Tacrolimus inhibits stress responses and hyphal formation via the calcineurin signaling pathway in Trichosporon asahii
Abstract
The pathogenic fungus Trichosporon asahii causes fatal deep-seated mycosis in immunocompromised patients. Calcineurin, which is widely conserved in eukaryotes, regulates cell growth and various stress responses in fungi. Tacrolimus (FK506), a calcineurin inhibitor, induces sensitivity to compounds that cause stress on the cell membrane and cell wall integrity. In this study, we demonstrated that FK506 affects stress responses and hyphal formation in T. asahii. In silico structural analysis revealed that amino acid residues in the binding site of the calcineurin–FKBP12 complex that interact with FK506 are conserved in T. asahii. The growth of T. asahii was delayed by FK506 in the presence of SDS or Congo red but not in the presence of calcium chloride. FK506 also inhibited hyphal formation in T. asahii. A mutant deficient of the cnb gene, which encodes the regulatory subunit B of calcineurin, exhibited stress sensitivities on exposure to SDS and Congo red and reduced the hyphal forming ability of T. asahii. In the cnb-deficient mutant, FK506 did not increase the stress sensitivity or reduce hyphal forming ability. These results suggest that FK506 affects stress responses and hyphal formation in T. asahii via the calcineurin signaling pathway.
Abbreviations
-
- CnA
-
- calcineurin catalytic subunit A
-
- CnB
-
- calcineurin regulatory subunit B
-
- FK506
-
- tacrolimus
-
- PCR
-
- polymerase chain reaction
-
- PDB
-
- Protein Data Bank
-
- TIMM
-
- Teikyo University Institute Medical Mycology
-
- UTR
-
- untranslated region
-
- WT
-
- wild type
-
- YPD
-
- yeast–peptone–dextrose
INTRODUCTION
Trichosporon asahii is the major causative agent of deep-seated trichosporonosis, which is common in patients with neutropenia and has a case fatality rate of over 70%.1, 2 T. asahii can change its morphology from yeast to hyphae and arthroconidia.3 Hyphae and arthroconidia are observed in lesion tissue on histopathological examination4 and play an important role in biofilm formation in T. asahii.5, 6 T. asahii needs to adapt to the host environment during infection. Therefore, inhibiting a crucial factor that regulates stress responses and hyphal formation in T. asahii could prevent infections.
The calcineurin signaling pathway is widely conserved in eukaryotes from yeast to humans and regulates various stress responses.7-9 Calcineurin is a calcium-dependent activated serine/threonine protein phosphatase that is composed of catalytic subunit A (CnA) and regulatory subunit B (CnB).10 The calcium–calmodulin complex, which is formed in response to increasing intracellular calcium concentrations, activates calcineurin.8, 11 The calcineurin signaling pathway controls hyphal formation, cell wall synthesis, drug resistance, and serum proliferation in Candida spp.—representative dimorphic fungi—and cell membrane and cell wall syntheses, cation homeostasis, and thermotolerance in Cryptococcus spp.—pathogenic basidiomycetes.12, 13 T. asahii, which is a dimorphic fungus and basidiomycete, also possibly exhibits similar stress responses.14
FK506 (tacrolimus), a calcineurin inhibitor, is an immunosuppressant agent intended to prevent allograft rejection in patients who undergo organ transplantation.15 It binds to its immunophilin FKBP12 to form the FK506–FKBP12 complex that inhibits calcineurin.16, 17 FK506 inhibits morphological changes in Candida spp.18-20 and leads to thermosensitivity in Cryptococcus neoformans.12, 21, 22
In this study, we demonstrated that FK506 and cnb deficiency caused sensitivity to stress inducers such as SDS and Congo red and reduced hyphal formation in T. asahii. Moreover, FK506 did not aggravate stress sensitivity or hyphal formation in the cnb-deficient mutant of T. asahii. Therefore, FK506 might affect T. asahii cells via the calcineurin signaling pathway.
MATERIALS AND METHODS
Comparing amino acid sequences
The structure of C. neoformans calcineurin–FKBP12 complex with FK506 (PDB ID: 6TZ8) was obtained from the Protein Data Bank (https://www.rcsb.org). The structural information was analyzed using PyMoL version 2.5.2, and the FK506 binding site was estimated. The amino acid sequences of CnA, CnB, and FKBP12 in C. neoformans and T. asahii were obtained from the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov). The GenBank IDs of CnA, CnB, and FKBP12 in C. neoformans are AFR97420.1, AFR95242.1, and AFR93187.1, respectively. The GenBank IDs of CnA, CnB, and FKBP12 in T. asahii are XP_014176335.1, XP_014180618.1, and XP_014177827.1, respectively. Amino acid sequence alignment was performed using CLUSTALW (https://www.genome.jp/tools-bin/clustalw).
Strains
The strains used in the present study are listed in Table 1. The T. asahii TIMM1318 (where TIMM stands for Teikyo University Institute Medical Mycology) strain obtained from the culture collection of TIMM was used as the parent strain. The cnb-deficient mutant was constructed via homologous recombination in T. asahii TIMM1318 according to a previously described method on C. neoformans.23 To generate the cnb-deficient strain, a DNA fragment (5′-UTR-NAT1–3′-UTR) containing the upstream 5′-untranslated region (UTR) and downstream 3′-UTR of cnb and the marker gene NAT1 was prepared using a polymerase chain reaction (PCR)-based method. To generate revertants, a DNA fragment (5′-UTR-cnb-hph-3′-UTR) containing the 5′-UTR, cnb, 3′-UTR, and the marker gene hph was prepared using the same PCR-based method. The sequences of primers used for the PCR amplification of each DNA fragment are listed in Table 2. DNA delivery into T. asahii cells was performed via biolistic transformation.24 In brief, T. asahii TIMM1318 or cnb-deficient mutant cell suspensions were each spread on yeast–peptone–dextrose (YPD; 1% yeast extract, 2% peptone, 2% glucose, 2% agar) plates supplemented with 0.7 M NaCl. DNA samples were prepared by combining 600 µg of gold microcarrier beads (0.6 µm; No. 165–2262; Bio-Rad, CA, USA) with 1–4 µg of DNA, 10 µL of 2.5 M CaCl2, and 2 µL of 1 M spermidine. The plates were then bombarded with the DNA-bound gold microcarrier beads in a Bio-Rad biolistic transformation apparatus at a helium gas pressure of approximately 1350 psi under a vacuum level of 29 in. of Hg. The plates were incubated at 27°C for 24 h, and liquified YPD medium with 0.7% agar containing nourseothricin (200 µg/mL) or hygromycin (200 µg/mL) was overlaid, and the plates were further incubated at 27°C for 3–5 days until antibiotic-resistant transformants appeared on the surface of the overlaid medium. Nourseothricin and hygromycin were purchased from Jena Bioscience GmbH (Thuringia, Germany) and FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan), respectively. The strains were grown on Sabouraud dextrose agar (1% peptone, 4% glucose, 1.5% agar) at 27°C.
| Strains | Relevant genotype | Background | Source |
|---|---|---|---|
| Wild-type | TIMM1318 | TIMM culture collection | |
| ∆cnb | cnb::NAT1 | TIMM1318 | This study |
| Rev10 | cnb::NAT1, cnb, hph | TIMM1318 ∆cnb | This study |
| Rev12 | cnb::NAT1, cnb, hph | TIMM1318 ∆cnb | This study |
| Primer | Sequence (5′-3′) |
|---|---|
| For cnb-deficient mutant | |
| F cnb (5′-UTR) | TGAACTAGTCCGTGATCTGCTGCACGTTCGGGTCC |
| R cnb (5′-UTR) | AAAGGGCCCAAGATCTAGTGATAGATGTGTGGAGA |
| F cnb (3′-UTR) | CTGGGATCCGCGCGCACACACGGATGTGAGCGTAA |
| R cnb (3′-UTR) | CGCGGTACCACTGTTCACCTCTGGCATTGTTACGA |
| For revertants | |
| TaCNA-F1/SpeI | TGAACTAGTCCGTGATCTGCTGCACGTTCGGGTCC |
| TaCNB-R2(-KpnI) | GTGCGTGCGCGGTACTCACGATCGGTGTAATAGC |
| TaCNB-F2(-KpnI) | GCTATTACACCGATCGTGAGTACCGCGCACGCAC |
| TaCNB-R6/ApaI | TACGCTCACATCCGTGTTTGGGCCCTTAGAACAGG |
| CnPactin(F)ApaI | TTTGGGCCCCCTGCGAGGATGTGAGCTGGAGAGCG |
| CnPact-hph(R) | GAGTTCAGGCTTTTTCATACGCGTAGACATGTTGG |
| CnPact-hph(F) | CCAACATGTCTACGCGTATGAAAAAGCCTGAACTC |
| hph-Ttrp1(R) | ACCGCCTTCACGAATTCCTATTCCTTTGCCCTCGG |
| hph-Ttrp1(F) | CCGAGGGCAAAGGAATAGGAATTCGTGAAGGCGGT |
| Ttrp1(R)BamHI | AAAGGATCCGAAGAGATGTAGAAACTAGC |
Hyphal formation assay
Sabouraud dextrose agar containing FK506 (0.1 µg/mL) or DMSO (control) was prepared. T. asahii cell suspensions (A630 = 0.1) were prepared using a spectrophotometer (AS ONE, Osaka, Japan), spotted onto the above media, and incubated at 27°C or 37°C for 4 days. The edges of the colonies were then observed using an optical microscope (Olympus corporation, Tokyo, Japan). FK506 was purchased from Cayman Chemical Company (MI, USA) and FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan).
Temperature tolerance test
Cell suspensions (A630 = 0.2) were serially diluted 10-fold. Cell suspensions of each concentration were spotted onto YPD agar plates and incubated at 27°C, 37°C, or 39°C for 24 or 48 h.
Drug sensitivity test
YPD agar containing SDS (0.005%), Congo red (0.1 and 1 mg/mL), CaCl2 (400 mM), or FK506 (0.1 µg/mL) were prepared. Cell suspensions (A630 = 0.2) were serially diluted 10-fold. Ten microliters of each concentration of the cell suspension were spotted onto the above media and incubated at 27°C or 37°C for 24 h.
RESULTS
Conservation of amino acids of the calcineurin–FKBP12 complex for FK506 binding
In C. neoformans, the binding site of the calcineurin–FKBP12 complex to FK506 was determined using crystal structure analysis (Figure 1a).25 We examined whether the amino acids of the calcineurin–FKBP12 complex are conserved in T. asahii. The homology of the amino acid sequences of the calcineurin–FKBP12 complex between C. neoformans and T. asahii was more than 60% for each protein (Table 3). The amino acids associated with FK506 binding in the calcineurin–FKBP12 complex of C. neoformans are depicted in Figure 1b. Trp382 (W382) of CnA, and Tyr53 (Y53), Asp64 (D64), Gln81 (Q81), Ile83 (I83), and Tyr109 (Y109) of FKBP12 in C. neoformans were close to FK506 (Figure 1b). The results of the amino acid alignment analysis indicate that these amino acids are conserved in the calcineurin–FKBP12 complex of T. asahii (Figure 1c). These findings also suggest that the calcineurin–FKBP12 complex of T. asahii interacts with FK506.

| Protein | Description | Query cover (%)a | Identity (%)a |
|---|---|---|---|
| CnA | Serine/threonine-protein phosphatase 2B catalytic subunit A1 | 99 | 77.23 |
| CnB | Calcium-dependent protein serine/threonine phosphatase | 100 | 94.86 |
| FKBP12 | Macrolide-binding protein FKBP12 | 100 | 63.87 |
- a Query cover and percentage identity were determined and compared with the amino acid sequence of C. neoformans.
Effects of FK506 on T. asahii
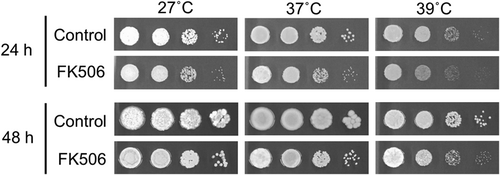
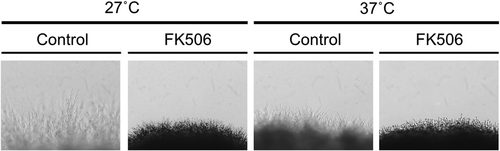
The calcineurin pathway regulates tolerance to high temperatures, membrane and cell wall stress, and cation homeostasis in Cryptococcus spp.13, 26, 27 We investigated whether FK506 induced stress sensitivity in T. asahii. The wild-type T. asahii strain TIMM1318 demonstrated retarded growth after FK506 treatment, especially at 39°C (Figure 2). In the presence of SDS, a cell membrane stress inducer, FK506 delayed the growth of T. asahii at both of 27°C and 37°C (Figure 3). In the presence of Congo red, a cell wall stress inducer, FK506 inhibited the growth compared with that seen in the absence of FK506 (control) at both temperatures (Figure 3). Although Congo red (1 mg/mL) affected the growth of T. asahii at 37°C in the absence of FK506, the growth inhibition was more severe in the presence of FK506 (Figure 3). By contrast, CaCl2, a calcium stress inducer, did not affect the growth in the presence or absence of FK506 (Figure 3). Moreover, we investigated whether FK506 affected the morphological change in T. asahii as the calcineurin signaling is required for hyphal growth of the representative dimorphic fungi, Candida spp.18, 20, 28 Hyphal formation of T. asahii was inhibited by FK506 at both 27°C and 37°C (Figure 4). These results suggest that FK506 has the potential to decrease stress tolerance and inhibit hyphal formation in T. asahii.

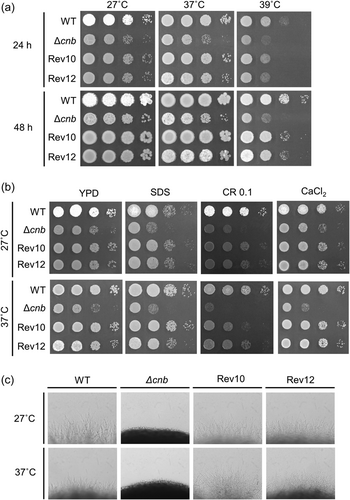
Phenotypic analyses of the cnb-deficient mutant under stress conditions
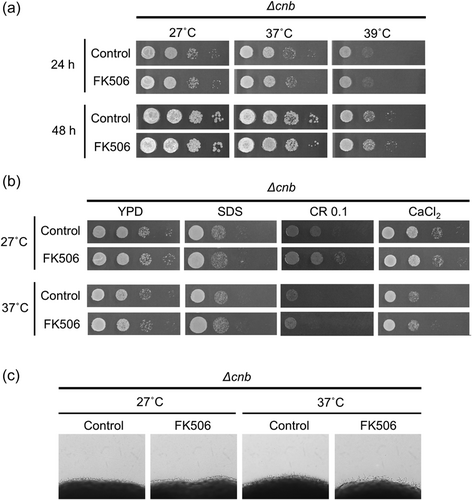
CnB is an essential component of the calcineurin pathway in C. neoformans.29 In Cryptococcus spp., the cnb gene-deficient mutant is sensitive to several stresses such as high temperature and cell wall damage.29, 30 To confirm the effect of FK506 was caused by inhibition of the calcineurin pathway, the same evaluations as those analyzed using the wild-type strain with FK506 treatment were performed using the cnb-deficient T. asahii mutant. The growth of the cnb-deficient mutant was delayed at 39°C (Figure 5a). Treatment with SDS, Congo red, and CaCl2 delayed the growth of the cnb-deficient mutant at both 27°C and 37°C (Figure 5b). The mutant also showed reduced hyphal formation at both temperatures (Figure 5c). These phenotypes in the cnb-deficient mutant were restored in the revertant strains (Rev10 and Rev12), in which the cnb gene was reintroduced into the genome of the cnb-deficient mutant (Figure 5). In the cnb-deficient mutant, the growth in the presence of the aforementioned stressors and hyphal formation ability were not further decreased by the addition of FK506 (Figure 6). These results suggest that FK506 affects T. asahii via the calcineurin signaling pathway.
DISCUSSION
In this study, we demonstrated that FK506 induced sensitivity to high temperature, SDS, and Congo red in T. asahii. Moreover, FK506 inhibited hyphal growth in T. asahii. The cnb-deficient mutant, with a defective calcineurin signaling pathway, sensitized to high temperature, SDS, and Congo red and reduced hyphal formation, compared with the wild-type strain. FK506 was ineffective against sensitivity to high temperature, SDS, and Congo red in the cnb-deficient mutant. Our findings suggest that FK506 inhibits stress responses and hyphal formation via the calcineurin signaling pathway in T. asahii.
Structural analyses of mammalian and fungal calcineurin molecules revealed amino acid residues in the binding site of the calcineurin–FKBP12 complex that interact with FK506.25, 31 Trp382 of CnA and Tyr53, Asp64, Gln81, Ile83, and Tyr109 of FKBP12 in C. neoformans were conserved in the calcineurin–FKBP12 complex of T. asahii (Figure 1c). Therefore, we assumed that FK506 might also interact with the calcineurin–FKBP12 complex of T. asahii and affect the cellular activities of this fungus.
Growth ability at over 37°C, which is human body temperature, is a key factor for virulence of pathogens. In basidiomycete Cryptococcus spp., calcineurin regulates thermotolerance.26, 27 C. neoformans and Cryptococcus gattii cannot grow above 37°C in the presence of calcineurin inhibitors or when the cells are calcineurin deficient.26, 27 However, FK506 did not affect the growth of Ascomycota fungus, Candida spp., except for some strains of Candida glabrata.9 In T. asahii, cell growth at 39°C was slightly delayed by FK506 treatment (Figure 2) and cnb deletion (Figure 5a). The results indicate that T. asahii, a Basidiomycota fungus, regulates thermotolerance through the calcineurin pathway, which is a common property of C. neoformans, although the regulation is weaker in T. asahii. While the cnb deletion strain delayed growth at 39°C, the growth of revertant strains did not recover as much as that of the wild-type strain (Figure 5a). To understand this phenomenon, the transcriptional expression level of the cnb gene was measured. The cnb gene expression was upregulated (3.8-fold) in Rev10 and downregulated (0.2-fold) in Rev12 compared with the wild type (Supporting Information Figure S1). Calcineurin forms a complex with Cna and Cnb subunits, and then dephosphorylates target molecules. The expression level of each subunit within a protein complex is ultimately determined in balance for appropriate cellular performance. Overexpression of only one subunit within a complex may trigger disruption of stoichiometric balance, which causes cellular defects.32 Rev10 may not express appropriate calcineurin function because of stoichiometric imbalance. By contrast, the calcineurin function of Rev12 did not reach the level of the wild type because of the lower expression level of the cnb gene.
In fungi, the calcineurin signaling pathway regulates several physiological maintenance activities and responses such as cell membrane and cell wall synthesis.12 FK506 treatment and cnb deletion caused increased sensitivity to SDS and Congo red in T. asahii (Figures 3 and 5b). A transient increase of Ca2+ concentration in cytoplasm is stress for cells, which is sensed by calmodulin. This subsequently activates calcineurin. The calcineurin signaling pathway also regulates calcium transport to reduce intracellular Ca2+ concentrations in Cryptococcus spp.13, 30, 33 By contrast, calcineurin functions as a negative regulator against Ca2+ tolerance in Saccharomyces cerevisiae and C. glabrata.34, 35 In this study, we investigated whether the calcineurin pathway of T. asahii responds to calcium stress. The effect of calcium stress on T. asahii differed between FK506 treatment (Figure 3) and cnb deletion (Figure 5b). Therefore, the lack of cnb might also affect the calcium homeostasis system, independent of the effects of FK506. Further studies are required to determine the exact mechanism.
T. asahii is a dimorphic fungus, which forms yeast, hyphae, and arthroconidia. In Candida spp., the representative dimorphic fungi, calcineurin signaling is required for hyphal growth,18, 20, 28 although the effect in Candida albicans depends on the experimental conditions and strain backgrounds.36, 37 In C. albicans, hyphal development is related to severe infections.38 Hyphae of T. asahii are observed in blood vessels and may cause necrotic thrombi.39 The present study results showed that FK506 treatment and cnb deletion inhibited hyphal formation in T. asahii (Figures 4 and 5c). By contrast, FK506 did not further decrease the growth and hyphal formation of the cnb lacking mutant (Figure 6). Unexpectedly, FK506 slightly improved the growth of the cnb-deficient mutant in the presence of Congo red. FK506 might also inhibit/promote calcineurin-independent pathways, which have an antagonistic function against cell wall stress. However, effects of FK506 on calcineurin-independent pathways were considered to be weak because FK506 inhibited the growth of the wild type in the presence of Congo red (Figure 3). These results suggest that FK506 inactivates the calcineurin molecule in T. asahii, resulting in the inhibition of stress tolerance and hyphal formation. Therefore, we hypothesize that calcineurin inhibitors are potential drugs against T. asahii infections. FK506 suppresses the immune system by blocking T-cell activation in humans, and this effect should be eliminated when used as a drug against fungal infections. Odom et al.22 have reported a nonimmunosuppressive FK506 analog (L-685,818) that exhibited the same antifungal action as FK506 against C. neoformans. Juvvadi et al.25 have developed the FK506 analog APX879 that inhibits the Aspergillus fumigatus calcineurin but has reduced immunosuppressive activity against murine CD4+ T cells. Future studies on the antimicrobial activity of nonimmunosuppressive FK506 analogs against T. asahii holds high significance.
In conclusion, FK506 inhibits calcineurin, which controls morphological changes and various stress responses in T. asahii. The inhibition of calcineurin in T. asahii leads to reduced hyphal formation that could be associated with T. asahii virulence. The findings suggest that FK506 derivatives, which do not affect mammalian calcineurin, are potential drugs to treat T. asahii infections.
ACKNOWLEDGMENTS
This study was supported in part by the Japan Society for the Promotion of Science (JSPS; grant number JP20K16000 to S. K.) and in part by the Research Program on Emerging and Re-emerging Infectious Diseases of the Japan Agency for Medical Research and Development (AMED; grant number JP20fk0108135h0201 to T.S.).
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data supporting the findings of this study are available from the corresponding author upon reasonable request.