Linearized hepatitis B surface antigen and hepatitis B core-related antigen in the natural history of chronic hepatitis B
Abstract
Changes in two novel HBV serological markers, linearized hepatitis B surface antigen (HQ-HBsAg) and hepatitis B core-related antigen (HBcrAg), in the natural history of chronic hepatitis B (CHB) have not been well characterized. Serum HQ-HBsAg and HBcrAg levels of 404 Asian treatment-naïve CHB patients were analysed in a cross-sectional manner. Patients were categorized into five groups: immune tolerant (IT group, n = 52), immune clearance (IC group, n = 105), hepatitis B e antigen (HBeAg)-negative hepatitis (ENH group, n = 97), HBeAg-negative quiescent group (ENQ group, n = 95) and CHB with hepatitis B surface antigen (HBsAg) seroclearance (SC group, n = 55). HQ-HBsAg and HBcrAg were measured and correlated with HBV DNA, HBsAg, HBV genotype and clinical parameters. HQ-HBsAg showed good correlation with HBsAg, especially in the ENQ group (r = 0.874, p <0.001). Correlation of HQ-HBsAg with HBV DNA was less prominent and weakest in the ENH group (r = 0.268, p 0.008). HBcrAg correlated best with HBV DNA in the ENQ group (r = 0.537, p <0.001). In the ENQ group, 42.1% of patients had undetectable HBcrAg; this subgroup of patients, when compared with those with detectable HBcrAg, had significantly lower median HBV DNA (3.17/4.48 log IU/mL, p <0.001) and HBsAg (5.05/5.96 log mIU/mL, p <0.001) levels. Forty per cent of the SC group patients had detectable HQ-HBsAg and/or HBcrAg up to 42 months after HBsAg seroclearance. When comparing anti-HBs positivity and median time after HBsAg seroclearance in the SC group with and without detectable HQ-HBsAg/HBcrAg, there was no significant difference (22.7% and 36.4%, respectively, p 0.284, and 76.5 and 93.2 months, respectively, p 0.245). HQ-HBsAg and HBcrAg showed unique patterns of distribution throughout the five disease phases of CHB, including high detectability rates after HBsAg seroclearance, opening up different possibilities for their applicability.
Introduction
Chronic hepatitis B (CHB) affects 350–400 million individuals worldwide, of which 15–40% will develop cirrhosis and its complications, including hepatocellular carcinoma (HCC) 1. Disease activity is reflected by serum hepatitis B virus (HBV) DNA levels. Serum HBV DNA >20 000 IU/mL, when compared with undetectable HBV DNA levels, increases the risk of cirrhosis and HCC by ten- and nine-fold, respectively 2, 3. Another biomarker advocated for the disease monitoring of CHB is the quantification of serum hepatitis B surface antigen (HBsAg). Serum HBsAg levels have been demonstrated to play a role in predicting CHB patients with quiescent disease activity 4, 5, risk of cirrhosis and HCC 5, 6, insignificant liver fibrosis 7, and subsequent HBsAg seroclearance 8.
The current lower detection limit for the majority of conventional serum HBsAg assays is 50 mIU/mL. When serum HBsAg falls into the undetectable range in CHB patients, HBV could still persist at low replicative and transcriptional levels 9. In addition, conventional HBsAg assays target only one HBsAg epitope, the common determinant ‘a’. Mutations within the determinant ‘a’ can give false-negative results 10. An emerging viral marker is the linearized HBsAg (HQ-HBsAg), which targets both the outer determinant ‘a’ of the surface genome and the epitope embedded inside the lipid bilayer of the viral envelope, with detection enhanced by adding detergents to the reaction buffer and by improving the assay tracer 11. The HQ-HBsAg assay may therefore detect HBsAg mutants more effectively and achieve an even lower limit of detection of 5 mIU/mL 12. Another potentially useful novel marker is the hepatitis B core-related antigen (HBcrAg), which detects an identical amino-acid sequence shared by the hepatitis B e antigen (HBeAg), hepatitis B core antigen and 22 kDa precore protein; production of this sequence is not dependent on HBV DNA formation 13. Serum HBcrAg correlates with disease activity 14 and could also play a role in predicting HCC development 15.
Our previous study demonstrated that the combined use of HBcrAg and HQ-HBsAg could detect HBV in >40% of CHB patients after HBsAg seroclearance 16. We now proceed to determine the serum HQ-HBsAg and HBcrAg titres in different disease phases of CHB and correlate them with established viral markers including serum HBV DNA and HBsAg in a large cohort of Asian CHB patients.
Methods
Patients
The present patient cohort was recruited from the Liver Clinic, Department of Medicine, The University of Hong Kong, Queen Mary Hospital, Hong Kong, from March 2010 to July 2013. All enrolled Asian CHB patients were treatment-naïve and had no evidence of concomitant liver disease, including chronic hepatitis C virus infection or significant intake of alcohol (20 g per day for women, 30 g per day for men). The patient cohort was divided into five groups: immune tolerant phase (IT group) (i.e. HBeAg-positive with normal alanine aminotransferase) (ALT); immune clearance phase (IC group) (i.e. HBeAg-positive with elevated ALT, HBeAg-negative hepatitis with elevated ALT) (ENH group), HBeAg-negative quiescent phase with normal ALT (ENQ group), and known CHB with documented HBsAg seroclearance (SC group). For the first four groups, all patients were positive, with HBsAg for at least 6 months prior to enrollment. For the SC group, patients had HBsAg-positivity documented at our clinic for at least 6 months and had undergone HBsAg seroclearance upon follow-up, and were then persistently HBsAg-negative with or without appearance of antibody to HBsAg (anti-HBs) for at least 6 months. HBsAg seroclearance was documented by the conventional HBsAg assay with the lower limit of detection <50 mIU/mL.
The upper limit of normal of ALT was set at 40 U/L. Patients enrolled into either the IT group or ENQ group had persistently normal ALT for 6 months prior to enrollment. Plasma samples were stored at −20°C until tested. This study was approved by the Institutional Review Board, the University of Hong Kong and West Cluster of Hospital Authority, Hong Kong.
Serum HQ-HBsAg
Serum HQ-HBsAg was measured using a chemiluminscent enzyme immunoassay (CLEIA) Lumipulse G1200 automated analyzer (Fujirebio Inc, Tokyo, Japan) as previously described 11. Briefly, plasma samples with denatured HBsAg were added to ferrite microparticles bound with anti-HBs monoclonal antibodies against the external determinant ‘a’ and the internal (normally embedded) bilipid layer epitope. After washing and incubation, 200 μL of substrate (AMPPD; 3-(2′-spiroadamantan)-4-methoxy-4-(3″-phosphoryloxy) phenyl-1, 2-dioxetane disodium salt) (Applied Biosystems, Bedford, MA, USA) was added. The relative intensity of chemiluminescence was measured and the concentration of HBsAg calculated by comparing with a standard curve. The dynamic range of HQ-HBsAg was 5–150 000 mIU/mL (0.70–5.18 log mIU/mL), and retesting was performed with a 200-fold dilution of samples among >150 000 mIU/mL as recommended 12.
Serum HBcrAg
Serum HBcrAg was measured using CLEIA as described previously 14. Briefly, sodium dodecyl sulphate pretreated serum was incubated with monoclonal antibodies against denatured HBcAg and HBeAg. After washing and incubation, the relative chemiluminescence intensity was measured and the HBcrAg concentration was calculated by comparison with a standard curve generated from known concentrations of recombinant HBeAg-containing peptides. The lower detection limit was 100 U/mL (2 log U/mL).
Other laboratory assays
Serum HBV DNA and HBsAg levels were measured using the Cobas Taqman assay (Roche Diagnostics, Branchburg, NJ, USA) and Elecsys HBsAg II assay (Roche Diagnostics, Gmbh, Mannheim, Germany), respectively, with a lower limit of detection of 20 IU/mL and 50 mIU/mL, respectively. HBV genotyping was performed using the INNO-LIPA HBV genotyping assay (Innogenetics, Ghent, Belgium).
Statistical analyses
Continuous variables were expressed as median (range). All serum viral marker results were expressed in logarithm. Statistical analyses were performed using SPSS version 19.0 (SPSS Inc, Chicago, IL, USA). The Mann–Whitney U-test or the Kruskal–Wallis test when appropriate, was used for comparison of continuous variables. The chi-square test was used for comparing categorical variables. Correlation between different clinical parameters was tested using Spearman's bivariate correlation. A two-sided p value of <0.05 was considered statistically significant.
Results
Four hundred and four treatment-naïve Asian CHB patients were recruited. The baseline patient characteristics as stratified by their grouping are presented in Table 1. Fifty-two (12.9%), 105 (26.0%), 95 (23.5%) and 97 (24.0%) patients were enrolled into the IT, IC, ENQ and ENH groups, respectively. We also enrolled 55 (13.6%) CHB patients with HBsAg seroclearance achieved (SC group). These 55 patients were HBsAg-positive in our clinic for a median duration of 80.7 (range, 24.6–262.2) months before HBsAg seroclearance, and were then tested at a median 19.1 (range, 6.2–42.7) months after HBsAg seroclearance, with 17 (30.9%) anti-HBs-positive at the time of testing.
| All patients (n = 404) | HBeAg-positive (n = 157) | HBeAg-negative (n = 192) | SC group (n = 55) | |||
|---|---|---|---|---|---|---|
| IT group (n = 52) | IC group (n = 105) | ENH group (n = 97) | ENQ group (n = 95) | |||
| Age, years | 46.4 (20.8–82.3) | 30.4 (24.4–74.0) | 37.2 (20.8–65.6) | 49.6 (24.8–67.4) | 52.0 (24.0–80.4) | 52.5 (28.4–82.3) |
| Male, n (%) | 267 (58.3) | 29 (55.8) | 67 (63.8) | 65 (67.0) | 65 (68.4) | 41 (74.5) |
| Genotypea | ||||||
| B, n (%) | 208 (51.5) | 30 (57.7) | 40 (38.1) | 38 (39.2) | 63 (66.3) | 37 (67.3) |
| C, n (%) | 196 (48.5) | 22 (42.3) | 65 (61.9) | 59 (60.8) | 32 (33.7) | 18 (32.7) |
| Albumin, g/L | 43 (25–50) | 44 (43–46) | 41 (25–50) | 42 (29–48) | 44 (33–49) | 44 (34–50) |
| Bilirubin, μM | 12 (2–94) | 10 (4–29) | 13 (2–52) | 13 (4–94) | 11 (4–50) | 10 (2–38) |
| ALT, U/L | 47 (5–2144) | 23 (9–39) | 130 (41–2144) | 93 (44–2083) | 28 (22–34) | 23 (4–60) |
| HBV DNA, log IU/mL | 5.99 (UD–9.53) | 8.29 (2.15–8.92) | 7.68 (3.95–9.53) | 6.05 (3.12–8.60) | 3.82 (UD–7.12) | UD (UD–2.09) |
| HBsAg, log mIU/mL | 6.22 (UD–8.89) | 7.49 (3.87–8.89) | 6.72 (3.10–8.88) | 6.14 (3.39–7.98) | 5.60 (1.83–7.19) | UD |
| HQ-HBsAg, log mIU/mL | 6.31 (UD–9.06) | 7.75 (4.40–9.06) | 7.01 (3.03–8.47) | 5.93 (1.06–7.59) | 5.71 (2.59–7.11) | UD (UD–1.20) |
| HBcrAg, log U/mL | 5.24 (UD–9.92) | 8.54 (3.01–9.00) | 7.92 (5.04–9.92) | 4.92 (2.47–7.69) | 2.60 (UD–3.71) | UD (UD–8.39) |
- UD, undetectable; HBsAg, hepatitis B surface antigen; HQ-HBsAg, linearized HBsAg; HBcrAg, hepatitis B core-related antigen; IT group, immune-tolerant group; IC group, immune-clearance group; ENH group, HBeAg-negative hepatitis group; ENQ group, HBeAg-negative quiescent group; SC group, chronic hepatitis B with HBsAg seroclearance.
- Lower limit of detection: HBV DNA, 1.30 log IU/mL; HBsAg, 1.70 log IU/mL; HQ-HBsAg, 0.70 log IU/mL; HBcrAg, 2 log U/mL.
- a Genotype in patients with very low or undetectable viral loads (e.g. the SC group) was determined using a previously stored plasma sample of higher viral load.
The distribution of serum HBV DNA and HBsAg levels in all five groups is depicted in Fig. 1(a–b). The IT group had the highest median HBV DNA and HBsAg levels, followed by the IC, ENH and then the ENQ group.
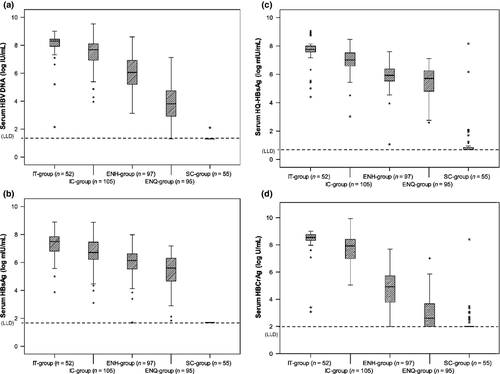
Serum HQ-HBsAg and HBcrAg levels among the whole cohort
Among the whole patient cohort (n = 404), there was a strong correlation between HQ-HBsAg levels and HBV DNA, HBsAg and HBcrAg levels (r = 0.762, 0.804 and 0.818, respectively, all p <0.001). Serum HBcrAg levels also correlated strongly with HBV DNA and HBsAg levels (r = 0.854 and 0.703, respectively, p <0.001). A weaker correlation was seen between HQ-HBsAg and HBcrAg levels with ALT (r = 0.210 and 0.341, respectively, p <0.001). Both HQ-HBsAg and HBcrAg had a moderate inverse correlation with age (r = −0.549 and −0.555, respectively, p <0.001).
When comparing median HBsAg and HQ-HBsAg levels among the whole patient cohort, there was no significant difference noted (6.22 and 6.31 log mIU/mL, respectively, p 0.411).
HBeAg-positive disease
The median HQ-HBsAg and HBcrAg levels and their distribution in HBeAg-positive disease are depicted in Table 1 and in Fig. 1(c,d). For HQ-HBsAg, the IT group had significantly higher median levels than the IC group (7.75 and 7.01 log IU/mL, respectively, p <0.001). Although HQ-HBsAg correlated well with HBsAg for both the IT and IC groups (Table 2A and Fig. S1, r = 0.639 and 0.503, respectively, p <0.001), median HQ-HBsAg levels were significantly higher than median HBsAg levels for both groups (IT group, 7.75 and 7.49 log mIU/mL, respectively, p 0.008; IC group, 7.01 and 6.72 log mIU/mL, respectively, p 0.021). No correlation exists between HQ-HBsAg and ALT (Table 2A).
| IT group (n = 52) | IC group (n = 105) | ENH group (n = 97) | ENQ group (n = 95) | |||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| (A) HQ-HBsAg | ||||||||
| Age (years) | −0.084 | 0.554 | −0.072 | 0.483 | −0.208 | 0.033 | −0.168 | 0.104 |
| HBV DNA (log IU/mL) | 0.520 | <0.001 | 0.268 | 0.008 | 0.427 | <0.001 | 0.442 | <0.001 |
| HBsAg (log mIU/mL) | 0.639 | <0.001 | 0.541 | <0.001 | 0.503 | <0.001 | 0.874 | <0.001 |
| HBcrAg (log U/mL) | 0.401 | 0.003 | 0.605 | <0.001 | 0.596 | <0.001 | 0.401 | <0.001 |
| ALT (U/L) | −0.076 | 0.598 | 0.020 | 0.848 | 0.150 | 0.128 | 0.112 | 0.284 |
| IT group (n = 52) | IC group (n = 105) | ENH group (n = 97) | ENQ group (n = 95) | |||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | r | p | p | r | p | |
| (B) HBcrAg | ||||||||
| Age (years) | 0.110 | 0.438 | −0.401 | −0.013 | 0.901 | <0.001 | −0.019 | 0.851 |
| HBV DNA (log IU/mL) | 0.369 | 0.007 | 0.484 | 0.537 | <0.001 | <0.001 | 0.472 | <0.001 |
| HBsAg (log mIU/mL) | 0.286 | 0.040 | 0.406 | 0.245 | 0.017 | <0.001 | 0.388 | <0.001 |
| HQ-HBsAg (log mIU/mL) | 0.401 | 0.003 | 0.596 | 0.401 | <0.001 | <0.001 | 0.605 | <0.001 |
| ALT (U/L) | 0.102 | 0.476 | 0.145 | 0.272 | 0.008 | 0.140 | 0.076 | 0.457 |
- HBsAg, hepatitis B surface antigen; HQ-HBsAg, linearized HBsAg; HBcrAg, hepatitis B core-related antigen; ALT, alanine aminotransferase; IT group, immune-tolerant group; IC group, immune-clearance group; ENH group, HBeAg-negative hepatitis group; ENQ group, HBeAg-negative quiescent group.
Median HBcrAg levels in the IT group were also significantly higher than in the IC group (8.54 and 7.92 log U/mL, respectively, p <0.001). The correlation of HBcrAg with other viral markers is depicted in Table 2(B). Correlation of HBcrAg with HBV DNA was moderate (IT group, r = 0.369, p 0.007; IC-group, r = 0.484, p <0.001). The pattern of HBcrAg distribution in HBeAg-positive disease is very similar to HBV DNA, as depicted in Fig. S2: very leptokurtic in the IT group (interquartile range 0.22 log U/mL, z = 28.3, and interquartile range 0.25 log IU/mL, z = 34.1, respectively, Fig. S2a) but more mesokurtic in the IC group (Fig. S2b). No correlation exists between HBcrAg and ALT (Table 2B).
HBeAg-negative disease
The median HQ-HBsAg and HBcrAg levels and their distribution in HBeAg disease are depicted in Table 1 and Fig. 1(c–d). For HQ-HBsAg, median levels in the ENQ group are significantly lower than in the ENH group (5.71 and 5.93 log IU/mL, respectively, p 0.005). Unlike HBeAg-positive disease, there was no significant difference between median HQ-HBsAg and HBsAg levels for both groups (ENQ group, 5.71 and 5.60 log mIU/mL, p 0.719; ENH group, 5.93 and 6.14 log mIU/mL, p 0.172). As depicted in Table 2(A), serum HQ-HBsAg correlated best with HBsAg in the ENQ group (r = 0.874, p <0.001). Correlation between HQ-HBsAg and HBV DNA was weaker, especially in the ENH group (r = 0.268, p 0.008). No correlation exists between HQ-HBsAg and ALT.
The median HBcrAg level in the ENQ group was significantly lower than that in the ENH group (2.60 and 4.92 log U/mL, respectively, p <0.001). Forty patients (42.1%) in the ENQ group had undetectable HBcrAg. This subgroup of patients, when compared with the ENQ group patients with detectable HBcrAg, had significantly lower median HBV DNA (3.17 and 4.48 log IU/mL, respectively, p <0.001), HBsAg (5.05 and 5.96 log mIU/mL, respectively, p <0.001) and HQ-HBsAg (5.03 and 6.05 log mIU/mL, respectively, p 0.012). Concerning the correlation of HBcrAg with other clinical parameters, HBcrAg correlated best with HBV DNA in the ENQ group (r = 0.537, p <0.001) and correlated best with HQ-HBsAg in the ENH group (r = 0.605, p <0.001). HBcrAg distribution in HBeAg-negative patients, as with the IC group patients with HBeAg-positive disease, had a mesokurtic distribution (Fig. S2).
HQ-HBsAg and HBcrAg after HBsAg seroclearance
The percentage of detectable serum HQ-HBsAg and HBcrAg in the SC group patients (n = 55) is depicted in Fig. 2(a). Sixteen (29.1%) and 12 (21.8%) had detectable HQ-HBsAg and HBcrAg, respectively, with the median duration after HBsAg seroclearance in these two groups being 16.2 (range 6.2–42) months and 19.2 (range 6.6–38.3) months, respectively. Only one patient (1.8%) had detectable HBV DNA. The levels of detectable HQ-HBsAg and HBcrAg with respect to the time of HBsAg seroclearance are depicted in Fig. 2(b). The median levels of detectable HQ-HBsAg and HBcrAg in the SC group were 1.18 (range 0.72 – 8.16) log mIU/mL and 2.70 (range 2.30 – 8.39) log U/mL, respectively.
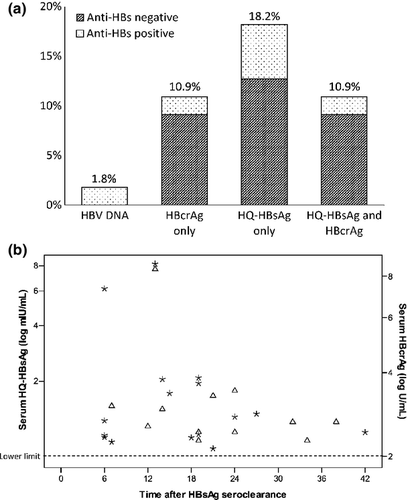
Altogether, 22 patients (40%) had detectable viral protein markers after HBsAg seroclearance. When comparing patients having either one or both detectable viral proteins (n = 22) with patients without detectable HQ-HBsAg and HBcrAg (n = 33), there was no significant difference in anti-HBs positivity (22.7% and 36.4%, respectively, p 0.284) or in the median duration after HBsAg seroclearance (76.5 and 93.2 months, respectively, p 0.245).
Discussion
Our present study provides the first detailed description of HQ-HBsAg and HBcrAg titres in a large cohort of Asian treatment-naïve CHB patients, and underlines the possible roles that both novel serologic markers could play in the disease monitoring of CHB (Table 3).
| HBcrAg | HQ-HBsAg |
|---|---|
| Proven | |
| Correlate strongly with disease activity | Lower HBsAg detection limit |
| Differentiate ENQ and ENH groups in HBeAg-negative disease | Better detection of HBsAg mutants |
| Detect occult HBV infection | Detect occult HBV infection |
| Potential (needs further evaluation) | |
| Predict occurrence of HCC | Replace conventional serum HBsAg quantification |
| Predict HBsAg seroclearance | Better delineation in durability of NA treatment cessation |
The role of quantitative serum HBsAg measurements in the management of CHB is continuously expanding 4-8, including during peginterferon 17 and nucleos(t)ide analogue (NA) therapy 18, 19. Besides showing strong correlation with serum HBsAg, the correlation of HQ-HBsAg with HBV DNA was similar to the interaction found between serum HBsAg and HBV DNA in previous studies 20, 21: moderate correlation in HBeAg-positive disease, but a weaker correlation in HBeAg-negative disease (Table 2A). This signifies serum HQ-HBsAg, with its advantages of a lower limit of detection and theoretically better detection of mutations compared with the ‘a’ determinant, could potentially replace serum HBsAg measurements in disease monitoring and prognostication. Serum HQ-HBsAg is significantly higher than HBsAg in HBeAg-positive disease, possibly due to the enhanced detection of minor viral populations with ‘a’ determinant mutations in patients with higher viral loads 11.
Serum HBcrAg has been previously shown to correlate strongly with intrahepatic markers of viral activity 14. In our present study, besides demonstrating good correlation with serum HBV DNA in all disease phases, we found that HBcrAg might be a good virological marker to be used to differentiate HBeAg-negative patients with active and inactive disease, as illustrated by the large difference of median values observed between the ENH group and the ENQ group, (4.92 and 2.60 log U/mL, respectively, a 2.3 log difference) (Table 1, Fig. 1d). In addition, 42.1% of patients in the ENQ group had undetectable HBcrAg. These results indicate that HBcrAg production is significantly lower in disease-quiescent HBeAg-negative patients compared with disease-active HBeAg-negative patients. It would hence be interesting to investigate whether there is any threshold HBcrAg level that could predict subsequent HBsAg seroclearance, as seen in the case of low HBsAg levels 8. Another possible role for HBcrAg would be in the prediction of HCC 15, 22, which could be explained by its good correlation with serum HBV DNA, and it potentially could complement well-established risk models for HCC 23, 24.
An important finding of our study is the detectability of HQ-HBsAg and HBcrAg among 40% of CHB patients achieving HBsAg seroclearance, validating the results of our previous study 16. The cumulative rate of HBsAg seroclearance has been reported to be 12.5% 25, with the seroclearance rate increasing after the age of 40 years 26. Despite HBsAg seronegativity, these individuals would still harbour occult HBV infection, with intrahepatic HBV DNA present at low replicative levels after HBsAg seroclearance 9, and could still develop HCC 27 and fulminant reactivation during immunosuppressive therapy 9, 28, 29. Since HQ-HBsAg and HBcrAg apply different virological methods in detecting HBV, their combined use could cumulatively improve the identification and differentiation of occult HBV infection from those with only past HBV exposure, allowing these individuals to benefit from HCC surveillance programmes or closer viral monitoring during immunosuppression.
Our study results may lay the foundation for the applicability of serum HQ-HBsAg and HBcrAg during peginterferon or NA therapy. Both markers could display on-treatment kinetics, which could affect treatment decisions, similar to HBsAg levels during peginterferon therapy 17. Although most patients would require long-term NA therapy to achieve continued virological suppression, treatment cessation after HBsAg seroclearance, seen in a minority of patients, has been shown to be durable 30. The role of HQ-HBsAg, with its lower detection limit, in defining the optimal time-frame for treatment cessation hence warrants further investigation.
Our study is limited by its cross-sectional nature, but longitudinal studies may be difficult given that patients in the IC and ENH groups are candidates for treatment. Our study also only included genotype B and C patients and, because HBsAg kinetics display inter-genotypic variability 17, 21, future studies should include patients with other HBV genotypes.
In conclusion, our study's two novel markers, serum HQ-HBsAg and HBcrAg, demonstrated a high degree of correlation with traditional viral markers in different disease phases of CHB. Unique patterns of serological distribution are present, including the combined 40% detectability among CHB patients with HBsAg seroclearance. These results may lay the foundation for future longitudinal studies investigating their applicability in both untreated and treated CHB.
Acknowledgements
The authors would like to thank John Chi-Hang Yuen, John Young and Karen Yu for their assistance in performing laboratory measurements.
Role of the Funding Source
The HBcrAg and linearized HBsAg measurements in our laboratory were supported by Fujirebio Inc.
Transparency Declaration
No conflicts of interest exist for any authors.




