Assessment of five screening strategies for optimal detection of carriers of third-generation cephalosporin-resistant Enterobacteriaceae in intensive care units using daily sampling
Abstract
There is no consensus on optimal screening procedures for multidrug-resistant Enterobacteriaceae (MDRE) in intensive care units (ICUs). Therefore, we assessed five strategies for the detection of extended-spectrum beta-lactamase (ESBL) and high-level expressed AmpC cephalosporinase (HL-CASE) producers. During a 3-month period, a rectal screening swab sample was collected daily from every ICU patient, from the first 24 h to the last day of ICU stay. Samples were plated on MDRE-selective media. Bacteria were identified using MALDI-TOF mass spectrometry and antibiograms were performed using disk diffusion. MDREs were isolated from 682/2348 (29.0%) screening samples collected from 93/269 (34.6%) patients. Incidences of patients with ESBL and HL-CASE producers were 17.8 and 19.3 per 100 admissions, respectively. In 48/93 patients, MDRE carriage was intermittent. Compared with systematic screening at admission, systematic screening at discharge did not significantly increase the rate of MDRE detection among the 93 patients (62% vs. 70%). In contrast, screening at admission and discharge, screening at admission and weekly thereafter, and screening at admission and weekly thereafter and at discharge significantly increased MDRE detection (77%, p 0.02; 76%, p 0.01; 86%, p <0.001, respectively). The difference in MDRE detection between these strategies relies essentially on the levels of detection of patients with HL-CASE producers. The most reasonable strategy would be to collect two samples, one at admission and one at discharge, which would detect 87.5% of the ESBL strains, 67.3% of the HL-CASE strains and 77.4% of all MDRE strains. This study should facilitate decision-making concerning the most suitable screening policy for MDRE detection in a given ICU setting.
Introduction
While third-generation cephalosporins (3GCs) are the antimicrobial agents used most commonly for infections due to Enterobacteriaceae, the level and spread of 3GC resistance has become a major public health concern 1-3. Two dominant mechanisms contribute to 3GC resistance in Enterobacteriaceae, the generally plasmid-mediated acquisition of extended-spectrum β-lactamase (ESBL)-encoding genes 1 and the high-level expression of AmpC cephalosporinase (HL-CASE) following either in vivo selected derepression of low-level expressed chromosome-encoded AmpC (LL-CASE) or, less frequently, acquisition of a plasmid-borne AmpC gene 1, 4, 5.
Resistance to 3GCs is often associated with resistance to other antibiotic families resulting in multidrug-resistant Enterobacteriaceae (MDRE). Before the spread of CTX-M-producing Escherichia coli, cross-contamination was the usual pathway for acquisition of ESBL-producing Enterobacteriaceae (ESBL strains) and hardly ever the consequence of in vivo selected mutation 1, 6. In contrast, in vivo selection remains the most common mode of HL-CASE production 7, 8. In recent years, a steady increase in the prevalence of ESBL strains has been observed in hospitals, long-term care facilities and in the community 9-11 while the prevalence of HL-CASE-producing Enterobacteriaceae (HL-CASE-strains) increased mainly in hospitals 5, 12, 13.
In intensive care units (ICUs), high MDRE prevalence has become a cause of increased morbidity and mortality, and a major concern with respect to the most appropriate treatment for infected patients 2, 14-17. Given the poor development of new drugs active against MDRE, the fight against their cross-transmission is of major importance. A better knowledge of the pattern of MDRE carriage in patients admitted to ICUs would help in selecting the best screening strategy in order to detect asymptomatic carriers, implement prompt isolation precautions and, thereby, limit MDRE spread 18, 19. While there is no definitive consensus on the most effective screening strategy for the detection of asymptomatic MDRE carriers 20, systematic screening at patient admission has been recommended 18, 19, 21. However, this strategy does not take into account MDRE acquisition during patient stay.
With the aim of defining the most appropriate screening strategy for detection of asymptomatic MDRE carriers in ICUs, we performed a prospective study using systematic daily sampling during the entire stay of ICU patients.
Methods
Settings and patients
All patients admitted to the medical and the surgical ICUs of an 830-bed acute-care teaching hospital between 3 April and 3 July 2011 were included in the study and surveyed until discharge. Patients already present in the ICUs on 3 April, patients who died during the first day of their stay and patients who refused to participate were not included. The study complied with confidentiality regulations and ethical standards, and, in agreement with French regulations, the institutional review board waived the need for informed consent (CCPPRB project number: ID-RCB 201-A00259-32, University Paris XI, February 2011).
Screening
Using the eSwab® system (Copan, Brescia, Italy), a rectal screening swab (RSS) was collected daily from each patient between the first 24 h (admission RSS) and the last day in the ICU (discharge RSS). For patients admitted late in the evening of day 1, if no RSS was collected, the RSS collected on the next morning (day 2) was considered the admission RSS. For patients who died, an RSS was not systematically collected on the last day, and the RSS of the day before was considered to be the discharge RSS. Rectal screening results were provided to physicians and nurses.
Microbiological tests
Each RSS was suspended in 1 mL of Liquid Amies (Copan), and 10 μL of the suspension were plated on two selective media (ChromID®, bioMérieux, Lyon, France, and Drigalski agar ceftazidime, Becton Dickinson, Franklin Lakes, NJ, USA) using an automated specimen-plating device (WASP®; Copan, Milan, Italy). Colonies were picked after 18 h of incubation at 37°C. Isolates were identified using matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry with a Microflex Bruker Daltonics/BioTyper™ version 2.0 system (Bruker Daltoniks, Bremen, Germany). Antimicrobial susceptibility indicative of ESBL and/or HL-CASE production was tested with the disk diffusion method on Mueller–Hinton agar (MH; Bio-Rad, Marnes-la-Coquette, France), according to European guidelines 22. An isolate was categorized as an ESBL producer when a zone of synergy between a 3GC or aztreonam and clavulanic acid disk was observed and as an HL-CASE producer when it displayed (i) resistance to 3GCs, (ii) absence of synergy between any 3GC or aztreonam and clavulanic acid, and (iii) at least a 5-mm increase in the inhibition zone diameters around the 3GC disks, observed on MH with and without cloxacillin (250 μg/mL). To reveal ESBL potentially masked by HL-CASE production, synergy was tested on MH with and without cloxacillin (250 μg/mL). All isolates categorized as ESBL and/or HL-CASE producers were also categorized collectively as MDRE.
Imported and acquired resistant strains
When the first RSS with an MDRE strain was collected on day 1 and/or day 2 (≤48 h), the corresponding strain was categorized as imported; when it was collected on or after day 3 (>48 h), the corresponding strain was categorized as acquired (by cross-transmission or in vivo selection). In a given patient, ESBL strains and HL-CASE strains could be imported or acquired independently.
Cross-transmission
When two isolates belonging to the same species and with the same antimicrobial resistance pattern were isolated from two patients hospitalized concomitantly in the same unit, strains were typed using randomly amplified polymorphic DNA analysis and the DiversiLab System (bioMérieux). Cross-transmission between two patients was assumed if their stays in the same unit overlapped for at least 1 day and if their isolates had the same antimicrobial pattern and similar DNA fingerprinting patterns.
Screening strategies
As an RSS was collected daily from each patient, we retrospectively and comparatively analysed the results of five potential MDRE detection strategies: screening only at admission (strategy A); screening only at discharge (D); screening at admission and discharge (AD); screening at admission and weekly thereafter (AW); screening at admission, weekly thereafter and at discharge (AWD). To calculate the weekly detection rate of MDRE carriers, we first determined the number of carriers who would have been detected, at each day of the week, if RSS had been collected at one given day. The seven values thus obtained were used to calculate the mean weekly detection rate.
Medical data
Demographic characteristics, severity scores at admission, need for mechanical ventilation and mortality were recorded for patients in the medical and surgical ICUs.
Statistical analysis
Continuous variables are presented as mean ± SD and categorical variables as n (%). Comparisons between groups (ICUs) were made using univariate analysis. For continuous variables, the Student's t-test was used for normally distributed variables and the Wilcoxon test for non-normally distributed variables. A chi-square test was used for categorical variables and for comparison of screening policies. SAS software version 9.2 (SAS Inc., Cary, NC, USA) was used for all statistical analyses.
Results
Patients
In total, 294 patients were admitted to the two ICUs during the study period. Small differences were observed between the two ICU populations (Table 1) but, taking into account that at admission carriage of ESBL strains and/or HL-CASE strains was not statistically different between the two populations, patients were pooled in one study group for further analysis.
| Factor | Surgical ICU | Medical ICU | Both ICUs pooled | p-Value |
|---|---|---|---|---|
| Number of patients | 84 | 185 | 269 | – |
| Age (years, mean ± SD)a | 59.4 (±18.7) | 66.5 (± 17.7) | 64.2 (±18.3) | 0.003b |
| Male sex (%) | 49 (58.3) | 109 (58.9) | 158 (58.7) | 0.93c |
| SAPS2 (mean ± SD) | 45.8 (±25.8) | 54.5 (±23.7) | 51.8 (±24.7) | 0.005d |
| Mechanical ventilation (%) | 54 (64.3) | 134 (72.4) | 188 (69.9) | 0.18c |
| Death (%) | 11 (13.1) | 47 (25.4) | 58 (21.6) | 0.04c |
| ESBL presence at admission (%)e | 12 (14.29) | 25 (13.51) | 37 (13.75) | 0.86c |
| HL-CASE presence at admission (%)f | 5 (5.95) | 18 (9.73) | 23 (8.55) | 0.30c |
| MDRE presence at admission (%)g | 17 (20.24) | 41 (22.16) | 58 (21.56) | 0.72c |
- a SD, standard deviation.
- b Parametric test.
- c Non-parametric test.
- d Chi-squared test.
- e ESBL, Enterobacteriaceae harbouring extended-spectrum β-lactamase.
- f HL-CASE, Enterobacteriaceae harbouring high-level expressed cephalosporinase.
- g MDRE, multidrug-resistant Enterobacteriaceae (ESBL and/or HL-CASE).
The 269 patients included in the study corresponded to 2413 patient days (mean length of stay, 8.6 days; range, 2–96 days). Compliance with providing an RSS was 97.3% (2348 RSS for 2413 patient days); compliance at admission and discharge was 99.9% in each case.
MDRE carriers
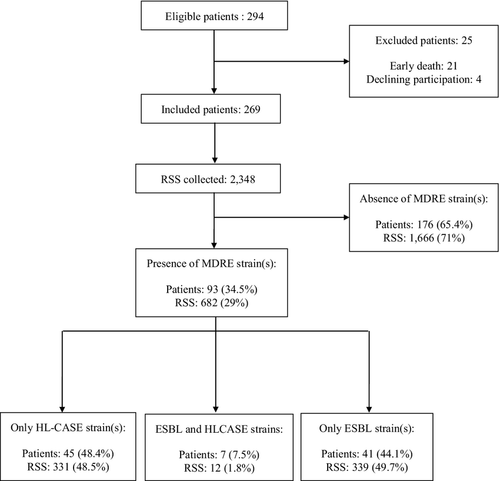
At least one MDRE strain was isolated from 682 RSS collected from 93 of 269 patients (34%) (Fig. 1). The incidence of MDRE carriers (n = 93) was 34.6 per 100 admissions. The incidence of patients with an ESBL strain (n = 48) was 17.8 per 100 admissions with a corresponding incidence density of 19.9 per 1000 patient days, while the incidence of patients with an HL-CASE strain (n = 52) was 19.3 per 100 admissions with a corresponding incidence density of 21.5 per 1000 patient days.
Distribution of MDRE strains
Table 2 illustrates the distribution of the 118 MDRE strains isolated during the study. E. coli and Klebsiella pneumoniae were the most common ESBL producers, while Enterobacter cloacae, E. coli and Citrobacter freundii were the most common HL-CASE producers.
| ESBL + straina | HL-CASE + strainb | No of strains | No (%) of strains isolated more than 48 h after admission | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Escherichia coli ESBL | + | + | + | + | 35 | 5 (14.3) | ||||||||||||
| Klebsiella pneumoniae ESBL | + | + | + | + | 16 | 6 (37.5) | ||||||||||||
| Other species ESBL | + | + | + | + | 5 | 0 | ||||||||||||
| Enterobacter cloacae HL-CASE | + | + | + | 18 | 12 (66.7) | |||||||||||||
| Escherichia coli HL-CASE | + | + | + | + | 14 | 5 (35.7) | ||||||||||||
| Citrobacter freundii HL-CASE | + | + | + | + | 10 | 3 (30.0) | ||||||||||||
| Other species HL-CASE | + | + | + | 20 | 14 (70.0) | |||||||||||||
| No of MDRE-patientsc | 29 | 10 | 2 | 4 | 1 | 1 | 1 | 14 | 8 | 4 | 16 | 1 | 3 | 2 | 3 | 1 | ||
- a ESBL, Enterobacteriaceae harbouring extended-spectrum β-lactamase.
- b HL-CASE, Enterobacteriaceae harbouring high-level over-expressed cephalosporinase.
- c Distribution of patients carrying ESBL strain(s) and/or HL-CASE strain(s). Seven patients who harboured ESBL strain(s) and HL-CASE strain(s) are counted twice.
Acquired/imported cases
Figure 2 shows the results of the daily collected RSS for each of the 93 patients with MDRE strains. Length of stay varied from 2 to 83 days. In 61/93 patients (65.6%), the first MDRE strain was isolated within 48 h of admission and considered as imported: 35 patients with only an ESBL strain, 22 patients with only an HL-CASE strain, and four patients with an ESBL and an HL-CASE strain. Among the four patients with an ESBL and an HL-CASE strain, patient 182 carried only an HL-CASE strain at admission and patient 183 carried only an ESBL strain at admission. Thus, at admission, the rate of ESBL-strain carriage was 14.1% (38/269) while that of HL-CASE strains was 9.3% (25/269).

In 32/93 (34.4%) patients, the first MDRE strain was acquired during the ICU stay more than 48 h after admission (see acquired cases, Fig. 2); that is, in six patients with only ESBL strains, in 23 patients with only HL-CASE strains and in three patients with both. In two patients, the first MDRE strain was imported, and at least one MDRE strain was acquired (Fig. 2, patients 182 and 183). Thus, the acquired/total case ratio was 10/48 (21%) for patients with ESBL strains (only E. coli and K. pneumonia) and 27/52 (52%) for patients with HL-CASE strains.
Daily sampling revealed that in 48/93 (52%) patients MDRE-strain carriage was intermittent (Fig. 2). Intermittent carriage concerned less often patients with ESBL (13/48, 27%) than those with HL-CASE strains (30/52, 58%; p <0.01). In patients with both ESBL and HL-CASE strains, these strains were found either sequentially (patients 6 and 182) or simultaneously (patients 185, 98, 38, 183 and 31).
Cross-transmission
Two episodes of cross-transmission with ESBL-producing K. pneumoniae involving five patients were observed during the study (first episode, patients 8 and 13; second episode, patients 182, 183 and 202). No cross-transmission between patients with HL-CASE strains was observed.
Screening strategy
When the results for all RSS collected were available, five potential MDRE screening strategies were retrospectively assessed. The results of these strategies are shown in Table 3.
| Screening strategy | Patients with MDRE-positive screening samples | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| ESBL carriers a (n = 48) | HL-CASE carriers b (n = 52) | MDRE carriers c (n = 93) | |||||||
| Nod | % | p Valuee | No | % | p Value | No | % | p Value | |
| RSS collected | |||||||||
| Admissionf | 37 | 77.1 | – | 23 | 44.2 | – | 58 | 62.4 | – |
| Discharge | 38 | 79.2 | 0.805 | 29 | 55.8 | 0.239 | 66 | 71.0 | 0.213 |
| RSS collected | |||||||||
| Admission + discharge | 42 | 87.5 | 0.181 | 35 | 67.3 | 0.018 | 72 | 77.4 | 0.025 |
| Admission + weekly mean valueg | 40 (38–42)h | 83.3 | 0.442 | 36 (34–40) | 69.2 | 0.010 | 74 (69–78) | 79.6 | 0.010 |
| RSS collected | |||||||||
| Admission + discharge + weekly mean value | 43 [42–44] | 89.6 | 0.100 | 41 [39–45] | 78.8 | <0.001 | 80 [77–82] | 86.0 | <0.001 |
- a Patients carrying Enterobacteriaceae strain(s) harbouring extended-spectrum β-lactamase (ESBL).
- b Patients carrying Enterobacteriaceae strain(s) harbouring high-level over-expressed cephalosporinase (HL-CASE).
- c Patients carrying ESBL and/or HL-CASE strain(s).
- d Number of patients.
- e The chi-squared test was used to compare different screening strategies with the reference policy (screening sample collected only at admission). Each strategy was considered as statistically different from the reference policy for a p value <0.05.
- f Detection of MDRE carriers at admission was chosen for reference.
- g We first assessed the number of MDRE patients detected if sampling had been done on a defined day of the week during their stay (seven values from Monday to Sunday). The mean value was then calculated from these seven values, and values between brackets correspond to extremes of the seven values. For instance, from the 48 ESBL patients, 23 ESBL patients were detected using a systematic RSS collected on Friday and 31 ESBL patients using an RSS collected on Thursday, and the mean value for the 7 days of the week was 27.
- h Extreme values for weekly screening.
Discussion
The results of the present study regarding the epidemiology of MDRE colonization in ICU patients can be summarized as follows: (i) the rate of MDRE carriage at admission is high, with identical prevalence of ESBL and HL-CASE strains; (ii) ESBL strains are mostly imported and HL-CASE strains mostly acquired; (iii) carriage of MDRE is intermittent in half of the cases; and (iv) the efficiency of the screening strategy varies with the screening policy implemented.
The prevalence of ESBL-strain carriers found in this study conducted in 2011 follows the trend observed in the last decade. At the end of the 1990s, when the dissemination of ESBLs was low, one report showed that only 1% of ICU patients were colonized with an ESBL strain 20. In the 2000s, when CTX-M-type ESBLs emerged, an increasing prevalence of patients with ESBL strains in ICUs, ranging from 8% in 2005 to 20% in 2010, was observed 24, 23. These studies also reported high rates of colonization with ESBL strains at admission, ranging from 9% to 15% 23, 24, similar to that found in our study (i.e. 14.1%).
The data regarding colonization with HL-CASE strains remain scarce. To the best of our knowledge, only one multicentre study conducted in 2005 reported incidence rates of MDRE colonization with ESBL and HL-CASE strains 24. In that study, the incidence of patients with MDRE ranged from 8.8 to 21.0/1000 patient-days and that of patients with ESBL strains from 1.4 to 10.9/1000 patient-days, depending on the ICU 24. The acquired case/total case ratio was 49% for ESBL and 60% for HL-CASE-strain carriers compared with 21% and 52%, respectively, in the present study. These results show that a significant proportion of patients admitted to the ICUs were colonized with an MDRE strain (mainly ESBL) while another significant proportion of patients acquired an MDRE strain under antibiotic selective pressure (mainly HL-CASE) during the ICU stay.
The present results of daily sampling provide new information about the variability of MDRE carriage in ICU patients. They reveal an intermittent MDRE-strain carriage that concerned 27% of patients with ESBL and 58% with HL-CASE strains. A similar intermittent carriage was previously reported in patients carrying methicillin-resistant Staphyloccocus aureus 25, 27, 26. A likely explanation for intermittent carriage of ESBL strains might be a switch between administration and withdrawal of different antibiotics during the ICU stay impacting on the density of resistant strains, as shown in an animal model 28.
With the daily collection of RSS, we investigated five potential screening strategies (Table 3) for MDRE detection. Overall, sampling at admission or sampling at discharge did not show significant differences, including ESBL and HL-CASE carriers. Increasing the number of RSS, with one collected at admission and one at discharge, increased the detection of MDRE carriers significantly, and the rate of detection was even greater with one RSS collected at admission, one at discharge and one weekly. The difference in detection of MDRE carriers between these strategies is mainly due to a better detection of patients with HL-CASE strains, in whom we found higher acquisition and intermittent-carriage rates. Taking into account our results and financial considerations, the most reasonable strategy would be to collect two samples, one at admission and one at discharge, which would detect 87.5% of the ESBL strains, 67.3% of the HL-CASE strains and 77.4% of all MDRE strains.
This present work has some limitations. The study covered a period of only 3 months and concerned only two ICUs and a limited number of patients. Moreover, the study design was prospective but data analysis and projections for screening strategies were performed retrospectively. It would therefore be interesting to compare the different strategies prospectively, either simultaneously in five similar ICUs or sequentially in one ICU. Nonetheless, to our knowledge this is the first report of systematic daily sampling in all patients of an ICU from admission until discharge.
In conclusion, this study revealed high MDRE prevalence rates in colonized patients at admission and high MDRE acquisition rates during their stay. We identified a significant intermittent MDRE carriage, especially in patients with HL-CASE strains. Testing different screening strategies, we found that none was perfect because with each of them a fraction of MDRE strains would go unidentified. Incidentally, isolation of all patients colonised with these organisms is not consensual, in particular of patients colonised with ESBL-producing E. coli or derepressed AmpC-producing Enterobacter. However, this study should facilitate decision-making concerning the most suitable screening policy for the detection of MDRE in a given ICU setting, in accordance with the local isolation policy.
Acknowledgements
We thank all companies that provided materials for this study free of charge: Siemens for the WASP, and bioMérieux and Becton Dickinson for ESBL media. Part of this research was presented at the 31st Réunion Interdisplinaire de Chimiothérapie Anti-Infectieuse (RICAI), Paris, France, 2011, Abstract number 174/44o.
Funding
This work was supported by a grant-in-aid from Assistance-Publique, Hopitaux de Paris.
Transparency Declaration
All authors declare that they have no conflict of interests with respect to the present study. For English language and style, the manuscript has been reviewed by a native English speaker, profiting from the above grant-in-aid.




