Horizontal Gene Transfer Systems for Spread of Antibiotic Resistance in Gram-Negative Bacteria
ABSTRACT
Antibiotic-resistant bacteria have become a significant global threat to public health due to the increasing difficulty in treatment. These bacteria acquire resistance by incorporating various antibiotic resistance genes (ARGs) through specialized gene transfer mechanisms, allowing them to evade antibiotic attacks. Conjugation, transformation, and transduction are well-established mechanisms that drive the acquisition and dissemination of ARGs in Gram-negative bacteria. In particular, the horizontal transfer of plasmids carrying multiple ARGs is highly problematic, as it can instantly convert susceptible bacteria into multidrug-resistant ones. Transduction, mediated by bacteriophages that package ARG-containing chromosomal DNA from host cells, also plays a crucial role in ARG spread without requiring direct cell-to-cell contact. Recently, a novel horizontal gene transfer (HGT) mechanism involving outer membrane vesicles (OMVs) has been identified as a key player in ARG dissemination. OMVs—nanoscale, spherical structures produced by bacteria during growth—have been found to carry small plasmids and chromosomal DNA fragments containing ARGs from their host bacteria. This newly discovered transfer process, termed “vesiduction,” enables intercellular DNA exchange and further contributes to the spread of antibiotic resistance. Additionally, mobile genetic elements such as transposons, insertion sequences, and site-specific recombination systems like integrons facilitate rearrangement of ARGs, including their translocation between chromosomes and plasmids. This review explores the molecular mechanisms underlying the HGT of ARGs, with a particular focus on clinically isolated antibiotic-resistant Gram-negative bacteria.
Abbreviations
-
- ARGs
-
- antimicrobial resistance genes
-
- BLNAR
-
- β-lactamase-negative ampicillin-resistant Haemophilus influenzae
-
- CT
-
- cholera toxin
-
- HGT
-
- horizontal gene transfer
-
- IS
-
- insertion sequence
-
- OMVs
-
- outer membrane vesicles
-
- pbp
-
- penicillin-binding protein
-
- PRGBS
-
- Streptococcus agalactiae with reduced susceptibility to penicillin
-
- ssDNA
-
- single-stranded DNA
-
- T4SS
-
- type IV secretion system
1 Introduction
Horizontal gene transfer (HGT) plays a crucial role in bacterial genetic evolution, allowing bacteria to adapt to harsh environmental conditions, such as antibiotic exposure [1, 2]. HGT occurs not only between closely related bacteria but also across different species, facilitating the spread of antibiotic resistance genes (ARGs) and contributing to bacterial drug resistance [3, 4].
-
Conjugation: Direct gene transfer (e.g., plasmid transfer) between bacteria through cell-to-cell contact.
-
Transformation: Uptake and incorporation of free DNA from the environment into bacterial genomes.
-
Transduction: Transfer of genetic material via bacteriophages, which inject DNA into bacterial cells and integrate it into their genomes.
Recently, a fourth mechanism, vesiduction, has been defined as another route for bacterial HGT [7-10]. This process involves outer membrane vesicles (OMVs)—spherical, nanosized particles released by Gram-negative bacteria. OMVs contain DNA fragments and plasmids derived from the host bacteria. Upon attachment to the bacterial surface, OMVs facilitate the uptake of their DNA contents into the cytoplasm. While incorporated plasmids can replicate autonomously, DNA fragments can ultimately integrate into the bacterial chromosome.
In this review, we examine how clinically relevant ARGs spread via conjugation, transformation, transduction, and vesiduction, using antibiotic-resistant Gram-negative bacteria as a model. Additionally, we discuss integrons and transposons, which not only enable the local accumulation of ARGs but also regulate their expression, further contributing to bacterial resistance.
2 Conjugation
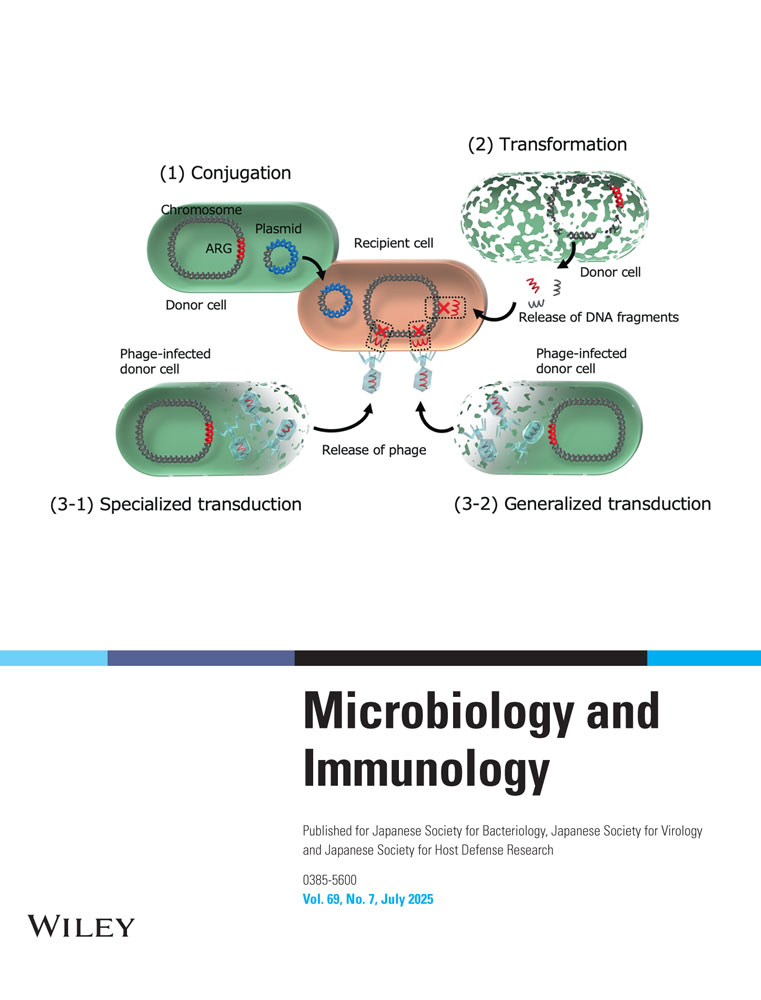
Conjugation was first identified in 1947 in Escherichia coli [11, 12]. It is a major mechanism driving HGT in Gram-negative pathogens, facilitating the spread of ARGs. This process requires direct contact between bacterial cells via a conjugation pilus, and subsequently forms nanotubes for plasmid transfer (Figure 1) [1, 13]. The genes responsible for pilus formation, which establish cell-to-cell contact, are located on conjugative plasmids. Once the donor and recipient cells are connected, a relaxase (also known as a Rep protein) nicks the plasmid at the origin of transfer (oriT) site, initiating the transfer of single-stranded DNA (ssDNA) [1, 13]. The ssDNA is then transferred from the donor to the recipient cell through membrane pores formed by the Type IV secretion system (T4SS). Both donor and recipient cells subsequently synthesize a complementary strand, completing the plasmid transfer.
In donor cells, rolling circle replication synthesizes the complementary strand [14]. At the nicked site, the exposed 3′-OH end serves as a primer for DNA polymerase, which extends strand synthesis using the intact circular strand as a template. In recipient cells, the transferred ssDNA circularizes and serves as a template for complementary strand synthesis.
In Enterobacterales and non-fermenters, conjugation plays a key role in plasmid-mediated ARG dissemination. Gram-negative bacterial plasmids are classified into 27 incompatibility groups, including IncA/C, B, F, H, I, L/M, K, P, T, O, X, W, R, and Y, along with their variants [15]. Among these, IncA/C, F, H, L, M, and P plasmids are widely distributed in Gram-negative bacteria and frequently carry ARGs [15]. However, clinically relevant ARGs—such as extended-spectrum β-lactamase genes (blaCTX-M, blaSHV), carbapenemase genes (blaIMP, blaNDM, blaKPC), 16S rRNA methyltransferase genes (armA, rmtB, rmtC), and colistin resistance genes (mcr)—are not exclusively linked to specific plasmid incompatibility groups. Nonetheless, certain trends can be observed. For example, IncF plasmids, which are highly prevalent in E. coli and Klebsiella pneumoniae, are often associated with β-lactamase genes such as blaTEM-1B, blaCTX-M (especially blaCTX-M-15), blaSHV, and blaKPC (particularly blaKPC-2) [15-17]. The blaNDM-1 gene is primarily linked to IncC plasmids [18-20], while colistin resistance genes mcr are frequently associated with IncH and IncX plasmids [21, 22]. Additionally, some minor ARGs exhibit strong correlations with specific plasmid incompatibility groups, such as blaCTX-M-8 with IncI1 plasmids [23, 24] and blaOXA-48 with IncL plasmids [25, 26].
Plasmid-mediated ARG transfer can occur among bacteria within localized environments in the human body, such as the intestinal tract. Reports have documented cases where multiple bacterial species harboring identical plasmid-carrying ARGs were isolated from a single patient, suggesting in vivo plasmid transfer [27, 28]. Furthermore, ARGs undergo molecular evolution such as mutations in β-lactamase genes leading to expanded substrate specificity [29, 30]. In addition, the ability of plasmids to transfer across bacterial species complicates the monitoring of drug-resistant bacteria. In clinical microbiology laboratories, antimicrobial resistance testing must account for the potential impact of conjugation to accurately assess resistance profiles.
3 Transformation
Natural transformation was first identified in Streptococcus pneumoniae in 1928 as a mechanism of HGT [31]. This process enables bacteria to incorporate exogenous, naked DNA into their genomes (either chromosome or plasmid DNA) when in a competent state (Figure 1). The uptake of DNA during natural transformation occurs in two stages. Initially, extracellular DNA is captured by a retractable type IV pilus, composed of pilin subunits that polymerize into a thin, flexible filament. The DNA then binds to competence proteins such as ComEA on the bacterial surface. In the second stage, one strand of the double-stranded DNA is transported into the cytoplasm through ComEC, while the other strand is degraded. Although the preference for ssDNA in transformation remains unclear, it is speculated that it is more resistant to restriction enzymes, thereby increasing the likelihood of successful genomic integration. Once inside the cytoplasm, the ssDNA undergoes homologous recombination via the RecA-dependent pathway.
Natural transformation has been observed in various human pathogenic Gram-negative bacteria, including Acinetobacter spp. [32, 33], Helicobacter pylori [34], Neisseria gonorrhoeae [35], Vibrio cholerae [36], and Haemophilus influenzae [37]. This mechanism plays a critical role in HGT and the acquisition of ARGs in these species. One notable example is the emergence of ceftriaxone-resistant N. gonorrhoeae, a drug-resistant pathogen classified by the WHO as a major public health threat requiring the development of new treatments [38]. This resistance has been linked to transformation-mediated gene acquisition [39]. Specifically, N. gonorrhoeae has incorporated all or part of the penA gene from commensal Neisseria species, such as Neisseria subflava, which naturally harbors resistance to ceftriaxone [40, 41]. The recombinant penA gene encodes an altered penicillin-binding protein (PBP) with reduced affinity for extended-spectrum cephalosporins, leading to increased resistance [42]. Interestingly, studies by Kanesaka et al. and Vincent et al. found no significant fitness cost in vitro for ceftriaxone-resistant transformants carrying the mosaic penA gene when competing with ceftriaxone-susceptible strains [40, 43]. This contrasts with S. pneumoniae, where the acquisition of mosaic pbp genes led to an increased fitness cost [44]. β-lactam resistance involving PBP modifications has also been observed in β-lactamase-negative ampicillin-resistant (BLNAR) H. influenzae [45] and Streptococcus agalactiae with reduced susceptibility to penicillin (PRGBS) [46]. However, in these species, resistance primarily arises from point mutations in endogenous pbp genes rather than through interspecies gene transfer via transformation.
Another example of transformation-driven antimicrobial resistance has been documented in Acinetobacter baumannii. Godeux et al. identified the transfer of large, chromosomally located resistance islands via transformation. These include AbaR4, which carries the carbapenem resistance determinant blaOXA-23 along with DNA fragments ranging from 27 to 90 kb, and AbaR1, which harbors multiple resistance genes—including aac(3), aph(3’), aad(3”), aad(2”), cat, tetA, blaOXA-10, and blaPRE-1—within approximately 120-kb DNA fragments [47].
4 Transduction
Transduction is the process of DNA transfer from one bacterium to another via bacteriophages, which can be categorized into two types (Figure 1) [48, 49]. The first type, temperate phages, can integrate their DNA into the bacterial chromosome and remain dormant within the host until triggered by external stress [50]. Upon activation, the phage DNA is excised from the bacterial genome, leading to the formation of new phage particles and the eventual lysis of the host cell. The second type, virulent phages [51], immediately begin replication upon injecting their DNA into the host, without integrating into the bacterial chromosome. Virulent phages have recently regained attention as a potential treatment for multidrug-resistant bacteria [51].
Transduction is classified into two forms: generalized transduction and specialized transduction (Figure 1) [49, 52]. Generalized transduction occurs when a bacteriophage mistakenly packages a fragment of bacterial DNA instead of its own genome. Upon infection of a recipient cell, the foreign DNA is injected and can be incorporated into the host genome through homologous recombination. In contrast, specialized transduction occurs when prophages excise themselves from the bacterial chromosome, carrying not only phage genes but also adjacent bacterial DNA. This phage particle can then transfer the combined genetic material to a new host.
While transduction is a well-documented mechanism of HGT in bacteria, experimental evidence for the transfer of ARGs in Gram-negative bacteria remains limited [53]. Zhang and Lejeune demonstrated that the AmpC β-lactamase gene (blaCMY-2) and tetracycline resistance genes (tetA and tetB) were transferred from Salmonella Heidelberg to Salmonella Typhimurium via generalized transduction. Although these resistance genes were not co-transferred, their individual transfer efficiencies were similar, suggesting that the process was mediated by transduction [54].
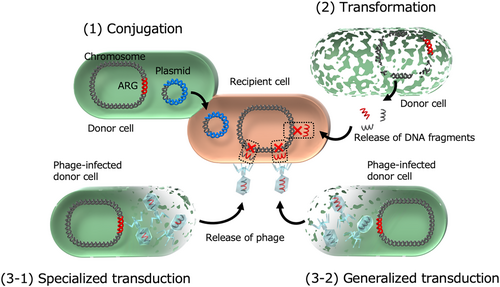
Another study provided evidence of ARG transfer through generalized transduction in a multidrug-resistant A. baumannii clinical isolate (Figure 2) [55]. Various ARGs—including β-lactamase gene (blaTEM-1), 16S rRNA methyltransferase gene (armA), aminoglycoside-modifying enzyme genes (aac, aph, and aad), macrolide phosphotransferase gene (mph), and tetracycline resistance gene (tetA)—were found to be transferred along with surrounding genetic regions, spanning up to 35 kb. Additionally, the transfer of an intrinsic quinolone resistance determinant, the gyrA (S81L) gene, was observed, accompanied by its neighboring genomic regions. Since antibiotic-resistant transformants did not emerge when recA-knockout A. baumannii was used as the recipient, homologous recombination was determined to be essential for ARG incorporation. Furthermore, ARG transfer was absent when donor A. baumannii lacked the phage capsid gene, confirming that a specific prophage mediated intercellular transfer through generalized transduction.
Phage-mediated ARG transfer is predicted to occur in various environments, including hospital effluents, urban wastewater, and rivers, as bacteriophages carrying ARGs—such as β-lactamase genes (blaTEM and blaCTX-M)—have been detected in these settings. Moreover, these genes have been successfully transferred to recipient E. coli cells, underscoring the environmental relevance of transduction in the spread of antibiotic resistance [56-58].
5 Vesiduction
OMVs are generally secreted from the bacterial cell membrane during growth and consist of outer membrane and periplasmic components. These vesicles play a key role in a process termed “vesiduction” [7-9]. OMVs are spherical, double-layered structures with an average diameter of 20–200 nm [59]. They contain biologically active components such as small molecules, cell wall components, proteins, and toxins, and mediate various physiological functions [60]. For example, in Pseudomonas aeruginosa, OMVs can transport small molecules involved in quorum sensing, facilitating cell–cell communication and promoting biofilm formation [61, 62]. In Enterobacterales and A. baumannii, OMVs carry β-lactamase enzymes that degrade β-lactam antibiotics, contributing to antimicrobial resistance [63-65]. These β-lactamase-containing OMVs act as vehicles for local enzyme dissemination and provide protection to nearby β-lactam-susceptible bacteria. Furthermore, OMVs function as carriers for bacterial toxins. For instance, OMVs containing cholera toxin (CT) from V. cholerae are readily absorbed by human intestinal cell lines [63]. The encapsulation of CT within OMVs confers resistance to degradation by intestinal proteases, thereby prolonging its biological toxicity in the intestinal tract [63].
OMVs have also been reported to encapsulate nucleic acids, including DNA fragments and plasmids, in Gram-negative bacteria such as N. gonorrhoeae, S. Typhimurium, and A. baumannii [66, 67]. Encapsulated DNA is protected from degradation by DNases, enhancing its stability over time and space. This OMV-mediated gene transfer, newly termed “vesiduction” by Soler et al., represents a fourth mode of HGT, alongside conjugation, transformation, and transduction (Figure 3). Unlike transduction by bacteriophages and plasmid transfer by conjugation, which require specialized machinery with a limited host range, OMV-mediated plasmid transfer is not strictly restricted by recipient species [68]. However, plasmid copy number and origin of replication influence transfer efficiency. This suggests that OMVs may play a crucial role in spreading genetic material across bacterial species beyond the three classical HGT mechanisms.
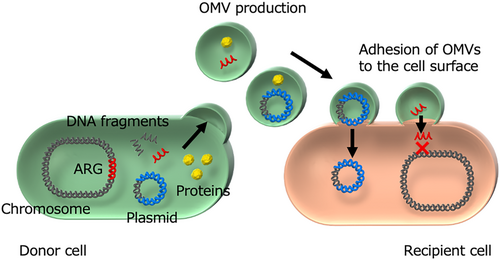
The first report of ARG transfer through vesiduction in clinically isolated Gram-negative bacteria was by Rumbo et al., who demonstrated the horizontal transfer of the carbapenemase gene blaOXA-24 via plasmid-containing OMVs in A. baumannii [67]. Their study confirmed that plasmids (pMMA2 and pMMCU3) carrying blaOXA-24 from clinical A. baumannii isolates (AbH12O-A2 and AbH12O-CU3) were packed into OMVs (20–100 nm in size). These OMVs successfully transferred the plasmids to the A. baumannii ATCC17978 strain, conferring carbapenem resistance comparable to that of the original clinical isolates. Notably, no resistant transformants appeared when the same plasmids were introduced as naked DNA, highlighting the essential role of OMV encapsulation in facilitating gene transfer.
ARG transfer via vesiduction has been observed in additional cases, including the spread of the extended-spectrum β-lactamase gene blaCTX-M-15 in Enterobacteriaceae [69] and the dissemination of carbapenemase genes (blaNDM-1 and blaKPC-2) in K. pneumoniae [70, 71]. By enabling the transfer of ARGs on non-conjugative plasmids, OMVs create new opportunities for the spread of antibiotic resistance, accelerating HGT. The plasmid sizes encapsulated within OMVs range from a few kilobases to approximately 100 kb, which is typical of plasmids carrying ARGs in Gram-negative bacteria.
The mechanisms by which cytoplasmic DNA is incorporated into OMVs are not fully understood. Some possible routes have been proposed. One involves the co-packaging of cytoplasmic DNA into OMVs due to the formation of double-layered OMVs, which encapsulate cytoplasmic, inner membrane, periplasmic, and outer membrane components. This type of OMV was first identified in Shewanella vesiculosa, a marine-derived bacterium [72, 73], and later observed in pathogenic bacteria such as N. gonorrhoeae, P. aeruginosa, and A. baumannii [73, 74]. The second mechanism involves cell lysis induced by phage-derived endolysins, which degrade the peptidoglycan layer and facilitate the formation of double-layered OMVs [75, 76]. This process leads to the inclusion of various cytoplasmic components, including DNA. Aktar et al. demonstrated that peptidoglycan deficiencies enhance plasmid DNA incorporation into OMVs, ensuring a clearer path from the cytoplasm to the extracellular space [73]. Additionally, chromosomally derived DNA has been found in OMVs. Bitto et al. identified chromosomally encoded virulence-related genes, such as exoS (ExoS toxin), spcS (chaperone SpcS), rlhB (biosurfactant), and narGHIJ, K1, K2 (membrane nitrite reductase operon), within OMVs of P. aeruginosa [66]. Interestingly, while chromosomal DNA fragments in OMVs encompass the entire chromosome, certain regions are found in higher abundance [66]. A similar phenomenon was observed in the marine cyanobacterium Prochlorococcus [77], though the underlying reasons for the selective enrichment of specific DNA regions remain unclear. In theory, chromosomally derived ARGs could be transferred via OMVs, but experimental confirmation is lacking. Unlike plasmid-borne ARGs, chromosomal ARG fragments would need to integrate into recipient genomes via recombination mechanisms such as homologous recombination. One potential vehicle for such transfer could be circularized ARG-containing transposons excised from chromosomal DNA, though this remains unproven. Wang et al. reported ARG transfer via OMVs in Riemerella anatipestifer, a causative agent of duck serositis [78]. Their study demonstrated OMV-mediated transfer of the tetracycline-inactivating enzyme gene tet(X), the 23S rRNA methyltransferase gene ermF, and the class A β-lactamase gene blaRASA, arranged at approximately 2.5-kb intervals on the DNA fragments. However, the direction of these fragments and their precise transfer mechanisms remain unclear, necessitating further research. The efficiency of ARG transfer via OMVs may depend on the nature of recipient cells. In naturally competent bacteria such as Acinetobacter, OMVs carrying ARGs attach to recipient cells, release their DNA upon lysis, and facilitate natural transformation. In contrast, for non-naturally competent bacteria, OMV-mediated gene transfer likely involves the fusion of OMVs with the outer membranes of recipient cells, followed by the release of OMV contents—including DNA—into the cytosol through currently unknown mechanisms. Further investigations are needed to elucidate the molecular details of OMV-mediated DNA transfer and its role in bacterial adaptation and antibiotic resistance.
6 Integrons
Beyond the mechanisms that facilitate horizontal transfer of ARGs—such as conjugation, transformation, transduction, and vesiduction—there are intracellular processes that facilitate the translocation of ARGs within a bacterial cell. One such representative mechanism is the integron, which can tandemly align multiple ARGs as gene cassettes without any gaps [79-81]. This characteristic makes integrons a major contributor to multidrug resistance in bacteria. Three types of integrons—Class I, Class II, and Class III—have been primarily identified in clinically isolated Enterobacterales and non-fermenting Gram-negative bacteria [82].
Regardless of the integron subtype, they commonly consist of 5′-conserved segments that include integrase genes (intI1, intI2, and intI3) [82]. These integrase genes govern the acquisition and excision of ARGs as gene cassettes by recognizing three recombination sites: attI, attC, and secondary sites. Promoter regions responsible for driving the expression of ARG cassettes are typically found within the integrase genes. Downstream of the gene cassettes, the 3′-conserved segments contain various genes: qacEΔ1-sul1-orf5 for Class I integrons, tnsA-E for Class II integrons, and an unclear composition for Class III integrons. Among the three types, Class I integrons are the most frequently observed in Gram-negative pathogens. ARG cassettes are known to circularize after excision, forming circularized gene cassettes with the attC region (formerly known as the 59-bp element) at their ends. These circularized gene cassettes can then be reintegrated between the attI and attC recombination sites.
Integrons are ancient genetic structures, first discovered in 1989 [83], yet they continue to play a crucial role in accumulating and organizing ARGs on plasmids or chromosomes in Gram-negative bacteria. Many clinically relevant ARGs, such as carbapenemase genes and aminoglycoside-modifying enzyme genes, are embedded within integrons, significantly contributing to bacterial multidrug resistance [81]. For instance, the IMP-type metallo-β-lactamase gene, a carbapenemase capable of hydrolyzing carbapenems—the last-resort β-lactams used for treating infections caused by Gram-negative bacteria—was first identified in 1994 as a gene cassette within a Class I integron of Serratia marcescens, and subsequently in Class III integron [84, 85]. To this day, IMP-type metallo-β-lactamase remains a prevalent carbapenemase in clinical isolates.
Recently, another carbapenemase gene, blaGES, has been increasingly detected as a gene cassette in integrons of Aeromonas spp. recovered from wastewater [86, 87]. Notably, in some cases, multiple copies of the same blaGES gene have been found within a single integron, suggesting that this redundancy may confer higher levels of carbapenem resistance to Aeromonas cells [88]. In any case, integrons continue to be key genetic structures that allow Gram-negative bacteria to withstand highly effective antimicrobial agents, such as carbapenems.
7 Transposons and Insertion Sequences
It goes without saying that transposons and insertion sequences are closely related to the transfer of ARGs and often play a dominant role in their expression. Given their high diversity, this section focuses on representative examples that govern the transfer and expression of dominant ARGs in Gram-negative pathogens.
One notable example is ISEcp1, which regulates the expression and transposition of downstream ARGs such as blaCTX-M [89, 90], blaCMY-2, blaKPC [91] and rmtC [92], and qnrB-like gene [93]. Another important element is the IS26-mediated composite transposon, which includes blaSHV-12 [94], blaTEM-1, qnrB19, and armA, as well as entire Class 1 integrons carrying multiple ARGs [95]. The Tn3000 transposon, flanked by two IS3000 copies at both ends, carries blaNDM-1 [96]. The dissemination of the blaNDM-1 gene has been associated with the composite transposon Tn125, which is flanked by two copies of ISAba125. Additionally, the mobilization of the colistin resistance gene mcr-1 was frequently mediated by ISAPl1 in the mid-2000s [97].
8 Origins of Horizontally Transferred ARGS
A variety of ARGs are horizontally transferred between bacterial species through the systems mentioned above. Table 1 presents a list of ARGs and their respective origins. Transposons and insertion sequences likely play a role in mobilizing such ARGs from host bacteria—where they are innately encoded on the chromosome—to HGT systems. Once such ARGs are associated with HGT systems, they can be rapidly disseminated among bacterial species.
| ARGs | Mechanisms | Resistance | Origins | References |
|---|---|---|---|---|
| blaCTX-M-1-group | ESBL | Cephalosporin | Kluyvera cryocrescens | [98] |
| blaCTX-M-2-group | ESBL | Cephalosporin | Kluyvera ascorbata | [98] |
| blaCTX-M-8-group | ESBL | Cephalosporin | Kluyvera georgiana | [98] |
| blaCTX-M-9-group | ESBL | Cephalosporin | Kluyvera georgiana | [98] |
| blaCTX-M-25-group | ESBL | Cephalosporin | Kluyvera georgiana | [98] |
| blaCMY-1 | Cephalosporinase | Cephalosporin | Aeromonas hydrophila | [99] |
| blaCMY-2 | Cephalosporinase | Cephalosporin | Citrobacter freundii | [99] |
| blaMIR-1 | Cephalosporinase | Cephalosporin | Enterobacter cloacae | [99] |
| blaMOX-1 | Cephalosporinase | Cephalosporin | Aeromonas hydrophila | [99] |
| blaLAT-1 | Cephalosporinase | Cephalosporin | Citrobacter freundii | [99] |
| blaFOX-1 | Cephalosporinase | Cephalosporin | Aeromonas caviae | [99] |
| blaDHA-1 | Cephalosporinase | Cephalosporin | Morganella morganii | [99] |
| blaACT-1 | Cephalosporinase | Cephalosporin | Enterobacter asburiae | [99] |
| blaACC-1 | Cephalosporinase | Cephalosporin | Hafnia alvei | [99] |
| blaCFE-1 | Cephalosporinase | Cephalosporin | Citrobacter freundii | [99] |
| blaOXA-48-like | Carbapenemase | Carbapenem | Shewanella spp. | [100] |
| fosA3 | Glutathione-S-transferase | Fosfomycin | Kluyvera georgiana | [101] |
| fosA4 | Glutathione-S-transferase | Fosfomycin | Kluyvera georgiana | [102] |
| fosA8 | Glutathione-S-transferase | Fosfomycin | Leclercia adecarboxylata | [103] |
| npmA | 16S rRNA methyltransferase | Aminoglycoside | Clostridioides difficile | [104] |
| mcr-1 | Phosphoethanolamine transferase | Colistin | Moraxella spp. | [105] |
| mcr-2 | Phosphoethanolamine transferase | Colistin | Moraxella pluranimalium | [106] |
| mcr-3 | Phosphoethanolamine transferase | Colistin | Aeromonas spp. | [107, 108] |
| mcr-4 | Phosphoethanolamine transferase | Colistin | Shewanella frigidimarina | [109] |
| mcr-9 | Phosphoethanolamine transferase | Colistin | Buttiauxella gaviniae | [110] |
| qnrA1 | Quinolone resistance protein | Quinolone | Shewanella algae | [111] |
| qnrS1 | Quinolone resistance protein | Quinolone | Vibrio splendidus | [112] |
| qnrB | Quinolone resistance protein | Quinolone | Citrobacter freundii complex | [113] |
- This table is adapted from Wachino et al., 2020, published in Journal of the Japanese Society for Clinical Microbiology.
9 Conclusion
Conjugation, transformation, transduction, and vesiduction are major driving forces behind the spread of ARGs both within and between bacterial species, occurring in various settings, including clinical environments and natural ecosystems. However, capturing the precise moment when ARG transfer occurs in these situations is extremely challenging. We are often only able to observe the aftermath of such transfer events and must retrospectively verify them through experimental approaches. Nevertheless, studying HGT systems in conjunction with antibiotic resistance mechanisms remains highly valuable. A deeper understanding of these transfer systems could facilitate the development of potential inhibitors to prevent ARG dissemination, ultimately helping to mitigate the impact of drug-resistant bacteria.
Acknowledgments
This review includes part of the research conducted at the Department of Bacteriology, Nagoya University Graduate School of Medicine. The author would like to thank the laboratory members at the time for their valuable support.
Disclosure
The author has nothing to report.
Conflicts of Interest
The author declares no conflicts of interest.
Open Research
Data Availability Statement
The data presented here is referenced in the related articles.