentration of 2 ng/mL. The final decision on a lethal concentration of insulin lispro is quite difficult to establish.
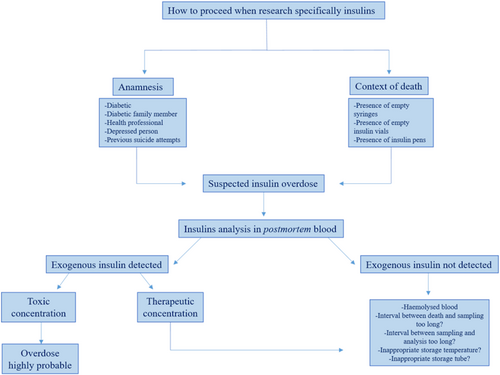
Many factors must be considered, among which the time factor, as indicated in the introduction, is of crucial importance. Figure 4 describes the parameters to be assessed when suspecting an insulin overdose. The time between administration and death, which in this case remains unknown. The time between death and sampling, as the longer this is, the more difficult it will be to establish a true insulin concentration at the time of death (in this case it was approximately 48 h). The biggest problem in this case was the interval between sampling and analysis, which was 9 months. In fact, insulin is rapidly degraded by insulinases released from erythrocytes as a result of hemolysis caused by postmortem decomposition. This degradation will continue in the tube if the sample is not stored properly. The storage of the sample and the type of tube used for preservation are also crucial in preventing post-sampling degradation.
In a study performed by our team [26], after testing the stability of insulin at +4°C and −20°C on various types of test tubes containing various types of preservatives, it was shown that if the sample was stored in tubes with EDTA and NaF, there was good stability even at +4°C, whereas if the sample was stored in dry tubes, there was a loss of 50% of the initial concentration already after 2 weeks. In our case, the blood was stored at +4°C for more than 9 months in dry tubes. Even if our study was conducted on insulin aspart and not on lispro, other insulin stability studies have shown that the stability of rapid insulins is similar [16, 26].
However, in the case described in this paper, the type of tube used (dry tube) not only do not contain any preservatives useful for storing insulin but are made of materials with which insulin forms bonds causing the phenomenon of adsorption and later aggregation. For all the difficulties encountered in this case, which combines practically most of the issues that can be observed in an insulin analysis, it cannot be excluded that, despite the therapeutic concentration of insulin lispro found in a diabetic subject under treatment, an overdose of insulin lispro happened (Table 1).
| Insulin lispro concentration | 1.1 ng/mL |
|---|---|
| Diabetic subject | Yes |
| Interval between administration and death | Unknown |
| Interval between death and sampling | 48 h |
| Interval between sampling and analysis | 9 months |
| Blood sample hemolyzed | Yes |
| Temperature of storage | +4°C |
| Type of tube | Dry tube |
| Conclusion | Overdose highly probable |
5 CONCLUSION
This case illustrates clearly the difficulties in the analytical characterization of insulin.
The long interval between collection and death, the hemolyzed state of the blood, and the storage of the sample in dry tubes have all together certainly contributed to a significant degradation of insulin. Nevertheless, the identification of insulin lispro, even at a low concentration, suggests that the concentration at the time of death was much higher and that it was probably a case of insulin lispro overdose.
This case illustrates the need for a complex approach in the interpretation of a postmortem insulin concentration. Many factors have to be assessed, such as the subject's medical history, circumstantial data and the time, mode, and state of preservation of the sample.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.




