Effect of mTOR inhibitors during CMV disease in kidney transplant recipients: Results of a pilot retrospective study
Abstract
mTOR inhibitors exert a preventive effect on cytomegalovirus (CMV) disease in CMV seropositive (R+) kidney transplant recipients, but their impact during the curative treatment of CMV disease in high-risk kidney transplant recipients has not been investigated. We aimed to evaluate the efficacy and tolerance of mTOR inhibitors compared with mycophenolic acid in 63 consecutive kidney transplant recipients (80% of D+R−) suffering from CMV disease with a persistent or a recurrent CMV DNAemia. In this monocentric retrospective study, 16 had their treatment converted to mTOR inhibitors and 47 did not. The Kaplan–Meier curves did not show any significant differences in CMV DNAemia eradication (77% vs. 88% respectively; hazard ratio (HR), 1.648 [95% confidence interval (CI), 0.913–2.973]; log-rank test, P = .132), DNAemia recurrence (36% vs. 47%; HR, 1.517 [95% CI, 0.574–4.007]; log-rank test, P = .448) and CMV clinical recurrence (17% vs. 27%; HR, 1.375 [95% CI, 0.340–5.552]; log-rank test, P = .677) between patients who received mTOR inhibitors and those who did not. These results were confirmed in uni- and multivariate time-dependent Cox regressions. In summary, conversion from mycophenolic acid to mTOR inhibitors seems inadequate for improving CMV clearance or in better preventing CMV recurrences during severe or persistent CMV disease.
Abbreviations
-
- ABMR
-
- antibody-mediated rejection
-
- CI
-
- confidence interval
-
- CMV
-
- cytomegalovirus
-
- CNI
-
- calcineurin inhibitor
-
- D+R−
-
- donor with CMV status positive/recipient with CMV status negative
-
- FCV
-
- foscavir
-
- GCV
-
- ganciclovir
-
- HR
-
- hazard ratio
-
- KTR
-
- kidney transplant recipient
-
- MPA
-
- mycophenolic acid
-
- mTORi
-
- mTOR inhibitors
-
- NA
-
- not applicable
-
- N
-
- number
-
- QNAT
-
- quantitative nucleic acid testing
-
- R+
-
- recipient with CMV status positive
-
- rATG
-
- rabbit anti-thymocyte globulins
-
- SD
-
- standard deviation
-
- TCMR
-
- T cell-mediated rejection
-
- VGCV
-
- valganciclovir
-
- Vδ2neg T lymphocyte
-
- V delta 2 negative T lymphocyte
-
- vs
-
- versus
1 INTRODUCTION
Despite the current prevention strategies, cytomegalovirus (CMV) is still a major issue in solid-organ transplant recipients. CMV disease is very frequent with an incidence reaching 15–20% in D+R−1-3 and 5% in R+ kidney transplant recipients (KTR).4 Moreover, despite appropriate antiviral treatment during CMV disease, failure to clear CMV occurs in 30% of patients. Finally, DNAemia and clinical recurrences still occur in 30% and 15% of KTR, respectively.5 Therefore, there is an unmet need for new strategies to better treat CMV disease and to avoid recurrence.
Over the past years, a growing body of evidence, including randomized controlled trials, has shown that the use of mTOR inhibitors (mTORi) had anti-CMV properties. In vitro, mTORi could directly inhibit CMV replication, potentiate the generation of CMV-specific memory CD8+ T-cells, and deviate the CMV-mediated immune evasion by blocking mTORC1 activity in myeloid cells.6, 7 In randomized trials, a reduction of post-transplant CMV events was reported in kidney transplant recipients receiving de novo mTORi, either in association with mycophenolic acid (MPA),8 or with calcineurin inhibitors (CNI).9-11 Based on these studies, CMV guidelines now propose the use of mTORi as a potential preventive approach to decrease CMV infection and disease in R+ kidney transplant recipients.12
While mTORi are able to exert a preventive effect on CMV disease in R+ kidney transplant recipients, their use during CMV disease to achieve faster CMV eradication has not been demonstrated. Two analyses have been made in 9 and 11 patients and it was reported that switching immunosuppression from CNI to a mTORi-based regimen could be proposed as a salvage therapy in ganciclovir-resistant CMV infection.13, 14 However, the use of mTORi in the clinical scenarios of CMV DNAemia persistence or recurrence has not been investigated, notably switching from MPA to mTORi with calcineurin inhibitors maintenance. Indeed, as mycophenolate mofetil (the precursor of MPA) has been shown to increase CMV invasive organ diseases in kidney transplant recipients,15 changing to mTORi could offer an option against the deleterious effect of MPA.
We therefore conducted a retrospective study to evaluate the efficacy and tolerance of a conversion from MPA to mTORi in kidney transplant recipients suffering from CMV disease with a persistent or a recurrent CMV DNAemia.
2 PATIENTS AND METHODS
2.1 Study design
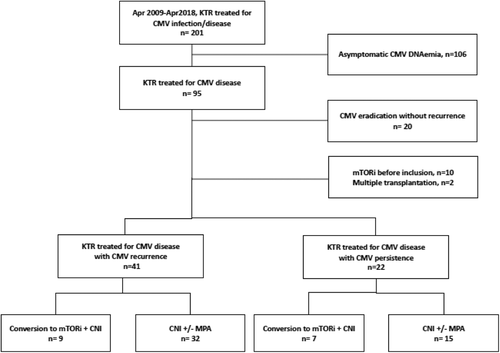
A monocentric retrospective study was conducted at the Bordeaux University Hospital, France, from April 2009 to April 2018. Among the consecutive 95 patients who underwent CMV disease during this period, first 20 patients were excluded with early CMV eradication without recurrence. Also excluded were the patients for whom mTORi had been already administered in other indications (n = 10), as well as multiple organ transplantations (n = 2). Consequently, 63 patients were included, either patients who had a persistent CMV DNAemia after 7 weeks of antiviral treatment (n = 22), based on the VICTOR study5 or those who had a CMV recurrence (n = 41; Figure 1). All patients were monitored for 1 year after meeting the inclusion criteria. This study was approved by the Institutional Review Board of the Bordeaux University Hospital.
2.2 Immunosuppression
Induction therapy was based on rabbit anti-thymocyte globulins (rATG) in sensitized patients, and basiliximab in non-sensitized patients. The immunosuppressive regimen was based on CNI with a tacrolimus target residual plasma concentration of 10–12 ng/mL for the first 3 months and then 5–10 ng/mL. The cyclosporine A target residual plasma concentration was 150–200 ng/mL for the first 3 months and then 75–125 ng/mL. MPA was used mainly as an antiproliferative drug until the clinical decision was made to switch to mTORi for the indication of CMV persistence or CMV recurrence during an outpatient visit. No standardized criteria were used to define patients eligible for mTORi conversion. Conversion from MPA to mTORi was decided clinically because mTORi has been demonstrated to prevent CMV infection better than MPA, to contribute to CMV-mediated evasion suppression,6 and consequently has been recently added in the latest CMV guidelines.12
We considered the beneficial effect of mTORi to outweigh its adverse events such aphtosis, edemas, and delay in healing. Those adverse events are described below. The mTORi target residual plasma concentration was then 4–7 ng/mL. All patients received everolimus as mTORi treatment, except one who received rapamycin.
The expanded criteria for donor and delayed graft function were defined as described previously.16 All acute rejections, which included both antibody-mediated and T cell-mediated acute rejections, were biopsy-proven. The glomerular filtration rate was expressed based on the MDRD formula.
2.3 CMV prevention
From April 2009 to June 2010, patients were preemptively followed and treated when the CMV quantitative nucleic acid testing (QNAT) was 2000 IU/mL as reported previously.17 From July 2010 to April 2018, D+R− and R+ patients received, respectively, 6 months and 3 months of universal prophylaxis using valganciclovir 900 mg once a day. The dose was carefully adjusted at each outpatient visit, following the manufacturer's recommendations, using the Cockcroft–Gault formula.
2.4 CMV monitoring
The CMV IgG serology (Enzygnost anti-CMV/IgM and IgG [Dade Behring, Marburg, Germany] and Acces CMV IgG and IgM [Beckman Coulter, Brea, CA]), were performed following the manufacturer's recommendations.
CMV QNAT was performed as described previously with a real-time polymerase chain reaction in whole blood,18 and from June 2012, using LightMix® kit human cytomegalovirus (hCMV; TIB MOLBIOL GmbH, Berlin, Germany). Before June 2012, QNAT was reported in copies/mL, however, the equivalence in IU/mL was retrospectively calculated by using the World Health Organization International Standard for hCMV, in order to homogenize all the results before performing the statistical analysis. The threshold of CMV DNAemia detectability was 250 IU/mL. The laboratory is following the program of Quality Control for Molecular Diagnostics (QCMD, Glasgow, Scotland) from 2004.
The baseline viral load was defined as the first positive DNAemia of the CMV event which led us to include the patient. During the viral monitoring of CMV disease, the assay was performed once a week during the first 7 weeks or until two consecutive negative CMV QNATs had occurred, then once a month until month 3. CMV QNAT was also systematically performed every year and when CMV disease was clinically suspected.
Antiviral drug resistance was suspected when persistent viral replication was observed after >2 weeks of appropriate antiviral therapy and was confirmed by full-length sequencing of the UL97 and UL54 genes,3 performed at the French National Cytomegalovirus Reference Center (Limoges, France).
2.5 Definitions of CMV events
CMV disease was defined as CMV syndrome or CMV tissue-invasive disease, consistent with the American Society of Transplantation and the CMV Drug Development Forum recommendations.19 Intravenous (i.v.) ganciclovir (5 mg/kg twice daily) or oral valganciclovir (900 mg twice daily) were given as the curative treatment. The dose was carefully adjusted at each outpatient visit, following the manufacturer's recommendations, using the Cockcroft–Gault formula.
“Post-prophylaxis” CMV disease was defined as the first episode of CMV disease occurring >3 months (100 days) after transplantation.
Early-onset disease was defined as the first episode of CMV disease occurring <3 months (100 days) after transplantation.20
CMV DNAemia eradication was defined as the occurrence of one negative CMV QNAT.
CMV DNAemia persistence was defined as no CMV eradication occurring after 7 weeks of antiviral treatment.
CMV DNAemia recurrence was assessed as a new positive CMV DNAemia (≥250 IU/mL) in patients with proven CMV DNAemia eradication. Clinical recurrence was defined as CMV DNAemia recurrence associated with viral syndrome or documentation of CMV in tissue from a relevant organ.12
2.6 Immunophenotyping
We have previously shown that Vδ2neg T lymphocytes are involved in the control of CMV21 since their expansion is correlated with CMV infection resolution.22 Consequently, Vδ2neg T lymphocytes kinetics were analyzed in patients with or without conversion to mTORi. The Vδ2neg T lymphocyte count was obtained by immunophenotypic determination. Flow cytometry was carried out on 100 µL anticoagulated whole blood taking into account at least 5000 total lymphocytes stained with anti-CD45, anti-pan-δ (clone IMMU 510; Beckman Coulter, Krefeld, Germany), and anti-TCR Vδ2 (clone 15D; Thermo Fisher Scientific, Rockford, IL). The percentages of cell populations were obtained using CELLQUEST software (BD Bioscience), and absolute counts of lymphocytes were obtained using the Single–Platform Lyse/No–Wash Trucount (BD Bioscience). In our center, the surveillance of Vδ2neg T lymphocytes was based on a measurement at day 0 of the graft; month 3, 6, and 12; and then every year. In the case of CMV persistence, the monitoring was performed at day 0 of CMV infection, day 49 and once a month in the 3 months following day 49. In the case of CMV recurrence, the surveillance of Vδ2neg T lymphocytes was performed on that day and then once a month in the three following months.
2.7 Statistical analysis
The Mann-Whitney test and the χ2 test were used when appropriate. Alternatively to the χ2 test, the Fisher's test was used for a low number of patients. P < .05 was considered statistically significant. The survival curves were estimated with Kaplan–Meier method and compared with the log-rank test.
We performed a univariate time-dependent Cox regression analysis for CMV DNAemia eradication, CMV DNAemia recurrence and clinical recurrence. Then, covariates with a < 0.25 P-value and “mTORi conversion” were included in a multivariate time-dependent Cox regression analysis. The results were expressed as hazard ratios (HR) with 95% confidence intervals (95% CI). Finally, we separately analyzed patients with persistent and recurrent CMV disease.
Analyses were performed with conventional statistical methods using the GraphPad Prism (version 6.0; GraphPad Software, San Diego, CA) and the RStudio statistical software (Version 1.1.423 – © 2009-2018 RStudio, Inc).
3 RESULTS
3.1 Baseline characteristics of patients
Sixty-three KTR with CMV disease were included at the time of persistent CMV DNAemia diagnosis or at the time of CMV DNAemia recurrence. Baseline characteristics are described in Table 1. Seventy percent of the patients were males with an average age of 56 years. Forty-one percent received rATG as induction treatment, 79.4% received tacrolimus, while 17.5% received cyclosporine A. Seventy percent had corticosteroids and 92% had MPA. Acute antibody-mediated rejection occurred before inclusion in 1.6% of patients and acute T cell-mediated rejection in 11% of patients.
| Characteristic | Value |
|---|---|
| Gender | |
| Female/Male | 19/63 (30%)/44/63 (70%) |
| Age (yr), mean ± SD | 56 ± 13 |
| Rank of transplantation | |
| 1 | 53/63 (84%) |
| >1 | 10/63 (15.8%) |
| Preemptive transplantation | |
| No/Yes | 7/63 (11%)/56/63 (89%) |
| Total ischemia time (hours), mean ± SD | 15 ± 7.7 |
| Delayed graft function | |
| No/Yes | 39/63 (62%)/24/63 (38%) |
| Expanded criteria donor | |
| No/Yes | 23/63 (37%)/34/63 (54%) |
| NA | 6/63(9%) |
| Living donor | |
| No/Yes | 56/63 (89%)/7/63 (11%) |
| Nephropathy | |
| Vascular | 18/63 (29%) |
| Glomerular | 17/63 (27%) |
| Unknown | 8/63 (13%) |
| Tubulo interstitial/others | 12/63 (19%) |
| Hereditary/others | 8/63 (13%) |
| Immunosuppressive regimen | |
| rATG induction | |
| No/Yes | 36/61 (59%)/25/61 (41%) |
| Cyclosporine A | |
| No/Yes | 52/63 (82.5%)/11/63 (17.5%) |
| Tacrolimus | |
| No/Yes | 13/63 (20.6%)/50/63 (79.4%) |
| MPA | |
| No/Yes | 5/63 (8%)/58/63 (92%) |
| Corticosteroids | |
| No/Yes | 19/63 (30%)/44/63 (70%) |
| Azathioprine | |
| No/Yes | 60/63 (95.2%)/3/63 (4.7%) |
| Previous ABMR | |
| No/Yes | 62/63 (98%)/1/63 (1.6%) |
| Previous TCMR | |
| No/Yes | 56/63 (89%)/7/63 (11%) |
- This table describes the baseline characteristics of patients at inclusion, regarding age, sex, rank of transplantation, graft characteristics, immunosuppressive regimen, and the history of acute rejection before inclusion.
- Abbreviations: ABMR, antibody-mediated rejection; CMV, cytomegalovirus; rATG, rabbit anti-thymocyte globulins; TCMR, T cell-mediated rejection; SD, standard deviation.
Among the 63 included patients, 16 were converted to mTORi (Table 2). The conversion to mTORi occurred 31 days after inclusion on average (median 21; 1st–3rd quartile 8–49 days). Among the 16 patients converted to mTORi, 56% still received ganciclovir or valganciclovir at the time of the conversion, versus 45% in the patients without mTORi conversion (P = .675). In the other patients, antiviral therapy was stopped.
| Characteristic | Conversion to mTORi, n = 16 | No conversion to mTORi, n = 47 | P-value |
|---|---|---|---|
| Recurrent/persistent | 9/16 (56%)/7/16 (44%) | 32/47 (68%)/15/47 (32%) | .391 |
| CMV status D+R-/R+ | 13/16 (81%)/3/16 (19%) | 36/47 (77%)/11/47 (23%) | .698 |
| Preemptive versus universal prophylaxis | 2/16 (12%)/14/16 (88%) | 13/47 (28%)/34/47 (72%) | .315 |
| Early-onset versus “post-prophylaxis” CMV disease | 2/16 (12%)/14/16 (88%) | 11/47 (23%)/36/47 (77%) | .486 |
| No/VGV/GCV/FCV at inclusion | .675 | ||
| 6/16 (37.5%) | 26/47 (55%) | ||
| 7/16 (43.75%) | 15/47 (32%) | ||
| 2/16 (12.5%) | 6/47 (13%) | ||
| 1/16 (6.25%) | 0 | ||
| Baseline CMV DNAemia (IU/ml); mean ± SD | 685720 ± 1447761 | 753024 ± 1924860 | .869 |
| Mutant strain | 14/16 (88%)/2/16 (12%) | 43/47 (91%)/4/47 (8.5%) | |
| Lymphocyte count (/µL); mean ± SD | 635 ± 647 | 785 ± 464 | .058 |
| rATG | 7 (43.8) | 18 (38.4) | .7 |
- This table describes CMV baseline characteristics of the donor and recipient status, type of preventive strategy, inclusion for CMV persistence or recurrence, the antiviral treatment, virus characteristics, and lymphocyte count.
- Abbreviations: CMV, cytomegalovirus; mTORi, mTOR inhibitors; rATG, rabbit anti-thymocyte globulins; SD, standard deviation.
The proportions of CMV diseases with persistent CMV DNAemia, D+/R− status, and of a mutant strain were not significantly different in the two groups (Table 2). The mean of the absolute lymphocytes counts was slightly higher in the group without mTORi conversion (635 vs. 785/µL, respectively), but it was not statistically significant (P = .058). The preventive strategy, the baseline viral load, the ratio of “post-prophylaxis” versus “early-onset disease” between the two groups were not significantly different either (Table 2).
3.2 mTOR inhibitors conversion is not associated with a faster CMV DNAemia eradication
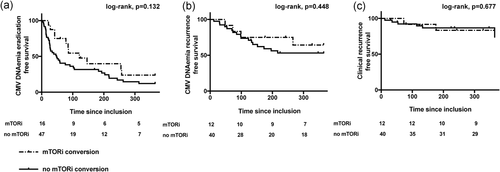
Univariate analysis showed no significant association between mTORi conversion and CMV DNAemia eradication (HR, 0.835; [95% CI, 0.411–1.696]; P = .618; Table 3a). Analysis of the 1-year survival curve showed no significant difference of CMV DNAemia eradication between patients with or without mTORi conversion (77% vs. 88% respectively; HR, 1.648 [95% CI, 0.913–2.973]; log-rank test, p = .132; Figure 2a).
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Covariate | HR | CI 95% | P-value | HR | CI 95% | P-value |
| Age | 1.001 | 0.981–1.021 | 0.949 | |||
| Sex | 0.972 | 0.530–1.784 | .928 | |||
| rATG | 0.861 | 0.479–1.549 | .619 | |||
| Corticosteroids | 1.195 | 0.651–2.19 | .566 | |||
| mTORi conversion | 0.835 | 0.411–1.696 | .618 | |||
| CMV status | 1.194 | 0.609–2.338 | .606 | |||
| Preventive strategy | 0.423 | 0.225–0.793 | .007 | |||
| Early-onset disease | 0.429 | 0.255–0.95 | .034 | |||
| Mutant strain | 0.540 | 0.194–1.504 | .239 | |||
| Baseline viral load | 0.473 | 0.269–0.831 | .009 | |||
| Persistent versus recurrent | 0.376 | 0.204–0.693 | .001 | 0.438 | 0.221–0.718 | .002 |
| TCMR before CMV disease | 1.526 | 0.644–3.609 | .336 | |||
| ABMR before CMV disease | 3.011 | 0.401–21.58 | .284 | |||
| Lymphocyte count | 1 | 1–1.001 | .074 | 1.0005 | 1–1.0011 | .068 |
- Baseline viral load was converted in a binary variable, taking the median value of 58,000 IU/mL and analyzing the correlation between baseline viral load higher or lower than this cutoff and CMV DNAemia eradication. Seven variables were analyzed kept for the initial multivariate analysis and the model was convergent.
- Abbreviations: ABMR, antibody-mediated rejection; CI, confidence interval; CMV, cytomegalovirus; HR, hazard ratio; mTORi, mTOR inhibitors; rATG, rabbit anti-thymocyte globulins; TCMR, T cell-mediated rejection.
In the multivariate analysis, only the “persistent CMV DNAemia versus recurrent CMV infection” covariate was associated with a lower rate of CMV DNAemia eradication (HR, 0.438 [95% CI, 0.221–0.718]; P = .002; Table 3A).
3.3 mTOR inhibitors is not associated with CMV recurrence
We then excluded the patients for whom CMV DNAemia eradication was not obtained (n = 11, four with mTORi conversion and seven without mTORi conversion), in order to analyze the covariates associated with both CMV DNAemia and clinical recurrences.
mTORi conversion was not significantly associated with either CMV DNAemia recurrence (HR, 0.942; [95% CI, 0.314–2.831]; P = .916; Table 3b), or clinical recurrence (HR, 1.524; [95% CI, 0.408–5.693]; P = .531; Table 3c). The CMV DNAemia recurrence (36% vs. 47%; HR 1.517 [95% CI, 0.574–4.007]; log-rank test, P = .448; Figure 2b) and the clinical recurrence (17% vs. 27%; HR, 1.375 [95% CI, 0.340–5.552]; log-rank test, P = .677; Figure 2c) were not significantly different in patients with and without mTORi conversion in the 1-year survival curves.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Covariate | HR | CI 95% | P-value | HR | CI 95% | P-value |
| Age | 1.006 | 0.974–1.038 | .726 | |||
| Sex | 0.535 | 0.223–1.282 | .161 | |||
| rATG | 3.956 | 1.625–9.63 | .002 | 3.581 | 1.449–8.848 | .005 |
| Corticosteroids | 1.402 | 0.546–3.595 | .482 | |||
| mTORi conversion | 0.942 | 0.314–2.831 | .916 | |||
| CMV status | 1.687 | 0.655–4.344 | .278 | |||
| Preventive strategy | 0.682 | 0.277–1.675 | .404 | |||
| Early-onset disease | 0.571 | 0.232–1.402 | .221 | |||
| Mutant strain | 2.093 | 0.618–7.087 | .235 | |||
| Baseline viral load | 1.197 | 0.518–2.762 | .674 | |||
| Persistent versus recurrent | 0.569 | 0.209–1.547 | .27 | |||
| TCMR before CMV disease | 0.295 | 0.039–2.196 | .233 | |||
| ABMR before CMV disease | 1.086 | 0.988–1.122 | .998 | |||
| Lymphocyte count | 0.680 | 0.294–1.572 | 0.368 | |||
- Baseline viral load was converted in a binary variable, taking the median value of 58,000 IU/mL and analyzing the correlation between baseline viral load higher or lower than this cutoff and CMV DNAemia eradication. Five variables were kept for the initial multivariate analysis and the model was convergent.
- Abbreviations: ABMR, antibody-mediated rejection; CI, confidence interval; CMV, cytomegalovirus; HR, hazard ratio; mTORi, mTOR inhibitors; rATG, rabbit anti-thymocyte globulins; TCMR, T cell-mediated rejection.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Covariate | HR | CI 95% | P-value | HR | CI 95% | P-value |
| Age | 1.013 | 0.968–1.06 | .578 | |||
| Sex | 0.290 | 0.092–0.911 | .0341 | |||
| rATG | 6.386 | 1.718–23.73 | .005 | 7.723 | 2.0422–29.2 | .002 |
| Tacrolimus | 1.233 | 0.333–4.558 | .754 | |||
| Corticosteroids | 2.559 | 0.559–11.71 | .226 | |||
| mTORi conversion | 1.524 | 0.408–5.692 | .531 | |||
| CMV status | 2.256 | 0.677–7.516 | .185 | |||
| Preventive strategy | 5.07 | 0.652–39.37 | .121 | |||
| Early-onset disease | 4.317 | 0.555–33.54 | .162 | 5.806 | 0.7313–46.1 | .096 |
| Mutant strain | 3.014 | 0.656–13.84 | .156 | |||
| Baseline viral load | 1.294 | 0.417–4.012 | .656 | |||
| Persistent versus recurrent | 0.894 | 0.241–3.313 | .867 | |||
| TCMR before CMV disease | 1.184e-08 | 0-Inf | .998 | |||
| ABMR before CMV disease | 2.959e-07 | 0-Inf | .998 | |||
| Lymphocyte count | 0.323 | 0.097–1.077 | .066 | |||
- Baseline viral load was converted in a binary variable, taking the median value of 58,000 IU/mL and analyzing the correlation between baseline viral load higher or lower than this cutoff and CMV DNAemia eradication. We put the seven most significantly associated variables to obtain a convergent model.
- Abbreviations: ABMR, antibody-mediated rejection; CI, confidence interval; CMV, cytomegalovirus; HR, hazard ratio; mTORi, mTOR inhibitors; rATG, rabbit anti-thymocyte globulins; TCMR, T cell-mediated rejection.
In the multivariate analyses, only rATG was significantly associated with an increased risk of both DNAemia (HR, 3.581; [95% CI, 1.449–8.848]; P = .005; Table 3b) and clinical recurrence (HR, 7.723; [95% CI, 2.042–29.2]; P = .002; Table 3c). The percentage of patients receiving rATG was not different in the patients with and without mTORi conversion (43.8% vs. 38.4% respectively, P = .7).
3.4 Analysis of the anti-CMV immune response using the Vδ2neg T lymphocyte expansion
Baseline characteristics were similar in patients with or without mTORi conversion in the two subgroups: “CMV persistence” and “CMV recurrence”, with the exception of lymphocyte counts which were lower in patients converted to mTORi in the CMV persistent subgroup (326 vs. 430/µL, respectively, P = .01; Table 4a, b).
| Characteristics | Conversion to mTORi, n = 7 | No conversion to mTORi, n = 15 | P-value |
|---|---|---|---|
| CMV status D+R-/R+ | 1 (14.3)/6(85.7) | 12 [80]/3 (20) | .746 |
| Preemptive versus universal prophylaxis | 1 (14.3)/6(85.7) | 0/15 | .318 |
| Early-onset versus “post-prophylaxis” CMV disease | 1 (14.3)/6(85.7) | 0/15 | .318 |
| No/VGV/GCV/FCV at inclusion | 3 (42.8)/2 (28.2)/2 (28.2)/1 (14.3) | 2 (13.3)/10(66.7)/3(20)/0 | .166 |
| Baseline CMV DNAemia (IU/mL); mean ± SD | 1.44 106 ± 2.39 105 | 1.62 106 ± 2.56 105 | .679 |
| Mutant strain | 1 (14.3)/6(85.7) | 2 (13.3)/13 (86.7) | |
| One year CMV DNAemia eradication | 5/7 (71.4) | 10/15 (66.7) | .823 |
| One year CMV DNAemia recurrence | 2/5 [40] | 3/10 (30) | .387 |
| Lymphocyte count (/µL), mean ± SD | 326 ± 198 | 430 ± 327 | .01 |
- Abbreviations: CMV, cytomegalovirus; HR, hazard ratio; mTORi, mTOR inhibitors; SD, standard deviation.
| Characteristic | Conversion to mTORi, n = 9 | No conversion to mTORi, n = 32 | P-value |
|---|---|---|---|
| CMV status | 7 (77.8)/2(22.8) | 23 (71.8)/9(28.2) | .724 |
| Preemptive versus universal prophylaxis | 1 (11.1)/8(89.9) | 9(28.2)/23(71.8) | .293 |
| Early-onset versus “post-prophylaxis” CMV disease | 1 (11.1)/8(89.9) | 8(25)/24[75] | .999 |
| Baseline CMV DNAemia (IU/mL); mean ± SD | 2.20 105 ± 3.24 105 | 5.3 106 ± 1.91 105 | .504 |
| Mutant strain | 1 (11.1)/8(89.9) | 0/32 | .219 |
| One year CMV DNAemia eradication | 6/9 (66.7) | 30/32 (93.7) | .061 |
| One year CMV DNAemia recurrence | 2/6 (33.3) | 15/30 [50] | .661 |
| Lymphocyte count (/µL); mean ± SD | 1 024 ± 848 | 762 ± 453 | .731 |
- This table describes the baseline CMV characteristics among patients with CMV recurrence regarding donor and recipient status, type of preventive strategy, inclusion for CMV persistence or recurrence, the antiviral treatment, virus characteristics, and lymphocyte count.
- Abbreviations: CMV, cytomegalovirus; HR, hazard ratio; mTORi, mTOR inhibitors; SD, standard deviation.
One-year CMV DNAemia eradication and CMV DNAemia recurrence were also similar in patients with or without mTORi conversion in these two subgroups (subgroup CMV persistence; P = .823 and .387 respectively. Subgroup CMV recurrence; P = .061 and .661 respectively, Table 4a, b).
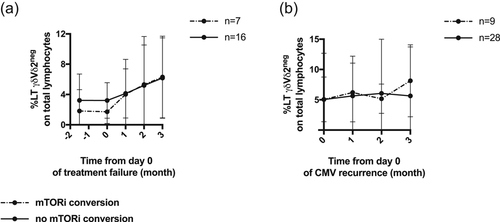
Finally, CMV-specific Vδ2neg T lymphocyte expansion were also similar in patients with or without mTORi conversion in these two subgroups (Figure 3a, b)
3.5 Rejection and graft survival
Since tacrolimus was mainly used, we compared the tacrolimus trough levels between the two groups at 1-, 3-, 6-, and 12-months post-inclusion, and observed that the tacrolimus trough level at month 3 was significantly lower in patients with mTORi conversion (Figure S1). Patients converted to mTORi exhibited few adverse events, as described in Table S1. Finally, acute rejection, eGFR, graft, and patient survival were compared 1-year post-inclusion and no significant difference was noted between the two groups (Table S2).
4 DISCUSSION
It is now widely accepted that the use of mTORi is a potential approach to decrease the incidence of CMV disease in R+ kidney transplant recipients. On the contrary, this retrospective study shows that the conversion from MPA to mTORi seems inadequate in improving CMV clearance or in better preventing CMV recurrences of persistent and recurrent CMV disease. Hypotheses can be proposed to explain this lack of efficacy in those scenarios.
First of all, this finding was observed in a very carefully selected population, displaying the most difficult CMV diseases to treat. For patients with persistent CMV DNAemia after 7 weeks of antiviral treatment, the management based on international recommendations remains controversial. Treating patients until obtaining a CMV QNAT below the lower limit of quantification on one highly sensitive assay, or two consecutive negative results if the assay is not highly sensitive is recommended.12 However, a cumulative (val)ganciclovir exposure exceeding 6 weeks is a strong risk factor for CMV antiviral drug resistance due to the emergence of UL97/UL54 mutations.3, 12 There is therefore an urgent need for new strategies in this scenario.
CMV recurrence is also a major issue. It is very frequent in patients with a lack of prior CMV immunity (D+/R−), a high baseline viral load (100,000 copies/mL), a failure to eradicate DNAemia by day 21 post-treatment, a weak viral load decrease during CMV therapy, or lymphopenia.23, 24 Our cohort of 63 kidney transplant recipients exhibited all of these characteristics. About 10% of our cohort exhibited CMV diseases with mutant strains which are also associated with a higher risk of recurrences.25 Testing a new strategy to improve CMV DNAemia eradication and to prevent recurrence in these patients was therefore very relevant but also very challenging.
Secondly, the lack of mTORi conversion efficacy could be related to the absence of tacrolimus minimization. In Transform and Athena studies, the lower incidence of CMV events could be related to everolimus or to the reduced dose of tacrolimus.9, 26, 27 In our study, most of the patients converted to everolimus had a tacrolimus blood concentration over 5 ng/mL because physicians are reluctant to decrease the tacrolimus trough level. Indeed, it has been associated with de novo donor-specific antibodies emergence and a lower graft survival.28 It probably resulted in a state of over-immunosuppression which could have contributed to this absence of effect on CMV.
Thirdly, the analysis of the CMV-specific Vδ2neg T lymphocyte expansion following the CMV disease or recurrence showed that conversion to mTORi was not associated either with a stronger or with a faster anti-CMV immune response.20 The protective effect of mTORi against CMV could depend on the immunological status of the recipient, and mainly benefit patients with preformed CMV-specific immunity. Two randomized studies have shown that mTORi attenuated the incidence of CMV disease mainly in R+ patients.10, 11 At the time of transplant, most R+ patients had memory CMV-specific T cells that could control the virus.29 mTORi may influence this immune-mediated response in R+ recipients, since it has been reported that sirolimus exerted dose-dependent immunostimulatory effects on CD8+ memory T cells in both mice and macaques exposed to viral pathogens.7, 30 More recently, an in vitro study demonstrated that mTORi significantly improved preformed CMV-specific effector memory T-cell function.31 Here, we focused mainly on naive D+R− KTR, for whom mTORi have controversial effects.30, 32, 33 From day 0 post transplantation to the end of universal prophylaxis, most of the D+R− KTR did not develop any CMV-specific T cells.34 Moreover, persistent CMV DNAemia or recurrence is also observed in D+R− patients with a lack of CMV immunity.22 Interestingly, mTORi did not influence naive T cells in vitro, nor their overall activation marker expression after CD3/CD28 coactivation.31 Our clinical data also suggest an inability of mTORi to control CMV infection in naive patients.
Finally, our study has some limits. It is a retrospective and monocentric study with a low number of patients. Very few R+ recipients develop CMV persistence or recurrence. CMV diseases in these patients are usually easily manageable. It is therefore impossible to include more R+ patients displaying the chosen inclusion criteria. Even in D+R− patients, these difficult CMV scenarios (CMV persistence or CMV recurrence) are not frequent enough to include more patients.
Moreover, the absence of standardized criteria to convert patients to mTORi could introduce potential hidden bias. One may expect a trend to consider patients with more difficult CMV disease to treat as eligible for conversion to mTORi but no difference in clinical CMV parameters was observed between the two groups. Prophylactic strategies changed over time but were equally balanced between the two groups with or without mTORi conversion. The assay to monitor DNAemia also changed over time but the threshold of CMV DNAemia detectability of 250 IU/mL did not change, which allowed us to analyze equally the eradication and recurrences all over the period. We may also point out a delay in the conversion to mTORi after inclusion. However, despite these drawbacks, our study is the first clinical experiment of the use of mTORi, compared with maintaining the standard immunosuppressive regimen: mTORi were quite well tolerated with no impact on graft function, rejection, and graft survival but showed no effect in such challenging scenarios.
In conclusion, the use of mTOR inhibitors did not lead to any improvement in the outcomes of CMV disease with persistent DNAemia or recurrence in that high-risk population.
Testing the conversion of mTORi in less severe CMV diseases, in R+ patients with preformed CMV immunity, or at an earlier stage of CMV disease (as soon as the first positive CMV QNAT is detected) should be tested in the future.
ACKNOWLEDGMENTS
We thank Catherine Rio, Bordeaux's center nurse coordinator. We also acknowledge the technicians of the Bordeaux University Hospital Laboratories of Virology for their significant contribution to this study, and the medical doctors involved in patient care.
CONFLICT OF INTEREST
The authors declare that there are no conflict of interests.